
REVIEW
J. Cent. South Univ. (2019) 26: 268-288
DOI: https://doi.org/10.1007/s11771-019-4000-3
Industrial wastes applications for alkalinity regulation in bauxite residue: A comprehensive review
XUE Sheng-guo(Ѧ����)1, WU Yu-jun(����)1, LI Yi-wei(����ΰ)1, KONG Xiang-feng(�����)1,
ZHU Feng(���)1, WILLIAM Hartley2, LI Xiao-fei(������)1, YE Yu-zhen(Ҷ����)1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Crop and Environment Sciences Department, Harper Adams University, Newport,Shropshire TF10 8NB, United Kingdom
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
Bauxite residue is a highly alkaline material generated from the production of alumina in which bauxite is dissolved in caustic soda. Approximately 4.4 billion tons of bauxite residues are either stockpiled or landfilled, creating environmental risks either from the generation of dust or migration of filtrates. High alkalinity is the critical factor restricting complete utilization of bauxite residues, whilst the application of alkaline regulation agents is costly and difficult to apply widely. For now, current industrial wastes, such as waste acid, ammonia nitrogen wastewater, waste gypsum and biomass, have become major problems restricting the development of the social economy. Regulation of bauxite residues alkalinity by industrial waste was proposed to achieve ��waste control by waste�� with good economic and ecological benefits. This review will focus on the origin and transformation of alkalinity in bauxite residues using typical industrial waste. It will propose key research directions with an emphasis on alkaline regulation by industrial waste, whilst also providing a scientific reference point for their potential use as amendments to enhance soil formation and establish vegetation on bauxite residue disposal areas (BRDAs) following large-scale disposal.
Key words:
Cite this article as:
XUE Sheng-guo, WU Yu-jun, LI Yi-wei, KONG Xiang-feng, ZHU Feng, WILLIAM Hartley, LI Xiao-fei, YE Yu-zhen. Industrial wastes applications for alkalinity regulation in bauxite residue: A comprehensive review [J]. Journal of Central South University, 2019, 26(2): 268�C288.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4000-31 Introduction
Bauxite residue, a highly alkaline solid waste, is generated by adding concentrated caustic soda to dissolve bauxite during the alumina production process [1�C4]. The global bauxite residues inventory reached approximately 4.6 billion tons in 2018, with a production rate of approximately 200 million tons per annum, which is the largest emission of smelting waste in the non-ferrous metal industry [5�C7]. Currently, disposal of bauxite residues is to stockpile, which consumes a lot of manpower and financial resource, taking up large areas of land, polluting the environment, and causing immeasurable spill disasters due to its mismanagement [8�C13]. It has been reported that in some areas of China, Hungary, India and Vietnam have all suffered ground and surface water pollution due to bauxite residues dam-failures, which subsequently brought about significant negative impacts on environmental safety and residents living in the surrounding areas [14�C16].
At present, bauxite residues reuse is largely concentrated on valuable metal recycling, construction materials, and environmental restoration materials, production and manufacturing [17�C22]. However, because of the strong alkaline nature of bauxite residues, problems such as the production of alkali aggregates and corrosion of steel parts during the process of actual application, restrict the utilization of bauxite residues [23�C25]. In reality, the global re-use of bauxite residues is less than 10% due to their high pH (10�C13) and leachate production (12�C14) [26�C31]. Therefore, alkaline regulation is the major challenge affecting bauxite residues reuse. Dealkalization is the main process which regulates alkalinity and includes methods such as amelioration by gypsum, neutralization with seawater, pressure leaching with carbon dioxide, and water and acid leaching [32�C34]. Amelioration with gypsum has been used for grassland restoration, precipitating carbonates and rapidly forming calcite; this suppresses the solubility of solid phase alkalinity to reduce pH and aluminum solution concentration, but this strategy takes a long-time in order to achieve revegetation [35, 36]. Seawater neutralization is similar to that of gypsum, with addition of seawater reducing pH, but an influx of Na-salts is also introduced into the residue which damages its physico-chemical structure; furthermore, this method requires the site to be close to the coast [37, 38]. Water leaching relies on influencing factors such as leaching time, temperature, stage and liquid to solid ratio; however, its dealkalization rate is less than 50% and large volumes of water are consumed in the process [39]. Acid leaching can effectively remove most of the free alkali and even dissolve calcium and aluminum in the bauxite residues [40�C42]. Pressure leaching using carbon dioxide can react with hydroxide to form carbonate and bicarbonate, but the processes may affect the dissolution of other chemical alkali, such as tricalcium aluminate (TCA). However, leaching with carbon dioxide is difficult to operate because of the high pressure atmosphere which needs to be maintained throughout the process [43, 44]. Although alkalinity reduction is noticeable using the methods described above, the chemicals applied to the bauxite residue are costly and problematic to widely implement due to the large volumes of bauxite residues.
For the meantime, industrial wastes such as acid, brine, ammonia nitrogen waste water, acid waste gas and gypsum, are effectively used to regulate bauxite residues alkalinity, but have become major environmental problems in relation to social and economic development. Consequently, alkaline regulation of bauxite residues by industrial wastes to achieve ��waste control by waste�� requires reconsideration and alternative methods in order to achieve both social and environmental considerations. Numerous strategies have been adopted in an attempt to understand bauxite residues remediation by industrial waste and the objectives of this paper are to review the alkaline sources and transformations of bauxite residues, analyze the characteristics of typical industrial wastes and their potential restoration approaches for bauxite residues alkalinity regulation. Nevertheless, the focus should be to facilitate bauxite residues soil formation, improve the stability of alkaline regulation and establish vegetation, all of which still requires further research.
2 Bauxite residue generation and its alkaline composition
2.1 Resources and distribution
In 1887, Karl Josef Bayer discovered that aluminum hydroxide precipitated from a sodium aluminate solution in crystalline form, and subsequently the ��Bayer process�� was developed [45]. Globally, the main alumina extraction processes include the Bayer method, sintering and Bayer-sintering methods. The characteristics and principles of the different alumina processes are summarized in Table 1 [46�C49].
In 1892, Bayer plants were established in England, France, Italy and Germany. Over the next 30 years, plants were established in the United States, Japan and the Soviet Union to produce alumina [6]. The development of the aluminum industry led to the rapid increase in production of bauxite residues. Estimates of global bauxite residues annual production and cumulative inventory in the period between 1900 and 2018 are shown in Figure 1 [6, 7]. In 1900, the global inventory was estimated to be 13 million tons, increasing to an estimated 22 million tons in 1940. At the beginning of the second industrial revolution, aluminum became an indispensable strategic metal for national economic development, as humanity entered the ��electrical age��. With an increasing aluminum demand for national defense construction, the largest nonferrous metal in production and consumption resulted in its annual production and cumulative inventory rising rapidly. Since the 1980s, Australia, Brazil, Spain and Ireland have gradually built larger factories due to technological developments and growing economies. Since 1985, bauxite residue has been produced at a rate of approximately 48.5 million tons per year, but this material has not been effectively utilized. In 2018, the global inventory reached 4.6 billion tons, with an annual growth rate of approximately 200 million tons.
Table 1 Characteristics and principles of different alumina processes
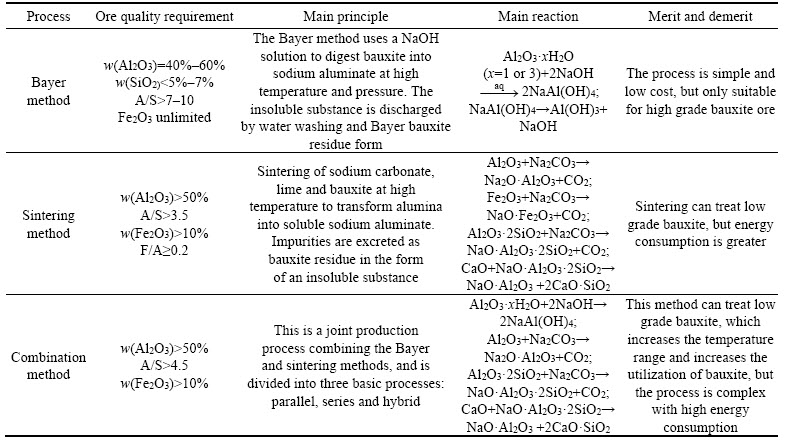
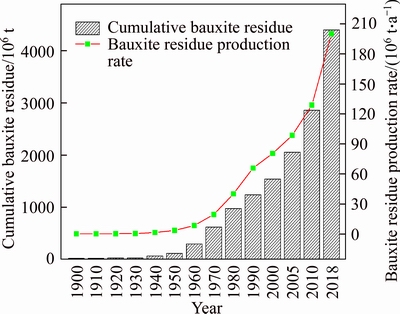
Figure 1 Global annual production and cumulative inventory of bauxite residue between 1900 and 2018
Global annual production distribution of bauxite residues between 1995 and 2018 is presented in Figure 2 [7]. In 2018, annual production distribution was estimated to be as follows: China (57.50%), Australia (16.20%) and Brazil (8.61%). The development of national economy of China during the 21st century led to the rapid development of the aluminum industry together with the generation of large volumes of bauxite residues; this accounted for 7.65% of the global annual production in 1995 and increased to 57.50% in 2018 with an annual production of 69 million tons of alumina.
2.2 Alkaline sources and transformations
The physical and chemical properties of bauxite residues vary according to the composition of bauxite and the alumina extraction process [50, 51]. However, the chemical composition of bauxite residue mainly includes Na2O, Al2O3, Fe2O3, SiO2, TiO2 and CaO. Approximately 95% of the global aluminum enterprises are currently using the Bayer process to produce aluminum oxide. Consequently, there are differences in the mineral and chemical compositions of different bauxite residue disposal areas (BRDAs) (Table 2). Alkaline substances are mainly divided into insoluble alkali and soluble chemical alkali (Table 3) [52�C56]. The insoluble alkali are mainly composed of sodium, cancrinite, calcite, hydrogarnet, diaspora, gibbsite and TCA (Tri-calcium aluminate) with strong acid neutralization capabilities, which are typical desilicon products of the alumina production process [57, 58]. In the process of extracting alumina, soluble alkali is generated from the reaction of caustic soda and bauxite. The residual sodium hydroxide forms strongly alkaline slurry, such as sodium aluminate and sodium silicate, which easily reacts with CO2 in the air and a small amount of SiO2 to generate sodium bicarbonate and soluble sodium silicate [51, 55]. The soluble chemical alkali is easy to dissolve and forms a large number of free alkaline anions in the liquid phase, leading to an increase in pH. During long-term natural storage, there is a complex transformation relationship between soluble and insoluble alkali (Table 3). SANTINI et al found that by monitoring the dissolution rate of trica (TCA) in bauxite residues, insoluble alkali was highly significant in reducing alkalinity [26, 59, 60]. There are two main approaches to regulating alkalinity: one is to convert the insoluble alkali into soluble alkali by destroying the chemical structure and then removal by water leaching; the other is to transform soluble alkali into a dissoluble substance by precipitation reaction, then subsequently suppress the dissolution of insoluble alkali.

Figure 2 Global annual production distribution of bauxite residue between 1995 and 2018
3 Analysis of industrial waste management practices
3.1 Characteristics of industrial wastes
Industrial waste is generally divided into water, gas and residue, and they are collectively termed as ��the three wastes��. Wastewater is recognized as either sewage or spent liquor which contains various raw materials, intermediate by-products and other contaminants from industrial processes associated with water loss. Wastewater is complex in which discharge rates may vary and it can have a diverse range of pollutants and concentrations, some of which may be toxic, a high environmental risk and the difficulty of monitoring pollutant migration after its discharge. Waste gas contains a wide variety of pollutants, has a complexy composition, high and elevated contaminant concentrations, can widely contaminate surrounding areas and is of great harm to humans. Waste residues produced from solid wastes during the process of industrial manufacture may contain poisonous, combustible or caustic byproducts, infectious diseases or other hazardous substances. Long-term stockpiling of waste residues may lead to pollution of surrounding water sources and the atmosphere. Waste residues are typically contaminated, difficult to treat and produce in large quantities [71]. Table 4 displays the main characteristics of some typical industrial wastes. Industrial wastes are largely generated from processes involving papermaking, chemical production, steel, metallurgy, electric power, food, mining and textiles [72]. Non-ferrous metal smelting and sulfuric acid production produce large quantities of acidic waste water.In addition to acid residues, the acidic waste water generally contains Cl�C, SO42�C and usually concentrated waste acid from the neutralization of alkaline waste [73]. Gelatin production or lime precipitation, for example, will produce calcium sulfate, phosphorus calcium brine and ammonia nitrogen wastewater, rich in calcium, magnesium or ammonium and will react with alkaline anions such as CO32�C, HCO3�C, AlO2�C, OH�C in bauxite residues to reduce the content of free alkali [10, 74]. Acid gases, such as CO2, SO2 and H2S produced by chemical plants, thermal power plants, oil refineries and pharmaceutical plants, react in water to form weak acids that neutralize free alkali, and dissolve bound alkali, thereby reducing their content [75�C77]. Gypsum is an industrial waste that has different sources of origin such as phosphogypsum, fluorogypsum and soda gypsum [78], but is generally more than 80% CaSO4. Calcium sulphate can be used to precipitate CO32�C and HCO3�C alkaline anions in bauxite residue and the calcium�Csodium substitution reaction can promote conversion of chemical bonded alkali into more stable minerals, thereby inhibiting the dissolution of the chemical bonded alkali [63]. Other waste sources produced from biomass mainly include agricultural, forestry and urban wastes, which are estimated to reach 10�C15 billion tons worldwide [79]; biomass can produce large volume of acid during the process of microbial fermentation [3].
Table 2 Alkaline minerals and chemical composition in mass fraction of bauxite residue in typical alumina plants
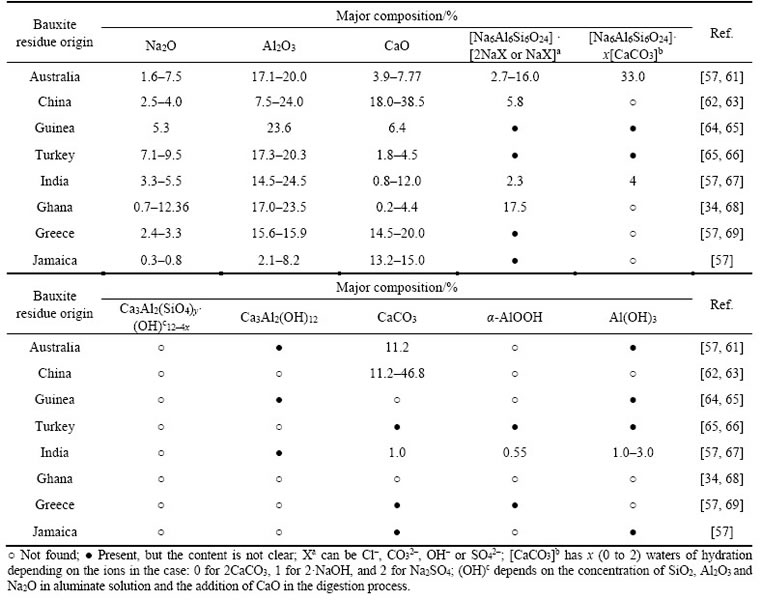
Table 3 Generation reactions and transformation of alkali in bauxite residue [30, 63, 70]
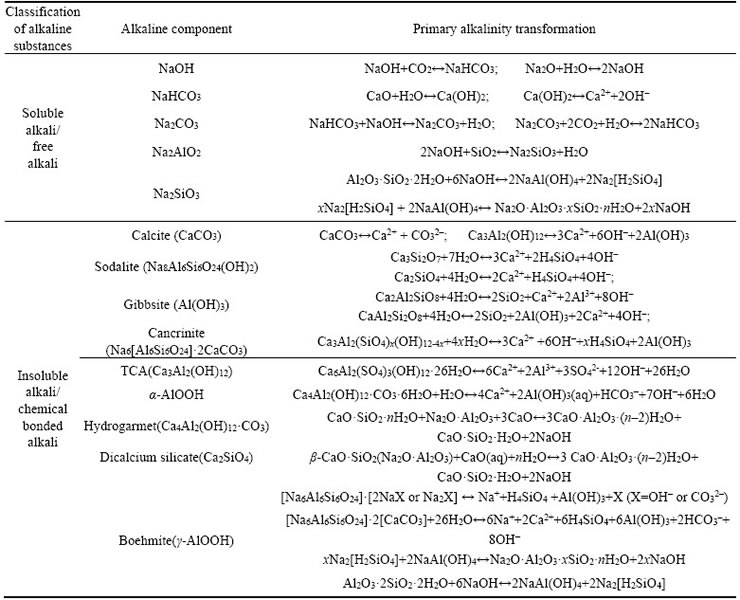
Table 4 Main characteristics of typical industrial wastes applied to regulate alkalinity

3.2 Conventional treatment of industrial wastes
The large volume of industrial waste discharged into the environment requires proper handling and failure to meet the approved discharge standards leads to pollution which consequently threatens both ecological and human health [92]. As a result, there has been increased attention to ��the three wastes��. For example, the United States of America, Britain and Japan all have a long history of industrial development, together with years of adjustment to environmental protection policies [93, 94]. In China, production of solid waste has formed a guiding philosophy for reduction of harm [95].
Currently, the treatment of industrial solid waste residues mainly includes landfill, compost processing and burning. Landfill occupies large areas, has lower waste resource utilization efficiency, and forms secondary pollutants. Hence, it has been unable to meet the requirements of urban development. The treatment is unstable. Burning is common practice, although it has higher operation costs, it is only suitable to certain regions, and the production of secondary pollutants, such as dioxins and heavy metals, restricts its practical application [96]. Waste gas treatment practices include active carbon adsorption, catalytic combustion, acid-base neutralization and plasma treatment [97]. Three processing methods, physical, chemical and biological, exist for waste water treatment. The method is based on the properties of the contaminants and is primary divided into physical absorption, physical separation and electrolysis. Chemical methods involve the reaction and transformation of pollutants, such as acid-base neutralization and redox process. Biological treatment involves adsorption and degradation of organic contaminants by microoganisms [98].
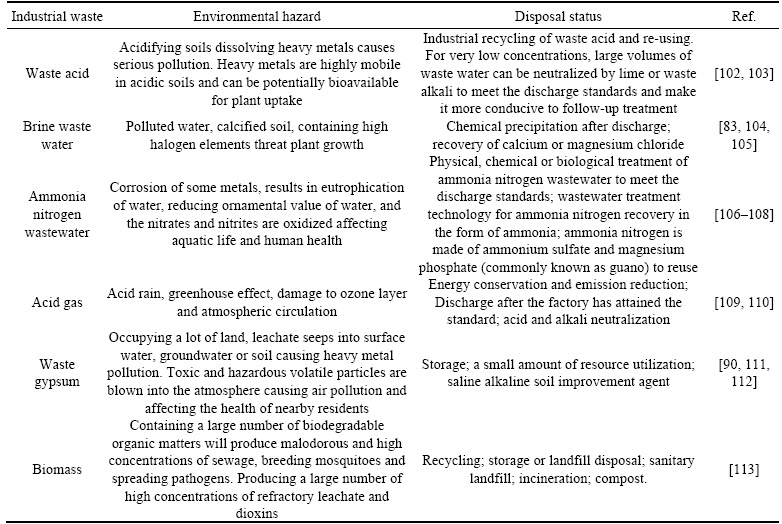
Table 5 shows the environmental hazards and disposal status of typical industrial waste. Conventional treatment of industrial waste recovers toxic substances and once a standard safe concentration has been reached, the water is discharged [99]. Exhaust gas treatments mainly involve desulfurization and denitrification processes [100]. Industrial solid waste disposal is mainly through stockpiling and then reuse, such as using waste gypsum to produce cement, bricks and environmental materials, or biogas production from biomass fermentation [101]. Nevertheless, industrial waste has disposal difficulties and so developing strategies for ��waste control by waste�� is an economically feasible way to promote the consumption of industrial wastes whilst solving waste pollution problems. Therefore, regulation of alkalinity in bauxite residues by addition of industrial waste not only promotes the sustainable use of waste products from other sources, but also assists in the production of soil formation at BRDAs to improve their ecological status.
Table 5 Environmental hazards and disposal status of industrial wastes
4 Incorporation of industrial waste for alkaline regulation
Management problems still exist for large quantities of industrial wastes as they are potentially hazardous materials and have high concentrations of toxic metals and other harmful substances within them. Expensive treatment costs, together with stringent standards and disposal regulations and restricted disposal sites, all provide an excellent opportunity to exploit these waste streams as useful amendments to control and regulate bauxite residue alkalinity, essentially providing potential technical, economic and environmental benefits.
4.1 Waste water
4.1.1 Waste acid neutralization
Adsorbed alkali, free alkali and chemical alkali in bauxite residues can be easily leached by neutralization of waste acid (H2SO4, HCl, HNO3, H3PO4) [1]. WANG et al [76] revealed that the lattice structure of bauxite residues changed when treated with waste hydrochloric acid, and the efficiency of dealkalization was more than 80% using carbon dioxide. The pH of bauxite residues decreased from 12.5 to 4.6�C8.0 by titration with hydrochloric acid and was maintained for 20�C50 h to achieve the reaction equilibrium [114]. Waste acid can also accelerate transformations in bauxite residue phases, improve its physical structure, promote the formation of large aggregates and sodium salts in the leaching liquid, and can be crystallized and recycled [115]. Alkalinity regulation by waste acid is founded on a series of neutralization reactions with soluble and insoluble alkali as shown below [116, 117].
OH�C + H+��H2O (1)
2CO32�C+2H+��H2O+CO2�� (2)
Al(OH)4�C+H+��Al(OH)3+H2O (3)
SiO32�C+2H+��H2SiO3 (4)
[Na6Al6Si6O24]��[2NaOH]+8H++10H2O��8Na++6Al(OH)3+ 6H2SiO3 (5)
Na8(Al6Si6O24)CO3+24H+��8Na++6Al3++6Si(OH)4+H2O+CO2�� (6)
However, the pH of bauxite residues increases after washing with water. The main reason for this is due to the strong buffering ability of chemical bonded alkali [118]. Consequently, alkalinity can be effectively regulated by adding appropriate quantities of waste acid to reduce pH to approximately 4.5 and then water to raise it to 6.0 [32]. Furthermore, it has also been reported that alkaline regulation with HPOx wastewater and the introduction of P, Ca and other elements needed for plant grow, is beneficial for vegetation establishment.
4.1.2 Brine neutralization
Soluble alkali can be converted into soluble minerals, such as calcite (CaCO3), hydrocalumite (Ca4Al2(OH)12��CO3), tricalcium aluminate (Ca3Al2(OH)12��CaCO3��5H2O), hydrotalcite (Mg6Al2(CO3)(OH)16��4H2O) and brucite (Mg3(OH)6) by adding Ca2+ and Mg2+ wastewater to bauxite residues, stabilizing soluble alkali and inhibiting dissolution of insoluble alkali [63]. CLARK et al [119] demonstrated that bauxite residues pH could be reduced from 13.1 to 7.5 when the liquid�Csolid ratio was 5:1, by regulating alkalinity with Ca2+ and Mg2+ in brine. This was due to precipitation reactions of hydroxide (OH�C), carbonate (CO32�C), aluminum (Al(OH)4�C), Ca2+ and Mg2+. Addition of Ca2+ and Mg2+ not only regulated alkalinity, it reduced pH and EC, whilst also reducing active Al, thereby inhibiting the dissolution of tricalcium (TCA) in insoluble alkali. CLARH et al [119] treated bauxite residues with CaCl2 wastewater under optimal experimental conditions; they discovered that the quality fraction of Na was lower than 0.8% with 75% removal rate whilst the leaching liquid pH was approximately 7. HAN et al [120] revealed that the introduction of Ca2+ accelerated a pH decrease in bauxite residues whilst also precipitating carbonate to form calcium carbonate. Addition of Ca2+ and Mg2+ could effectively precipitate alkaline anions and increase acid neutralization. Furthermore, the effect of alkaline regulation exhibited characteristics of long-term stability providing the possibility of safe deposition of bauxite residues. The main reactions are as follows [57, 114, 121]:
Ca2++2OH�C Ca(OH)2 (7)
Ca(OH)2 (7)
Ca2++CO32�C CaCO3 (8)
CaCO3 (8)
3Ca2++4OH�C+2Al(OH)4�C Ca3Al2(OH)12 (9)
Ca3Al2(OH)12 (9)
4Ca2++4OH�C+2Al(OH)4�C+CO32�C Ca3Al2(OH)12��CaCO3 (10)
Ca3Al2(OH)12��CaCO3 (10)
Mg2++2OH�C Mg(OH)2 (11)
Mg(OH)2 (11)
Mg2++CO32�C MgCO3 (12)
MgCO3 (12)
3Mg2++4OH�C+2Al(OH)4�C Mg3Al2(OH)12 (13)
Mg3Al2(OH)12 (13)
4Mg2++4OH�C+2Al(OH)4�C+CO32�C Mg 3Al2(OH)12��MgCO3 (14)
Mg 3Al2(OH)12��MgCO3 (14)
6Mg2++4OH�C+2Al(OH)4�C+3SO42�C Mg3Al2(OH)12��3MgSO4 (15)
Mg3Al2(OH)12��3MgSO4 (15)
4.1.3 Ammonia nitrogen wastewater neutralization
Ammonia nitrogen wastewater, containing a high concentration of ammonium ions, is produced by many chemical processes. When ammonia nitrogen wastewater is introduced into BRDAs, the ammonium ion reacts with the main alkaline anions (CO32�C, HCO3�C, AlO2�C, OH�C), consuming the alkaline anions and reducing alkalinity. The main reactions are as follows [122�C124]:
NH4++H2O NH3��H2O+H+ (16)
NH3��H2O+H+ (16)
CO32�C+H2O HCO3�C+OH�C (17)
HCO3�C+OH�C (17)
HCO3�C+H2O H2CO3+OH�C (Incomplete hydrolysis) (18)
H2CO3+OH�C (Incomplete hydrolysis) (18)
AlO2�C+2H2O Al(OH)3+OH�C (19)
Al(OH)3+OH�C (19)
Under optimum experimental conditions, WANG et al [74] established that the concentration of Na2O in bauxite residues could be reduced to less than 1% when treated with ammonium chloride. ZHONG et al [125] and LI et al [126] discovered that the leaching rate of Na+ by ammonium chloride was the same as that of water, mainly due to the ion exchange capacity of the ammonium ion being greater than sodium.
4.2 Waste gas
4.2.1 Carbon dioxide (CO2)
Using large-scale CO2 from ammonia plants to neutralize bauxite residues was first used at Alcoa��s Kwinana Refinery, Western Australia [127]. The pH of bauxite residues treated by CO2 decreased after one day, eventually reaching a stable 7.5 in 1.013��105 Pa (1 atm) for 30 d [28]. This demonstrated that the initial neutralization reaction occurred in the liquid phase, and dissolution of tricalcium aluminate solid phase resulted in recovery of pH. All TCA could be dissolved by CO2 neutralization, and precipitated in the form of calcium carbonate and gibbsite. However, further observation of the reaction process revealed the formation of dawsonite (NaAl(CO3)(OH)2), which can be stable at pH 4.1 to 7.8 [128]. TANG et al [129] conducted an alkalinity regulation experiment with bauxite residues using 20% CO2 from lime kiln based on the characteristics of an aluminum plant in Shanxi, China. Under optimal experiment conditions, alkali removal rate reached 31%, demonstrating the effective removal of soluble alkali in bauxite residues. YIN et al [130] found that the process of desodium reaction in bauxite residues treated with CO2 from flue gas had a synergistic effect on sodium based solids with CO2�CH2O, OH�C�CCO2 system. The alkaline substances in bauxite residues reacted with CO2 and sodium decreased significantly. With reasonable design and appropriate operation, the rate of sodium desodium removal reached more than 70% whilst the pH was reduced to approximately 8.0. Carbon dioxide from waste gas dissolved in the solution to form carbonic acid, continuously released H+ and neutralized the alkaline ions in bauxite residues (Figure 3). Finally, the chemical balance between CO32�C and HCO3�C was formed in the bauxite residues liquid phase and the pH of the liquid phase reduced to less than 10.0. As a result, regulation of pH by CO2 was achieved [122].
4.2.2 Sulphur dioxide (SO2)
Refineries in Japan (Sumitomo) and Italy (EurAllumina) began using SO2 waste gas to neutralize bauxite residues during the mid-1970��s and recently in the early 2000��s [131]. FOIS et al [132] revealed that the suspended state of bauxite residues could efficiently absorb SO2 gas in a bubbling reactor to form Na2SO3 which was further oxidized to Na2SO4 (Figure 3). SO2 will increase the concentration of H+ to dissolve sodium minerals and promote solution Na+ leaching including desilication products. NAN et al [77] studied the dealkalization rate at low concentrations of SO2; the dealkalization rate reached 70%, fulfilling the requirements of cement production. At present, studies concentrating on flue gas desulfurization by bauxite residues still require further research in order to determine their industrial value.
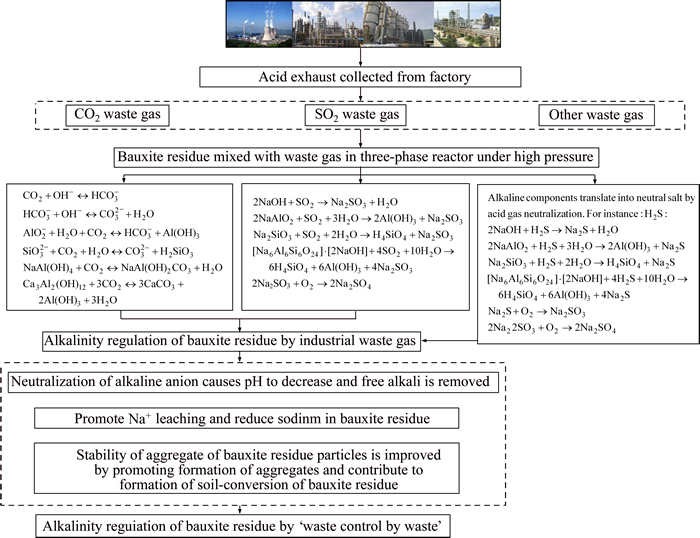
Figure 3 Mechanisms for regulation of alkalinity of bauxite residue by waste acid gas
4.2.3 Other acid waste gas
Industrial waste gas contains large concentrations of acid gases, such as H2S, NO2, POx, HF [133�C135]. The alkaline material in bauxite residues will transform into neutral salts following reaction of acid gas and hydrate to reduce the alkalinity of bauxite residues. Bauxite residue has strong alkalinity and a large specific surface area, and its ability to neutralize acid is very strong [136,137]. It is therefore difficult to regulate alkalinity with these gases mainly due to safety risks and operational constraints. However, using bauxite residues to absorb the exhaust gases NO2, POx, HF and other acid gases has promise for future research opportunities.
4.3 Waste residues
4.3.1 Waste gypsum
During the process of industrial production and flue gas desulfurization, large volume of waste gypsum are produced, including phosphogypsum, desulfurization gypsum and fluorine gypsum, which contain a lot of CaSO4 and acid. Regulation of alkalinity by phosphogypsum has been attempted many times; WONG et al [138] discovered that addition of phosphogypsum neutralized the alkalinity and reduced the concentration of sodium in bauxite residue, which was beneficial to the growth of herbaceous plants in BRDAs. JONES et al [139] studied the pH of bauxite residue and the impact of Na+ leaching by adding phosphogypsum after 6 and 10 weeks. As a result, pH decreased by two units and EPS (extracellular polymeric substances) also reduced, clearly due to soluble sodium which was much greater than residual sodium in bauxite residue, making conditions more conducive to vegetation restoration and improvement of the BRDAs. JONES et al [140] treated samples collected from the Kwinana and Alcoa aluminum plants with 2% phosphogypsum and two types of organic matter (solid and poultry excrement). The results revealed that bauxite residue treated with organic waste and phosphogypsum improved its physical and chemical properties, reduced alkalinity and supported plant growth. WANG et al [141] studied the effect of several factors on dealkalization, but the alkali content could not be reduced by desulfurization gypsum even under optimum conditions. WONG et al [142] evaluated the effect of waste gypsum, and found that it reduced pH, electrical conductivity (EC), Na and Al contents, and provided a continuous supply of Ca2+. This resulted in a lower ESP, improving soil conditions, increasing seedling germination rate and dry weight yields (Figure 4) [142�C144].
Waste gypsum can improve the structure of bauxite residue, reduce weight and firmness, increase porosity to enhance its permeability and reduce ground evaporation, which is favorable for salt leaching and reducing salt to surface aggregation [86]. Secondly, waste gypsum can reduce the pH of bauxite residue, increase the concentration of phosphate, Ca2+ and SO42+ and decrease the concentration of Na+, AlO2�C, CO32�C, HCO3�C, and improve both physical and chemical properties [115, 145, 146]. In addition, waste gypsum combined with organic waste and other ameliorants can improve the composition of nutrients providing enhanced conditions for vegetation establishment [147, 148].
4.3.2 Biomass
Alkaline regulation by biomass uses biological and microbial activity. Metabolites can reduce alkalinity and improve the physical properties of bauxite residue, which is a very promising method for in situ restoration of BRDAs. Figure 5 shows the mechanisms of regulating alkalinity of bauxite residue by biomass waste. REN et al [149] discovered that the pH of bauxite residue dropped from 10.60 to 9.61 and 8.96 respectively by adding 20% vinegar and furfural residue. Compared with vinegar residue alone, furfural residue reduced the pH, mainly due to the acid of furfural residue being stronger and containing approximately 5% free acid which has rich surface functional groups, such as ��NH2, ��OH, causing the pH to decline rapidly. COURTNEY et al [10] found that adding mushroom compost in bauxite residue could improve the physical and chemical properties of bauxite residue and reduce alkalinity to facilitate vegetation reestablishment. XENIDIS et al [150] assessed the improvement effect of gypsum, municipal sludge, and calcium phosphate on the bauxite residue matrix and found that they all promoted plant growth. Many researchers have carried out BRDAs stability studies using humus, straw, biomass sawdust, citric acid residue and bagasse, which all can improve the physical and chemical properties of bauxite residue to a certain extent and reduce alkalinity [151�C153].

Figure 4 Mechanisms of waste gypsum possibly contributing to pH neutralization in bauxite residue
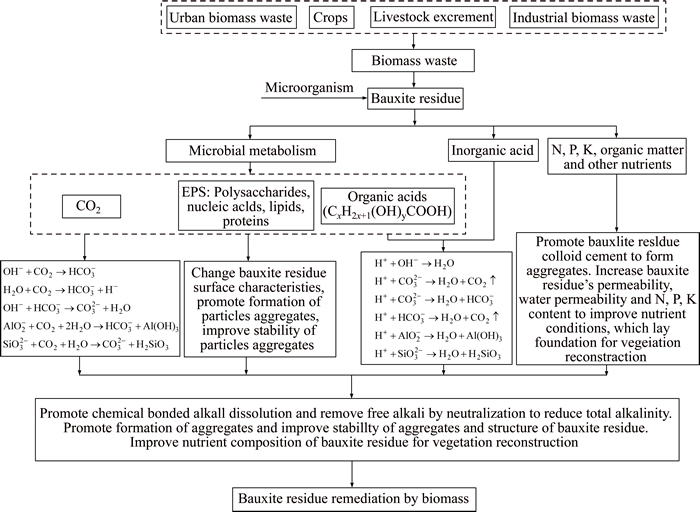
Figure 5 Mechanisms of regulating alkalinity in bauxite residue by biomass waste (EPS: extracellular polymeric substances)
4.3.3 Other waste residue
In addition, some works have used municipal sludge, compost, zeolite, fluoride gypsum, clinker and fly ash to improve the matrix of bauxite residue [138, 154�C156]. These industrial solid wastes are mainly rich in organic matter and calcium. Addition of organic matter can provide nutrients, form a stable complex with polyvalent metal ions, facilitate the agglomeration of bauxite residue particles, and provide energy for microorganisms which improves their activity [123, 157]. Calcium ions can react with alkaline substances in bauxite residue to form tricalcium aluminate and displace sodium to reduce alkalinity, which is conducive to the reconstruction of vegetation by improving the stability of bauxite residue microaggregates and increasing bauxite residue permeability [63, 158]. Although the addition of these materials can improve the physical and chemical properties of bauxite residue, maintaining the growth of vegetation on BRDAs still requires substantial financial support.
5 Problems associated with industrial waste alkaline regulation
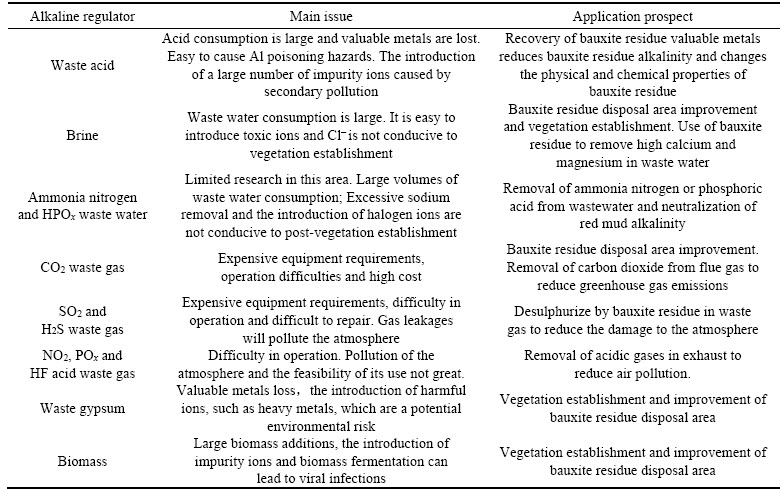
Regulating alkalinity using industrial waste has made some progress, although the methods described above still have constraints which limit them. Industrial wastes may allow improvement in regulating alkalinity, but as a result of their additions, nutrient imbalances may lead to undesirable effects on vegetation establishment [159]. At present, further studies of plant-bauxite residue-groundwater effects on the whole system are required and the effects of industrial waste on plant quality, the soil environment and groundwater quality following post-vegetation reconstruction are urgently required. Waste gypsum, ammonia nitrogen wastewater and other wastes, should be considered in an attempt to avoid salt increase and reduce the risk of salinization [158]. Furthermore, there are many harmful toxins and heavy metal ions present in some industrial wastes, which can produce secondary pollution. The main issues for alkaline regulation by industrial waste are presented in Table 6.
There are limited applications in which acid waste, Ca2+ and Mg2+ wastewater or ammonia nitrogen wastewater are used to regulate alkalinity. Waste acid neutralization is mainly based on metal recovery [160]; nevertheless, alkaline substances are complex and result in waste acid consumption larger and easier to dissolve toxic metals such as Al [161]. Calcium and Mg2+ wastewater and ammonia nitrogen wastewater will introduce toxic ions and a large concentration of anions, which has a negative effect on safe stockpiling and further uses. Limited research exists for the application of brine and most of the research focuses on brine from synthetic laboratory investigations. Although the technique can provide sufficient calcium and magnesium ions to reduce alkalinity, engineering applications are still required to solve problems such as inputs and cost. In the meantime, brine treatment introduces a large amount of Cl�C which can adversely affect its long-term uses and vegetation growth [40]. Consequently, brine needs to be combined with other alkaline regulators to control dosage and proportion, reduce cost and limit potential environmental risks.
Acid industrial waste gases can effectively convert alkaline anions and reduce the pH of bauxite residue. However, the anti-pressure and aseismic demands on the equipment are more difficult for the process to be widely used [27, 162].
Waste gypsum can not only reduce pH and salt content, but also promote formation of large particulate aggregates and increase the matrix permeability coefficient [120]. Research founded on alkaline transformation combined with plant establishment, microorganism activity and overall ecological restoration, should be the focus for future studies [163�C165]. Studies focusing on the improvement and acceleration of soil formation processes have important significance, although care with addition of waste gypsum which may introduce harmful heavy metal ions, may result in secondary pollution [166].
Biomass regulation is mainly used to produce acid by means of fermentation with microorganisms to effectively reduce pH, improve physical and chemical properties and nutrient conditions of bauxite residue, which are essential for the establishment of vegetation [167]. Biomass has good prospects for alkaline regulation, although the screening of alkali-resistant microorganisms is more complex and may lead to certain limitations.
Table 6 Main issues following addition of industrial wastes
6 Conclusions and future prospects
The increasing storage of bauxite residue can easily lead to major environmental problems and safe disposal of bauxite residue remains at a worldwide problem. Whether the addition of industrial wastes is environmentally friendly requires a focus on long-term and fixed-point tests, following logical research and verification studies. Problems for the regulation of alkalinity by wastewater have hindered its wider application; salinity is high. There is the influence of geographical location, and the risk of secondary pollution. Currently, waste gypsum and biomass show the most promise in aiding soil formation of bauxite residue and ecological rehabilitation. Nevertheless, alkaline regulation characteristics and existing problems have made it difficult to achieve long-term stability. Alkaline regulation by industrial waste application, achieved using the ��using waste to treat waste�� principle, makes bauxite residue environmental development a future possibility.
Incorporating appropriate wastes can transform free alkali and chemical bonded alkali by reducing bauxite residue alkalinity. This can improve the physical properties of bauxite residue, balance the nutrient supply to plants and improve beneficial microbial colonization and activity, which is an important precondition and the key link for alkaline regulation and soil formation of bauxite residue. In the future, regulation of bauxite residue alkalinity by industrial waste should be concerned with the following directions:
1) It should be economically and technologically feasible, by reducing pollutant emissions and the cost of industrial waste treatment to achieve the principal of ��waste control by waste��.
2) At bauxite residue disposal sites, monitoring and control of environmental indicators during restoration with industrial wastes should include the control of dust, monitoring of groundwater and surface water, and assessment of changes to toxic and harmful substances. Potential risks and harmful factors following industrial waste additions need further analysis. A feasible improvement in measures to control secondary pollution should also be considered.
3) Studies should focus on the process mechanisms and reaction dynamics of alkalinity regulation by industrial waste, in order to determine the best technological conditions, application range and effectively solve problems of excessive consumption.
4) Industrial wastes should work together with existing methods to improve the physical and chemical properties of bauxite residue, screen bauxite residue pioneer plants and use modified microorganisms, more tolerant of alkalinity, such as salt-tolerant and acid-producing bacteria. Future research should aim to establish an environment suitable for plant growth to phytostabilize the soil and improve the ecological environment of bauxite residue.
5) Research should concentrate on real natural storage facilities and be based on alkaline related ion reaction-migration behaviors and mineral dissociation equilibriums.
6) Studies should be central to soil formation processes in amended BRDAs, focusing on innovative technologies to reduce emissions which will help further our understanding, but this still maintains a challenge for the alumina industry.
References
[1] KONG Xiang-feng, LI Meng, XUE Sheng-guo, HARTLEY W, CHEN Cheng-rong, WU Chuan, LI Xiao-fei, LI Yi-wei. Acid transformation of bauxite residue: Conversion of its alkaline characteristics [J]. Journal of Hazardous Materials, 2017, 324: 382�C390. DOI: 10.1016/j.jhazmat.2016.10.073.
[2] XU Bing-an, SMITH P, WINGATE C, SILVA L D. The effect of calcium and temperature on the transformation of sodalite to cancrinite in bayer digestion [J]. Hydrometallurgy, 2010, 105: 75�C81. DOI: 10.1016/j.hydromet.2010.07.010.
[3] ZHU Feng, ZHOU Jia-yi, XUE Sheng-guo, HARTLEY W, WU Chuan, GUO Ying. Aging of bauxite residue in association of regeneration: A comparison of methods to determine aggregate stability & erosion resistance [J]. Ecological Engineering, 2016, 92: 47�C54. DOI: 10.1016/j.ecoleng.2016.03.025.
[4] SANTINI T C, KERR J L, WARREN L A. Microbially- driven strategies for bioremediation of bauxite residue [J]. Journal of Hazardous Materials, 2015, 293: 131�C157. DOI: 10.1016/j.jhazmat.2015.03.024.
[5] ZHU Xiao-bo, LI Wang, GUAN Xue-mao. An active dealkalization of red mud with roasting and water leaching [J]. Journal of Hazardous Materials, 2015, 286: 85�C91. DOI: 10.1016/j.jhazmat.2014.12.048.
[6] POWER G, GRAFE M, KLAUBER C. Bauxite residue issues: I. Current management, disposal and storage practices [J]. Hydrometallurgy, 2011, 108: 33�C45. DOI: 10.1016/j.hydromet.2011.02.006.
[7] USGS (United States Geological Survey). Mineral commodity summaries: Bauxite and alumina [R]. Washington: United States Government Printing Office, 2016.
[8] PEPPER R A, COUPERTHWAITE S J, MILLAR G J. Comprehensive examination of acid leaching behaviour of mineral phases from red mud: Recovery of Fe, Al, Ti, and Si [J]. Minerals Engineering, 2016, 99: 8�C18. DOI: 10.1016/j.mineng.2016.09.012.
[9] RENFORTH P, MAYES W M, JARVIS A P, BURKE I T, MANNING D A C, GRUIZ K. Contaminant mobility and carbon sequestration downstream of the Ajka (Hungary) red mud spill: The effects of gypsum dosing [J]. Science of the Total Environment, 2012, 421�C422: 253�C259. DOI: 10.1016/j.scitotenv.2012.01.046.
[10] COURTNEY R, KIRWAN L. Gypsum amendment of alkaline bauxite residue-plant available aluminium and implications for grassland restoration [J]. Ecological Engineering, 2012, 42: 279�C282. DOI: 10.1016/j.ecoleng. 2012.02.025.
[11] ZHU Feng, LIAO Jia-xin, XUE Sheng-guo, HARTLEY W, ZOU Qi, WU Hao. Evaluation of aggregate microstructures following natural regeneration in bauxite residue as characterized by synchrotron-based X-ray micro-computed tomography [J]. Science of the Total Environment, 2016, 573: 155�C163. DOI: 10.1016/j.scitotenv.2016.08.108.
[12] DING Xu-han, XU Guang, KIZIL M, ZHOU Wei, GUO Xing-yun. Lignosulfonate treating bauxite residue dust pollution: Enhancement of mechanical properties and wind erosion behavior [J]. Water, Air, & Soil Pollution, 2018, 229: 214. DOI: 10.1007/s11270-018-3876-0.
[13] DING Xu-han, XU Guang, ZHOU Wei, KURUPPU M. Effect of synthetic and natural polymers on reducing bauxite residue dust pollution [J]. Environmental Technology, 2018, 1�C10. DOI:10.1080/09593330.2018. 1505963.
[14] XUE Sheng-guo, KONG Xiang-feng, ZHU Feng, HARTLEY W, LI Xiao-fei, LI Yi-wei. Proposal for management and alkalinity transformation of bauxite residue in China [J]. Environmental Science and Pollution Research, 2016, 23: 12822�C12834. DOI: 10.1007/s11356-016-6478-7.
[15] GELENCSER A, KOVATS N, TUROCZI B, ROSTASI B, HOFFER A, IMRE K, NYIRO-KOSA I, CSAKBERENYI- MALASICS D, TOTH A, CZITROVSZKY A, NAGY A, NAGY S, ACS A, KOVACS A, FERINCZ A, HARTYANI Z, POSFAI M. The red mud accident in Ajka (Hungary): Characterization and potential health effects of fugitive dust [J]. Environmental Science & Technology, 2011, 45: 1608�C1615. DOI: 10.1021/es104005r.
[16] ZHU Feng, HUANG Nan, XUE Sheng-guo, HARTLEY W, LI Yi-wei, ZOU Qi. Effects of binding materials on microaggregate size distribution in bauxite residues [J]. Environmental Science and Pollution Research, 2016, 23: 23867�C23875. DOI: 10.1007/s11356-016-7626-9.
[17] PONTIKES Y, ANGELOPOULOS G N. Bauxite residue in cement and cementitious applications: Current status and a possible way forward [J]. Resources Conservation and Recycling, 2013, 73: 53�C63. DOI: 10.1016/j.resconrec.2013. 01.005.
[18] YANG Shao-xin, ZHANG Yi-he, YU Jie-mei, HUANG Tai-zhong, TANG Qi, CHU P K, QI Lei. Multi-functional honeycomb ceramic materials produced from bauxite residues [J]. Materials & Design, 2014, 59: 333�C338. DOI: 10.1016/j.matdes.2014.02.061.
[19] SHI Li, PENG Xian-jia, LUAN Zhao-kun, WEI Ning, WANG Qi, ZHAO Ying. Use of activated red mud to remove phosphate and heavy metals from the effluent of biologically treated swine wastewater [J]. Acta Scientiae Circumstantiae, 2009, 29(11): 2282�C2288. (in Chinese)
[20] SAMOUHOS M, TAXIARCHOU M, TSAKIRIDIS P E, POTIRIADIS K. Greek ��red mud�� residue: A study of microwave reductive roasting followed by magnetic separation for a metallic iron recovery process [J]. Journal of Hazardous Materials, 2013, 254�C255: 193�C205. DOI: 10.1016/j.jhazmat.2013.03.059.
[21] BHATNAGAR A, VILAR V J P, BOTELHO C M S, BOAVENTURA R A R. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater [J]. Environmental Technology, 2011, 32: 231�C249. DOI: 10.1080/09593330.2011.560615.
[22] ZOU Qi, AN Wen-hui, WU Chuan, LI Wai-chin, FU An-qin, XIAO Rui-yang, CHEN Hui-kang, XUE Sheng-guo. Red mud-modified biochar reduces soil arsenic availability and changes bacterial composition [J]. Environmental Chemistry Letters, 2018, 16(3): 615�C622.
[23] KAWAMURA M, TAKEUCHI K, SUGIYAMA A. Mechanisms of expansion of mortars containing reactive aggregate in NaCl solution [J]. Cement and Concrete Research, 1994, 24: 621�C632. DOI: 10.1016/0008- 8846(94)90186-4.
[24] LINDGARD J, THOMAS M D A, SELLEVOLD E J, PEDERSEN B, ANDI -
- AKIR O, JUSTNES H, RONNING T F. Alkali�Csilica reaction (ASR)��Performance testing: Influence of specimen pre-treatment, exposure conditions and prism size on alkali leaching and prism expansion [J]. Cement and Concrete Research, 2013, 53: 68�C90. DOI: 10.1016/j.cemconres.2013.05.017.
AKIR O, JUSTNES H, RONNING T F. Alkali�Csilica reaction (ASR)��Performance testing: Influence of specimen pre-treatment, exposure conditions and prism size on alkali leaching and prism expansion [J]. Cement and Concrete Research, 2013, 53: 68�C90. DOI: 10.1016/j.cemconres.2013.05.017.
[25] HUANG Ling, LI Yi-wei, XUE Sheng-guo, ZHU Feng, WU Chuan, WANG Qiong-li. Salt composition changes in different stacking ages of bauxite residue [J]. The Chinese Journal of Nonferrous Metals, 2016, 26(11): 2433�C2439. (in Chinese)
[26] LIU Wan-chao, CHEN Xiang-qing, LI Wang-xing, YU Yan-fen, YAN Kun. Environmental assessment, management and utilization of red mud in China [J]. Journal of Cleaner Production, 2014, 84: 606�C610. DOI: 10.1016/j.jclepro.2014.06.080.
[27] SANTINI T C, HINZ C, RATE A W, CARTER C M, GILKES R J. In situ neutralisation of uncarbonated bauxite residue mud by cross layer leaching with carbonated bauxite residue mud [J]. Journal of Hazardous Materials, 2011, 194: 119�C127. DOI: 10.1016/j.jhazmat.2011.07.090.
[28] KHAITAN S, DZOMBAK D A, LOWRY G V, FAN M H, VIDIC R. Mechanisms of neutralization of bauxite residue by carbon dioxide [J]. Journal of Environmental Engineering, 2009, 135: 433�C438. DOI: 10.1061/(ASCE)EE.1943- 7870.0000010.
[29] SANTINI T C, FEY M V. Synthesis of hydrotalcite by neutralization of bauxite residue mud leachate with acidic saline drainage water [J]. Applied Clay Science, 2012, 55: 94�C99. DOI: 10.1016/j.clay.2011.10.011.
[30] ZHANG Yi-he, WANG Xin-ke, LV Feng-zhu, ZHOU Feng-shan, TONG Wang-shu, HU Ying-mo, ZHANG An-zhen, LU Rong-rong. Study progress of alkali removal from red mud and novel functional materials [J]. Chinese Journal of Environmental Engineering, 2016, 10(7): 3383�C3390. (in Chinese)
[31] BURKE I T, PEACOCK C L, LOCKWOOD C L, STEWART D I, MORTIMER R J G, WARD M B, RENFORTH P, GRUIZ K, MAYES W M. Behavior of aluminum, arsenic, and vanadium during the neutralization of red mud leachate by HCl, gypsum, or seawater [J]. Environmental Science & Technology, 2013, 47: 6527�C7535. DOI: 10.1021/es4010834.
[32] KISHIDA M, HARATO T, TOKORO C, OWADA S. In situ remediation of bauxite residue by sulfuric acid leaching and bipolar-membrane electrodialysis [J]. Hydrometallurgy, 2016, 170: 58�C67. DOI: 10.1016/j.hydromet.2016.04.012.
[33] HANAHAN C, MCCONCHIE D, POHL J, CREELMAN R, CLARK M, STOCKSIEK C. Chemistry of seawater neutralization of bauxite refinery residues (red mud) [J]. Environmental Engineering Science, 2004, 21: 125�C138. DOI: 10.1089/109287504773087309.
[34] MENZIES N W, FULTON I M, MORRELL W J. Seawater neutralization of alkaline bauxite residue and implications for revegetation [J]. Journal of Environmental Quality, 2004, 33: 1877�C1884. DOI: 10.2134/jeq2004.1877.
[35] XUE Sheng-guo, ZHU Feng, KONG Xiang-feng, WU Chuan, HUANG Ling, HUANG Nan, WILLIAM H. A review of the characterization and revegetation of bauxite residues (red mud) [J]. Environmental Science and Pollution Research, 2016, 23: 1120�C1132. DOI: 10.1007/s11356-015- 4558-8.
[36] COURTNEY R G, TIMPSON J P. Nutrient status of vegetation grown in alkaline bauxite processing residue amended with gypsum and thermally dried sewage sludge�C A two year field study [J]. Plant and Soil, 2004, 266: 187�C194. DOI: 10.1007/s11104-005-0872-0.
[37] BARBHUIYA S A, BASHEER P A M, CLARK M W, RANKIN G I B. Effects of seawater-neutralised bauxite refinery residue on properties of concrete [J]. Cement and Concrete Composites, 2011, 33: 668�C679. DOI: 10.1016/ j.cemconcomp.2011.03.010.
[38] TUAZON D, CORDER G D. Life cycle assessment of seawater neutralised red mud for treatment of acid mine drainage [J]. Resources Conservation and Recycling, 2008, 52: 1307�C1314. DOI: 10.1016/j.resconrec.2008.07.010.
[39] ZHANG Ran, ZHENG Shi-li, MA Shu-hua, ZHANG Yi. Recovery of alumina and alkali in Bayer red mud by the formation of andradite-grossular hydrogarnet in hydrothermal process [J]. Journal of Hazardous Materials, 2011, 189: 827�C835. DOI: 10.1016/j.jhazmat.2011.03.004.
[40] LIANG Wen-tao, COUPERTHWAITE S, KAUR G, YAN Cheng, JOHNSTONE D W, MILLAR G J. Effect of strong acids on red mud structural and fluoride adsorption properties [J]. Journal of Colloid and Interface Science, 2014, 423: 158�C165. DOI: 10.1016/j.jcis.2014.02.019.
[41] SUSHIL S, BATRA V S. Modification of red mud by acid treatment and its application for CO removal [J]. Journal of Hazardous Materials, 2012, 203: 264�C273. DOI: 10.1016/ j.jhazmat.2011.12.007.
[42] DOYE I, DUCHESNE J. Neutralisation of acid mine drainage with alkaline industrial residues: Laboratory investigation using batch-leaching tests [J]. Applied Geochemistry, 2003, 18: 1197�C1213. DOI: 10.1016/S0883- 2927(02)00246-9.
[43] SI Chun-hua, MA Ying-qun, LIN Chu-xia. Red mud as a carbon sink: Variability, affecting factors and environmental significance [J]. Journal of Hazardous Materials, 2013, 244�C245: 54�C59. DOI: 10.1016/j.jhazmat.2012.11.024.
[44] BODOR M, SANTOS R M, GERVEN T V, VLAD M. Recent developments and perspectives on the treatment of industrial wastes by mineral carbonation��A review [J]. Central European Journal of Engineering, 2013, 3: 566�C584. DOI: 10.2478/s13531-013-0115-8.
[45] PERRY K W, RUSSELL A S. Advances and prospects in alumina technology [J]. JOM, 1982, 34: 48�C53. DOI: 10.1007/BF03338119.
[46] LIU Yong, LIN Chu-xia, WU Yong-gui. Characterization of red mud derived from a combined Bayer process and bauxite calcination method [J]. Journal of Hazardous Materials, 2007, 146: 255�C261. DOI: 10.1016/j.jhazmat.2006.12.015.
[47] LIU Wan-chao, YANG Jia-kuan, XIAO Bo. Review on treatment and utilization of bauxite residues in China [J]. International Journal of Mineral Processing, 2009, 93: 220�C231. DOI: 10.1016/j.minpro.2009.08.005.
[48] XU Bing-an, SMITH P, SILVA L D. The Bayer digestion behaviour of transition aluminas formed from roasted gibbsite [J]. International Journal of Mineral Processing, 2013, 122: 22�C28. DOI: 10.1016/j.minpro.2013.04.003.
[49] XU Guang, DING Xu-han, KURUPPU M, ZHOU Wei, BISWAS W. Research and application of non-traditional chemical stabilizers on bauxite residue (red sand) dust control, a review [J]. Science of the Total Environment, 2018, 616: 1552�C1565. DOI: 10.1016/ j.scitotenv.2017.10.158.
[50] BEARDEN B N, PETERSEN L. Influence of arbuscular mycorrhizal fungi on soil structure and aggregate stability of a vertisol [J]. Plant and Soil, 2000, 218: 173�C183. DOI: 10.1023/a:1014923911324.
[51] HAMDY M K, WILLIAMS F S. Bacterial amelioration of bauxite residue waste of industrial alumina plants [J]. Journal of Industrial Microbiology & Biotech, 2001, 27: 228�C233. DOI: 10.1038/sj.jim.7000181.
[52] PARADIS M, DUCHESNE J, LAMONTAGNE A, ISABEL D. Long-term neutralisation potential of red mud bauxite with brine amendment for the neutralisation of acidic mine tailings [J]. Applied Geochemistry, 2007, 22: 2326�C2333. DOI: 10.1016/j.apgeochem.2007.04.021.
[53] SURYAVANSHI A K, SCANTLEBURY J D, LYON S B. Mechanism of Friedel��s salt formation in cements rich in tri-calcium aluminate [J]. Cement and Concrete Research, 1996, 26: 717�C727. DOI: 10.1016/S0008-8846(96)85009-5.
[54] PAN Xiao-lin, YU Hai-yan, TU Gan-feng. Reduction of alkalinity in bauxite residue during Bayer digestion in high-ferrite diasporic bauxite [J]. Hydrometallurgy, 2015, 151: 98�C106. DOI: 10.1016/j.hydromet.2014.11.015.
[55] WHITTINGTON B I, FLETCHER B L, TALBOT C. The effect of reaction conditions on the composition of desilication product (DSP) formed under simulated Bayer conditions [J]. Hydrometallurgy, 1998, 49: 1�C22. DOI: 10.1016/S0304-386X(98)00021-8.
[56] MARTIN M A, FORTE G, OSTAP S, SEE J. The Mineralogy of Bauxite for Producing Smelter-Grade Alumina [J]. JOM, 2001, 53: 36�C40. DOI: 10.1007/ s11837-001-0011-1.
[57] GR FE M, POWER G, KLAUBER C. Bauxite residue issues: III. Alkalinity and associated chemistry [J]. Hydrometallurgy, 2011, 108: 60�C79. DOI: 10.1016/ j.hydromet.2011.02.004.
FE M, POWER G, KLAUBER C. Bauxite residue issues: III. Alkalinity and associated chemistry [J]. Hydrometallurgy, 2011, 108: 60�C79. DOI: 10.1016/ j.hydromet.2011.02.004.
[58] KONG Xiang-feng, TIAN Tao, XUE Sheng-guo, HARTLEY W, HUANG Long-bin, WU Chuan, LI Chu-xuan. Development of alkaline electrochemical characteristics demonstrates soil formation in bauxite residue undergoing natural rehabilitation [J]. Land Degradation & Development, 2018, 29: 58�C67. DOI: 10.1002/ldr.2836.
[59] LI Xiao-fei, YE Yu-zhen, XUE Sheng-guo, JIANG Jun, WU Chuan, KONG Xiang-feng, HARTLEY W, LI Yi-wei. Leaching optimization and dissolution behavior of alkaline anions in bauxite residue [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(6): 1248�C1255. DOI: 10.1016/S1003-6326(18)64763-6.
[60] KONG Xiang-feng JIANG Xing-xing, XUE Sheng-guo, HUANG Ling, HARTLEY W, WU Chuan, LI Xiao-fei. Migration and distribution of saline ions in bauxite residue during water leaching [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(3): 534�C541. DOI: 10.1016/S1003-6326(18)64686-2.
[61] CASTALDI P, SILVETTI M, SANTONA L, ENZO S, MELIS P. XRD, FTIL and thermal analysis of bauxite ore-Processing waste (red mud) exchanged with heavy metals [J]. Clays and Clay Minerals, 2008, 56: 461�C469. DOI: 10.1346/ccmn.2008.0560407.
[62] ZHANG Kun-yu, HU Hui-ping, ZHANG Li-juan, CHEN Qi-yuan. Surface charge properties of red mud particles generated from Chinese diaspore bauxite [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 1285�C1289. DOI: 10.1016/S1003-6326(08)60218-6.
[63] XUE Sheng-guo, LI Xiao-fei, KONG Xiang-feng, WU Chuan, LI Yi-wei, LI Meng, LI Chu-xuan. Alkaline regulation of bauxite residue: A comprehensive review [J]. Acta Scientiae Circumstantiae, 2017, 37: 2815�C2828. (in Chinese)
[64] ATASOY A. An investigation on characterization and thermal analysis of the Aughinish red mud [J]. Journal of Thermal Analysis & Calorimetry, 2005, 81: 357�C361. DOI: 10.1007/s10973-005-0792-5.
[65] ATASOY A. The comparison of the bayer process wastes on the base of chemical and physical properties [J]. Journal of Thermal Analysis & Calorimetry, 2007, 90: 153�C158. DOI: 10.1007/s10973-005-7671-y.
[66] ALP A, GORAL M S. The effects of the additives, calcination and leach conditions for alumina production from red mud [J]. Scandinavian Journal of Metallurgy, 2003, 32: 301�C305. DOI: 10.1111/j.1600-0692.2003.00656.x.
[67] SAMAL S, RAY A K, BANDOPADHYAY A. Proposal for resources, utilization and processes of red mud in India��A review [J]. International Journal of Mineral Processing, 2013, 118: 43�C55. DOI: 10.1016/j.minpro.2012.11.001.
[68] NEWSON T, DYER T, ADAM C, SHARP S. Effect of structure on the geotechnical properties of bauxite residue [J]. Journal of Geotechnical and Geoenvironmental Engineering, 2006, 132: 143�C151. DOI: 10.1061/(ASCE)1090- 0241(2006)132:2(143).
[69] PETROPULU M O, LYBEROPULU T, OCHSENKUHN K M, PARISSAKISA G. Recovery of lanthanides and yttrium from red mud by selective leaching [J]. Analytica Chimica Acta, 1996, 319: 249�C254. DOI: 10.1016/0003- 2670(95)00486-6.
[70] KONG Xiang-feng, GUO Ying, XUE Sheng-guo, HARTLEY W, WU Chuan, YE Yu-zhen, CHENG Qing-yu. Natural evolution of alkaline characteristics in bauxite residue [J]. Journal of Cleaner Production, 2017, 143: 224�C230. DOI: 10.1016/j.jclepro.2016.12.125.
[71] TAI Jun, ZHANG Wei-qian, CHE Yue, FENG Di. Municipal solid waste source-separated collection in China: A comparative analysis [J]. Waste Management, 2011, 31: 1673�C1682. DOI: 10.1016/j.wasman.2011.03.014.
[72] CHAABAN M A. Hazardous waste source reduction in materials and processing technologies [J]. Journal of Materials Processing Technology, 2001, 119: 336�C343. DOI: 10.1016/S0924-0136(01)00920-7.
[73] TWIDWELL L G, HWANG J R, DUFRESNE R E. Industrial waste disposal. Excess sulfuric acid neutralization with copper smelter slag [J]. Environmental Science and Technology, 1976, 10: 687�C691. DOI: 10.1021/ es60118a004.
[74] WANG Yun-shan, YANG Gang, ZHANG Jin-ping. Novel process for sodium elimination from red mud of alumina production [J]. Nonferrous Metals, 2010, 62(3): 61�C64. (in Chinese)
[75] SNYDER C S, BRUULSEMA T W, JENSEN T L, FIXEN P E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects [J]. Agriculture Ecosystems & Environment, 2009, 133: 247�C266. DOI: 10.1016/j.agee.2009.04.021.
[76] WANG Zhi, HAN Min-fang, ZHANG Yi-he, ZHOU Feng-shan. Study on the dealkalization technics of Bayer- process red mud with CO2 by carbonation [J]. Bulletin of the Chinese Ceramic Society, 2013, 32(9): 1851�C1861. (in Chinese)
[77] NAN Xiang-li, ZHANG Ting-an, WU Yi-quan, DOU Zhi-he. A study on absorption of low-concentration SO2 by bayer red mud [J]. Journal of Northeastern University, 2010, 31(7): 986�C989. (in Chinese)
[78] SINGH M, GARG M. Cementitious binder from fly ash and other industrial wastes [J]. Cement and Concrete Research, 1999, 29: 309�C314. DOI: 10.1016/S0008-8846(98)00210-5.
[79] BUDINOVA T, SAVOVA D, TSYNTSARSKI B, ANIA C O, CABAL B, PARRA, J B, PETROV N. Biomass waste-derived activated carbon for the removal of arsenic and manganese ions from aqueous solutions [J]. Applied Surface Science, 2012, 30: 999�C1000. DOI: 10.1016/ j.apsusc.2008.12.013.
[80] XU Jing, LU Shu-guang, FU Dan. Recovery of hydrochloric acid from the waste acid solution by diffusion dialysis [J]. Journal of Hazardous Materials, 2009, 165: 832�C837. DOI: 10.1016/j.jhazmat.2008.10.064.
[81] JEONG J, KIM M S, KIM B S, KIM S K, KIM W B, LEE J C. Recovery of H2SO4 from waste acid solution by a diffusion dialysis method [J]. Journal of Hazardous Materials, 2005, 124: 230�C235. DOI: 10.1016/j.jhazmat.2005.05.005.
[82] WANG Guang-hui, QIANG Min, WANG Guang-hua, HE Xuan-ming, LIU Zhi-ping, WEI Song-bo, DU Gong-liu. Experimental study on Ca2+ and Mg2+ removal from circulating water with alkaline wastewater from thermal power plant [J]. Journal of Wuhan Yejin Univeristy of Science and Technology, 2001, 24(2): 138�C144. (in Chinese)
[83] KRUTHIKA N L, KARTHIKA S, RAJU G B, PRABHAKAR S. Efficacy of electrocoagulation and electrooxidation for the purification of wastewater generated from gelatin production plant [J]. Journal of Environmental Chemical Engineering, 2013, 1: 183�C188. DOI: 10.1016/ j.jece.2013.04.017.
[84] G RSKA J S, CICHON A, MIKSCH K. Nitrogen removal from wastewater with high ammonia nitrogen concentration via shorter nitrification and denitrification [J]. Waster Science and Technology, 1997, 36: 73�C78. DOI: 10.1016/ S0273-1223(97)00000-0.
RSKA J S, CICHON A, MIKSCH K. Nitrogen removal from wastewater with high ammonia nitrogen concentration via shorter nitrification and denitrification [J]. Waster Science and Technology, 1997, 36: 73�C78. DOI: 10.1016/ S0273-1223(97)00000-0.
[85] HUANG Jun, CHEN Jian-zhong. Recent advances on the treatment technologies of ammonia-nitrogen wastewater [J]. Technigues and Equipment for Environmental Pollution Control, 2002, 3(1): 65�C68. (in Chinese)
[86] GRISMER M E, COLLISON R S. The zeolite-anammox treatment process for nitrogen removal from wastewater��A review [J]. Water, 2017, 9: 901�C915. DOI: 10.3390/ w9110901.
[87] BACHU S, WATSON T L. Review of failures for wells used for CO2, and acid gas injection in Alberta, Canada [J]. Energy Procedia, 2009, 1: 3531�C3537. DOI: 10.1016/ j.egypro.2009.02.146.
[88] CHIEN T W, CHU H. Removal of SO2, and NO from flue gas by wet scrubbing using an aqueous NaClO2 solution [J]. Journal of Hazardous Materials, 2000, 80: 43�C57. DOI: 10.1016/S0304-3894(00)00274-0.
[89] CHANDARA C, AZIZLI K A M, AHMAD Z A, SAKAI E. Use of waste gypsum to replace natural gypsum as set retarders in portland cement [J]. Waste Management, 2009, 29: 1675�C1679. DOI: 10.1016/j.wasman.2008.11.014.
[90] RUTHERFORD P M, DUDAS M J, SAMEK R A. Environmental impacts of phosphogypsum [J]. Science of the Total Environment, 1994, 149: 1�C38. DOI: 10.1016/ 0048-9697(94)90002-7.
[91] MOSIER N, WYMAN C, DALE B, ELANDER R, LEE Y Y. Features of promising technologies for pretreatment of lignocellulosic biomass [J]. Bioresource Technology, 2005, 96: 673�C686. DOI: 10.1016/j.biortech.2004.06.025.
[92] OLIVEROS E, LEGRINI O, HOHL M, MULLER T, BRAUN A M. Industrial waste water treatment: Large scale development of a light-enhanced Fenton reaction [J]. Chemical Engineering and Processing, 1997, 36: 397�C405. DOI: 10.1016/S0255-2701(97)00011-1.
[93] AIKEN D V, F RE R, GROSSKOPF S, PASURKA C A. Pollution abatement and productivity growth: Evidence from Germany, Japan, the Netherlands, and the United States [J]. Environmental & Resource Economics, 2009, 44: 11�C28. DOI: 10.1007/s10640-008-9256-2.
RE R, GROSSKOPF S, PASURKA C A. Pollution abatement and productivity growth: Evidence from Germany, Japan, the Netherlands, and the United States [J]. Environmental & Resource Economics, 2009, 44: 11�C28. DOI: 10.1007/s10640-008-9256-2.
[94] SHAH F, ZILBERMAN D, LICHTENBERG E. Optimal combination of pollution prevention and abatement policies: The case of agricultural drainage [J]. Environmental and Resource Economics, 1995, 5: 29�C49. DOI: 10.1007/ BF00691908.
[95] DU Wu-peng, GAO Qing-xian, ZHANG En-chen, MIAO Qi-long, WU Jiao-guo. The emission status and composition analysis of municipal solid waste in China [J]. Research of Environmental Science, 2006, 19(5): 85�C90. (in Chinese)
[96] SLOOT H A V D, KOSSON D S, HJELMAR O. Characteristics, treatment and utilization of residues from municipal waste incineration [J]. Waste Management, 2001, 21(8): 753�C765. DOI: 10.1016/S0956-053X(01)00009-5.
[97] BURGESS J E, PARSONS S A, STUETZ R M. Developments in odour control and waste gas treatment biotechnology: A review [J]. Biotechnology Advances, 2001, 19: 35�C63. DOI: 10.1016/S0734-9750(00)00058-6.
[98] MO Jia-hao, YANG Qi, ZHANG Na, ZHANG Wen-xiang, ZHENG Yi, ZHANG Zhi-en. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment [J]. Journal of Environmental Management, 2018, 227: 395�C405. DOI: 10.1016/j.jenvman.2018.08.069.
[99] SUSHMA, KUMARI M, SAROHA A K. Performance of various catalysts on treatment of refractory pollutants in industrial wastewater by catalytic wet air oxidation: A review [J]. Journal of Environmental Management, 2018, 228: 169�C188. DOI: 10.1016/j.jenvman.2018.09.003.
[100] PAMBOR M, WIESNER M. Study of the process design and flue gas treatment of an industrial-scale energy-from- waste combustion plant [J]. Industrial & Engineering Chemistry Research, 2007, 46: 2648�C2656. DOI: 10.1021/ ie060929d.
[101] RAUT S P, RALEGAONKAR R V, MANDAVGANE S A. Development of sustainable construction material using industrial and agricultural solid waste: A review of waste- create bricks [J]. Construction & Building Materials, 2011, 25: 4037�C4042. DOI: 10.1016/j.conbuildmat.2011.04.038.
[102] CHEN Xiang-ping, CHEN Yong-bin, ZHOU Tao, LIU De-pei, HU Hang, FAN Shao-yun. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries [J]. Waste Management, 2015, 38: 349�C356. DOI: 10.1016/j.wasman.2014.12.023.
[103] ZHANG Gui-qing, ZHANG Qi-xiu, ZHOU Kang-gen. Acid recovery from waste sulfuric acid by diffusion dialysis [J]. Journal of Central South University of Technology, 1999, 6(2): 103�C106. DOI: 10.1007/s11771-999-0008-4.
[104] VIEIRA A M S, BERGAMASCO R, GIMENES M L, NAKAMURA C V, FILHO B P D. Microbial populations of an upflow anaerobic sludge blanket reactor treating wastewater from a gelatin industry [J]. Environmental Technology Letters, 2001, 22: 1477�C1485. DOI: 10.1080/ 09593332208618182.
[105] KONG Xiu-qin, SHI Xiao-feng, REN Rui-fang, ZHAO Feng, XING Xin-hui. Experimental research on treating gelatin wastewater by the joint process of photosynthetic bacteria and activated sludge method [J]. Environmental Engineering, 2010, 28(3): 39�C42. (in Chinese)
[106] DIWANI G E, RAFIE S E, IBIARI N N E, AILA H I E. Recovery of ammonia nitrogen from industrial wastewater treatment as struvite slow releasing fertilizer [J]. Desalination, 2007, 214: 200�C214. DOI: 10.1016/j.desal. 2006.08.019.
[107] HUANG Zhi-jin, HUANG Guang-tuan, SHI Chun-qiong, JI Jing-yu. Effect of DO on high concentration of ammonia nitrogen wastewater treatment in membrane bioreactor [J]. Environmental Science & Technology, 2010, 33(1): 138�C141. (in Chinese)
[108] WANG Yu, PELKONEN M, KOTRO M. Treatment of high ammonium-nitrogen wastewater from composting facilities by air stripping and catalytic oxidation [J]. Water Air & Soil Pollution, 2010, 208: 259�C273. DOI: 10.1007/s11270-009-0164-z.
[109] Pooladi-DARVISH M, HONG H, Stocker R, Bennion B, Theys S. Chromatographic partitioning of H2S and CO2 in acid gas disposal [J]. Journal of Canadian Petroleum Technology, 2008, 10: 52�C57. DOI: 10.2118/ 130064-PA.
[110] Srivastava R K, Jozewicz W. Flue gas desulfurization: The state of the art [J]. Air & Waste Management Association, 2001, 12: 1676�C1688. DOI: 10.1080/10473289.2001.10464387.
[111] Nelson W H. Gypsum waste disposal: Land vs sea or recycling [J]. Chemical Ecology, 1990, 4: 247�C258. DOI: 10.1080/02757549008035239.
[112] Tayibi H, Choura M, Lopez F A, Alguacil F J, LOPEZDELGADO A. Environmental impact and management of phosphogypsum [J]. Environmental Management, 2009, 8: 2377�C2386. DOI: 10.1016/ j.jenvman.2009.03.007.
[113] LIU Xiao, GAO Xing-bao, WANG Wei, ZHENG Lei, ZHOU Ying-jun, SUN Yi-fei. Pilot-scale anaerobic co- digestion of municipal biomass waste: Focusing on biogas production and GHG reduction [J]. Renewable Energy, 2012, 44: 463�C468. DOI: 10.1016/j.renene.2012.01.092.
[114] Khaitan S, Dzombak D A, Lowry G V. Chemistry of the acid neutralization capacity of bauxite residue [J]. environmental engineering science, 2009, 26: 873�C881. DOI: 10.1089/ees.2007.0228.
[115] Chvedov D, Ostap S, Le T. Surface properties of red mud particles from potentiometric titration [J]. Colloids and Surfaces A. Physicochemical and Engineering Aspects, 2001, 182: 131�C141. DOI: 10.1016/S0927-7757(00)00814-1.
[116] Yang Yang, Wang Xue-wen, Wang Ming-yu, Wang Hua-guang, Xian Peng-fei. Iron recovery from the leached solution of red mud through the application of oxalic acid [J]. International Journal of Mineral Processing, 2016, 157: 145�C151. DOI: 10.1016/j.minpro.2016.11.001.
[117] Couperthwaite S J, Johnstone D W, Millar G J, Frost R L. Neutralization of acid sulfate solutions using bauxite refinery residues and its derivatives [J]. Industrial & Engineering Chemistry Research, 2013, 52: 1388�C1395. DOI: 10.1021/ie301618p.
[118] Zhang Guo-li, Li Shao-chun, Zhang Xin-yuan, Wang Zhi-kai. Comparison study on different de-alkalization processes of red mud by Bayer process [J]. Inorganic Chemicals Industry, 2012, 44(18): 40�C42. (in Chinese)
[119] CLARK M W, JOHNSTON M, REICHELT-BRUSHETT A J. Comparison of several different neutralisations to a bauxite refinery residue: Potential effectiveness environmental ameliorants [J]. Applied Geochemistry, 2015, 56: 1�C10. DOI: 10.1016/j.apgeochem.2015.01.015.
[120] Han Y S, JI S, Lee P K, Oh C. Bauxite residue neutralization with simultaneous mineral carbonation using atmospheric CO2 [J]. Journal of Hazardous Materials, 2016, 326: 87�C93. DOI: 10.1016/j.jhazmat.2016.12.020.
[121] Mayes W, Younger P, Aumonier J. Buffering of alkaline steel slag leachate across a natural wetland [J]. Environmental Science & Technology, 2006, 40: 1237�C1243. DOI: 10.1021/es051304u.
[122] Jones G, Joshi G, Clark M, McConchie D. Carbon capture and the aluminium industry: Preliminary studies [J]. Environmental Chemistry, 2006, 3: 297�C303. DOI: 10.1071/ EN06018.
[123] Zhu Feng, Xue Sheng-guo, Hartley W, Huang Ling, Wu Chuan, Li Xiao-fei. Novel predictors of soil genesis following natural weathering processes of bauxite residues [J]. Environmental Science and Pollution Research, 2016, 23: 2856�C2863. DOI: 10.1007/s11356-015-5537-9.
[124] Dilmore R, Lu P, Allen D, Soong Y, Hedges S. Sequestration of CO2 in mixtures of bauxite residue and saline wastewater [J]. Energy & Fuels, 2008, 22: 1325�C1333. DOI: 10.1021/ef7003943.
[125] ZHONG Chen, XIA Ju-pei. Study on leaching Na+ in red mud from bayer process [J]. Bulletin of the Chinese Ceramic Society, 2013, 32(10): 2012�C2015. (in Chinese)
[126] LI Yi-wei, JIANG Jun, XUE Sheng-guo, MILLAR G, KONG Xiang-feng, LI Xiao-fei, LI Meng, LI Chu-xuan. Effect of ammonium chloride on leaching behavior of alkaline anion and sodium ion in bauxite residue [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(10): 2125�C2134. DOI: 10.1016/S1003-6326(18)64857-5.
[127] Philipsborn H V, Kuhnast E. Gamma spectrometric characterisation of industrially used african and australian bauxites and their red mud tailings [J]. radiation protection dosimetry, 1992, 45: 741�C743. DOI: https://doi.org/10.1093/ oxfordjournals.rpd.a081642.
[128] Su Chun-ming. in situ infrared speciation of adsorbed carbonate on aluminum and iron oxides [J]. Clays and Clay Minerals, 1997, 45: 814�C825. DOI: 10.1346/CCMN.1997. 0450605.
[129] TANG Xiao-hui, XU Gang, LIU Run-zao, LI Shi-qi, XU Kai. Simulation experiments for dealkalization of red mud by limekiln gas [J]. Journal of Chongqing University, 2015, 38(5): 142�C150. (in Chinese)
[130] Yi Yuan-rong, Han Min-fang. Characteristics and mechanism of sodium removal by the synergistic action of flue gas and waste solid [J]. journal of environmental sciences, 2012, 33: 2522�C2527. (in Chinese)
[131] Fuller R D, Nelson E D P, Richardson C J. Reclamation of red mud (bauxite residues) using alkaline- tolerant grasses with organic amendments [J]. journal of environmental quality, 1982, 11: 533�C539. DOI: 10.2134/ jeq1982.00472425001100030040x.
[132] Fois E, Lallai A A, Mura G. Sulfur dioxide absorption in a bubbling reactor with suspensions of bayer red mud [J]. Industrial & Engineering Chemistry Research, 2007, 46: 6770�C6776. DOI: 10.1021/ie0616904.
[133] Sahu R C, Patel R, RAY B C. Removal of hydrogen sulfide using red mud at ambient conditions [J]. Fuel Process Technol, 2011, 92: 1587�C1592. DOI: 10.1016/j.fuproc. 2011.04.002.
[134] SHULTZ F, BERBER J. Hydrogen sulfide removal from hot producer gas with sintered absorbents [J]. Air Repair, 1969, 20: 93�C96. DOI: 10.1080/00022470.1970.10469380.
[135] BHATTACHARYYA A, RAJANIKANTH B S. Biodiesel exhaust treatment with HFAC plasma supported by red mud: Study on DeNOx and power consumption [J]. Energy Procedia, 2015, 75: 2371�C2378. DOI: 10.1016/j.egypro.2015. 07.168.
[136] SOUZA K C D, ANTUNES M L P, COUPERTHWAITE S J, CONCEICAO F T D, BARROS T R D, FROST R. Adsorption of reactive dye on seawater-neutralised bauxite refinery residue [J]. Journal of Colloid and Interface Science, 2013, 396: 210�C214. DOI: 10.1016/j.jcis.2013.01.011.
[137] WU Chuan, HUANG Liu, XUE Sheng-guo, HUANG Yu-ying, HARTLEY W, CUI Meng-qian, WONG M H. Arsenic sorption by red mud-modified biochar produced from rice straw [J]. Environmental Science and Pollution Research, 2017, 24: 18168�C18178. DOI: 10.1007/s11356- 017-9466-7.
[138] WONG J, GOEN H. Sewage sludge as organic ameliorant for revegetation of fine bauxite refining residue [J]. Resources, Conservation and Recycling, 1994, 11: 297�C309. DOI: 10.1016/0921-3449(94)90097-3.
[139] JONES B E H, HAYNES R J, PHILIPS I R. Influence of amendments on acidification and leaching of Na from bauxite processing sand [J]. Ecological Engineering, 2015, 84: 435�C442. DOI: 10.1016/j.ecoleng.2015.09.054.
[140] JONES B E H, HAYNES R J, PHILIPS I R. Influence of organic waste and residue mud additions on chemical, physical and microbial properties of bauxite residue sand [J]. Environmental Science and Pollution Research, 2011, 18: 199�C211. DOI: 10.1007/s11356-010-0364-5.
[141] WANG Li-ying, LI Xiao-lei, ZHAI Er-an, TAO Feng, SUN Feng, ZHANG Zuo-cai. Study on new technology of red mud alkali removal by desulfurization gypsum [J]. Science & Technology Information, 2010(21): 48�C96. (in Chinese)
[142] WONG J W C, HO G E. Use of waste gypsum in the revegetation on red mud deposits: A greenhouse study [J]. Waste Management and Research, 1993, 11: 249�C256. DOI: 10.1006/wmre.1993.1024.
[143] KOPITTKE P M, MENZIES N W, FULTON I M. Gypsum solubility in seawater, and its application to bauxite residue amelioration [J]. Soil Research, 2005, 42: 953�C960. DOI: 10.1071/SR04034.
[144] ZHU Feng, CHENG Qing-yu, XUE Sheng-guo, LI Chu-xuan, HARTLEY W, WU Chuan, TIAN Tao. Influence of natural regeneration on fractal features of residue microaggregates in bauxite residue disposal areas [J]. Land Degradation and Development, 2018, 29: 138�C149. DOI: 10.1002/ldr.2848.
[145] COURTNEY R G, TIMPSON J P. Reclamation of fine fraction bauxite processing residue (red mud) amended with coarse fraction residue and gypsum [J]. Water Air and Soil Pollution, 2005, 164: 91�C102. DOI: 10.1007/s11270-005- 2251-0.
[146] HO G E, MATHEW K, NEWMAN P W G. Leachate quality from gypsum neutralized red mud applied to sandy soils [J]. Water Air and Soil Pollution, 1989, 47: 1�C18. DOI: 10.1007/BF00468992.
[147] JONES B E H, HAJNES R J, PHILIPS I R. Addition of an organic amendment and/or residue mud to bauxite residue sand in order to improve its properties as a growth medium [J]. Journal of Environmental Management, 2012, 95: 29�C38. DOI: 10.1016/j.jenvman.2011.09.014.
[148] XUE Sheng-guo, LI Meng, JIANG Jun, MILLAR G J, LI Chu-xuan, KONG Xiang-feng. Phosphogypsum stabilization of bauxite residue: Conversion of its alkaline characteristics [J]. Journal of Environmental Sciences, 2019, 77: 1�C10. DOI: 10.1016/ j.jes.2018.05.016.
[149] REN Jie, LIU Ji-dong, CHEN Juan, LIU Xiao-lian, LI Fa-sheng, DU Ping. Effects of vinegar and furfural residue on metal stability in bauxite residue [J]. Research of Environmental Sciences, 2016, 29(12): 1895�C1903. (in Chinese)
[150] XENIDIS A, HAROKOPOU A D, MYLONA E, BROFAS G. Modifying alumina red mud to support a revegetation cover [J]. JOM, 2005, 57: 42�C46. DOI: 10.1007/s11837-005- 0214-y.
[151] LI Xiao-ping. Study on side slope environment problems and the countermeasure of Pingguo red mud disposal Field [J]. Nonferrous Metal (Mining Section), 2007, 59(2): 29�C31. (in Chinese)
[152] KHAITAN S, DZOMBAK D A, SWALLOW P S, SCHMIDT K, FU J. Field evaluation of bauxite residue neutralization by carbon dioxide, vegetation, and organic amendments [J]. Journal of Environmental Engineering, 2010, 136: 1045�C1053. DOI: 10.1061/(ASCE)EE.1943- 7870.0000230.
[153] KLAUBER C, GRAFE M, POWER G. Bauxite residue issues: II. Options for residue utilization [J]. Hydrometallurgy, 2011, 108: 11�C32. DOI: 10.1016/ j.hydromet.2011.02.007.
[154] WONG J W C. Effects of gypsum and sewage sludge amendment on physical properties of fine bauxite refining residue [J]. Soil Science, 1991, 152: 326�C332. DOI: 10.1097/00010694-199111000-00003.
[155] COURTNEY R G, JORDAN S N, HARRINGTON T. Physico-chemical changes in bauxite residue following application of spent mushroom compost and gypsum [J]. Land Degradation and Development, 2009, 20: 572�C581. DOI: 10.1002/ldr.926.
[156] ZHU Feng, HOU Jing-tou, XUE Sheng-guo, WU Chuan, WANG Qiong-li, HARTLEY W. Vermicompost and gypsum amendments improve aggregate formation in bauxite residue [J]. Land Degradation and Development, 2017, 28: 2109�C2120. DOI: 10.1002/ldr.2737.
[157] ZHU Feng, LI Xiao-fei, XUE Sheng-guo, HARTLEY W, WU Chuan, HAN Fu-song. Natural plant colonization improves the physical condition of bauxite residue over time [J]. Environmental Science and Pollution Research, 2016, 23: 22897�C22905. DOI: 10.1007/s11356-016-7508-1.
[158] LEHOUX A P, LOCKWOOD C L, MAYES W M, STEWART D I, MORTIMER R J, GRUIZ K, BURKE I T. Gypsum addition to soils contaminated by red mud: Implications for aluminium, arsenic, molybdenum and vanadium solubility [J]. Environmental Geochemistry and Health, 2013, 35: 643�C656. DOI: 10.1007/s10653-013- 9547-6.
[159] BRAY A W, STEWART D I, COURTNEY R, ROUT S P, HUMPHREYS P N, MAYES W M, BURKE I T. Sustained bauxite residue rehabilitation with gypsum and organic matter 16 years after Initial Treatment [J]. Environmental Science & Technology, 2018, 52: 152�C161. DOI: 10.1021/acs.est.7b03568.
[160] ZHANG Na, LI Hong-xu, LIU Xiao-ming. Recovery of scandium from bauxite residue-red mud: A review [J]. Rare Metals, 2016, 35: 887�C900. DOI: 10.1007/s12598-016- 0805-5.
[161] JOHNSTON M, CLARK M W, MCMAHON P, WARD N. Alkalinity conversion of bauxite refinery residues by neutralization [J]. Journal of Hazardous Materials, 2010, 182: 710�C715.
[162] BORRA C R, BLANPAIN B, PONTIKES Y, BINNEMANS K, VANGERVEN T. Recovery of rare earths and other valuable metals from bauxite residue (red mud): A review [J]. Journal of Sustainable Metallurgy, 2016, 2: 365�C386. DOI: 10.1007/s40831-016-0068-2.
[163] ZHU Feng, LI Yu-bing, XUE Sheng-guo, HARTLEY W, WU Hao. Effects of iron-aluminium oxides and organic carbon on aggregate stability of bauxite residues [J]. Environmental Science and Pollution Research, 2016, 23(9): 9073�C9081.
[164] LIAO Jia-xin, JIANG Jun, XUE Sheng-guo, CHENG Qing-yu, WU Hao, RAJENDRAN M, HARTLEY W, HUANG Long-bin. A novel acid-producing fungus isolated from bauxite residue: the potential to reduce the alkalinity [J]. Geomicrobiology Journal, 2018, 35(10): 840�C847. DOI: 10.1080/01490451.2018.1479807.
[165] XUE Sheng-guo, YE Yu-zhen, ZHU Feng, WANHG Qiong-li, JIANG Jun, HARTLEY W. Changes in distribution and microstructure of bauxite residue aggregates following amendments addition [J]. Journal of Environmental Sciences, 2019, 78: 276�C286. DOI: 10.1016/j.jes.2018.10.010.
[166] JACOMINO V M F, OLIVEIRA K A P D, TADDEI M H T, SIQUEIRA M C, CAMEIRO M E D P. Radionuclides and heavy metal contents in phosphogypsum samples in comparison to Cerrado soils [J]. Revista Brasileira De Ciencia Do Solo, 2009, 33: 1481�C1488. DOI: 10.1590/ S0100-06832009000500038.
[167] BANNING N C, PHILIPS I R, JONES D, MURPHY D V. Development of microbial diversity and functional potential in bauxite residue sand under rehabilitation [J]. Restoration Ecology, 2011, 19: 78�C87. DOI: 10.1111/j.1526-100X.2009. 00637.x.
(Edited by YANG Hua)
���ĵ���
��ҵ������Գ�����Ե��ص�Ӧ���о���չ
ժҪ����������������ҵ���������в�����ǿ���Է�������������Լ������Դ�����õ���ҪӰ�����ӡ������Ѽ��о������ѿ�չ��ʮ�꣬�����������Բ�ʼ��δ��ʵ��Ӧ�á����ù�ҵ��������س�����Լȿɴ������ٷ���Ĵ��÷��ã�Ҳ�ɲ������õ���̬����Ч�档�������������������Դ�Լ���ҵ�����ﴦ�÷�ʽ�Ļ����ϣ�����ˡ��Է��ηϡ����о�˼·��̽���˳�����Ե��ص�ƿ�����⣬�����˵���ҵ�������������ˮ�ͷ������Գ�����Ե�ת�����ƣ�ָ����Ӧ�ù�ҵ�����������������о����ص㷢չ�����⽫Ϊ��������������úͶѳ�����̬��ʵ���ṩ��ѧ������
�ؼ��ʣ����ࣻ����ת�������Ե��أ���ҵ���������������
Foundation item: Projects(41877551, 41842020) supported by the National Natural Science Foundation of China; Project(201509048) supported by the Environmental Protection��s Special Scientific Research for Chinese Public Welfare Industry
Received date: 2018-10-23; Accepted date: 2018-12-12
Corresponding author: XUE Sheng-guo, PhD, Professor; Tel: +86-13787148441; E-mail: sgxue70@hotmail.com, sgxue@csu.edu.cn; ORCID: 0000-0002-4163-9383
Abstract: Bauxite residue is a highly alkaline material generated from the production of alumina in which bauxite is dissolved in caustic soda. Approximately 4.4 billion tons of bauxite residues are either stockpiled or landfilled, creating environmental risks either from the generation of dust or migration of filtrates. High alkalinity is the critical factor restricting complete utilization of bauxite residues, whilst the application of alkaline regulation agents is costly and difficult to apply widely. For now, current industrial wastes, such as waste acid, ammonia nitrogen wastewater, waste gypsum and biomass, have become major problems restricting the development of the social economy. Regulation of bauxite residues alkalinity by industrial waste was proposed to achieve ��waste control by waste�� with good economic and ecological benefits. This review will focus on the origin and transformation of alkalinity in bauxite residues using typical industrial waste. It will propose key research directions with an emphasis on alkaline regulation by industrial waste, whilst also providing a scientific reference point for their potential use as amendments to enhance soil formation and establish vegetation on bauxite residue disposal areas (BRDAs) following large-scale disposal.


