DOI:10.19476/j.ysxb.1004.0609.2017.11.24
H2O2/SO2催化还原六价硒及硒单质形成机理
郭学益,徐润泽,田庆华,李 栋
(中南大学 冶金与环境学院,长沙 410083)
摘 要:
研究采用双氧水催化二氧化硫还原六价硒获得单质硒的新方法。通过对催化还原过程中的溶液电位、离子浓度、产物形貌及晶型变化规律的研究,揭示催化还原过程中硒单质晶型转变机理。结果表明:在反应温度小于60 ℃时硒还原率可达到98%以上,六价硒还原产物为无晶型红硒单质;反应温度高于60 ℃时还原产物为斜方晶型的黑硒单质,其还原反应历程为SeO42-还原产生红硒,红硒进一步被二氧化硫过还原为Se2-离子,Se2-离子与还原中间产物SeO32-发生归中反应最终生成斜方晶型的黑硒单质。
关键词:
文章编号:1004-0609(2017)-11-2370-09 中图分类号: 文献标志码:A
硒属于稀散金属元素,具有独特的半导体、光电感应特性,在冶金[1-2]、化学、光电学[3-5]、医学[6]等领域被广泛应用,我国作为全球主要硒进口国[7],硒资源高效利用以及综合回收具有重要意义。目前,国内从铜、镍阳极泥等二次资源中回收硒占硒产量的90%以上[8]。回收技术主要利用硒化合物易被氧化分解的特性,破坏其原有结构使硒以硒单质或二氧化硒的形式分离[9-10],后经收集或还原过程产生粗硒[11-13]。但氧化分离过程中硒极易被过氧化成六价的硒化合物[14-15],这类高价硒化合物的存在大幅降低了现有(以四价硒为处理对象)工艺的硒回收率。
特别针对六价硒回收的技术目前有离子交换[16]、光催化还原[17]、铁离子还原[14, 18]、电积[19]、金属(如铝、锌、铜)置换[20]等。但上述方法具有成本高、回收工艺流程长、技术不成熟等缺点,工业化可行性普遍较低,简单可行的六价硒回收方法仍有待开发。
随着人类大规模有色金属冶炼、煤炭开采与使用,使得硒污染问题日益突出[21-22]。人们很早就意识到含硒煤发电污染问题[23],在煤燃烧过程中,硒挥发进入烟尘[24-25],随后在烟尘脱硫过程中被氧化为六价硒酸盐。这类硒酸盐难以利用常规废水处理技术消除[26]。
目前六价硒废水处理技术有膜分离[27-28]、活性污泥吸附[29]、碳纳米管吸附[30]、零价铁离子还原[31]、层状双金属氢氧化物吸附[32]、生物还原[33-34]等。但由于硫酸根与硒酸根存在吸附竞争这一无法根除的技术缺点[35]及处理费用昂贵等原因,六价硒废水处理及硒资源回收是近年来工业生产中亟待解决的技术难题之一。
在酸性溶液中,硒酸根离子被还原为硒单质的半反应如式(1)所示[17]:
SeO42-+8H++6e=Se+3H2O, φΘ=0.9 V (1)
在酸性溶液中溶解的二氧化硫发生还原反应的半反应如式(2)所示[1]:
SO42-+4H++(n-2)H2O+2e=SO2·nH2O, φΘ=0.2 V (2)
由式(1)和(2)可知,当二氧化硫为还原剂、SeO42-为氧化剂时,电池反应电势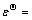 0.9-0.2=0.7 V,二氧化硫倾向于将SeO42- 还原为单质硒。反应总方程式及标准吉布斯自由能[36-38]如下:
0.9-0.2=0.7 V,二氧化硫倾向于将SeO42- 还原为单质硒。反应总方程式及标准吉布斯自由能[36-38]如下:
SeO42-+2H2O+3SO2=3SO42-+4H++Se (3)
△GΘ=-417.228 kJ/mol
上述热力学分析表明,溶液中二氧化硫具有将硒酸还原为硒单质的能力。但现有研究资料证实溶液体系中二氧化硫无法还原硒酸离子,只有在温度高于130 ℃的条件下,二氧化硫气体可以将硒酸还原为亚硒酸[1]。
本文作者首先发现并研究了双氧水催化二氧化硫还原六价硒的新技术,用催化的方式降低了二氧化硫还原六价硒的动力学阻力,为处理与回收六价硒资源提供了一种简单高效的新方法。在此,探索了初始溶液酸度、反应水浴温度、反应时间等因素对催化还原六价硒的影响,并通过进一步研究溶液电位变化、硒还原过程中产物物相变化,论证了还原过程中硒单质生成机理。迄今为止,没有发现任何关于双氧水催化二氧化硫还原Se(VI)及还原过程中硒单质生成机理的研究报道。
1 实验
1.1 实验原料
实验用硒酸溶液为二氧化硒(湖南鑫裕公司生产,99.85%)配置,氧化后通过电感耦等离子光谱仪检测溶液中含硒2.5 g/L,配合离子色谱确定溶液中有机玻璃全部为六价硒离子,二氧化硫用作还原剂,纯水由纯水仪(ZOOMWO,ZWL-HLPA1-60)制备,电阻率18 MΩ·cm,分析纯双氧水、浓硫酸、氢氧化钠来自国药集团。
1.2 实验方法
实验在四口瓶中进行,二氧化硫采用曝气头通入溶液中,高温反应(温度高于60 ℃)采用冷凝管回流水蒸汽。尾气处理装置由存有氨水的广口瓶、存有氢氧化钠的广口瓶和存有活性炭的U型管组成,避免剩余二氧化硫直接排放。
反应溶液硒离子浓度均为2.5 g/L,双氧水加入量为总溶液体积的10%。酸度条件实验中控制反应温度为25 ℃;温度-酸度条件实验中选用200 mL溶液反应,二氧化硫通气量为0.3 L/min,实验用水浴方式控制反应温度,其中0 ℃采用冰水浴;温度条件实验中溶液氢离子浓度控制为2 mol/L;反应历程实验选用硒浓度为1.25 g/L,二氧化硫通气量为1 L/min,氢离子浓度为6 mol/L,反应温度为60 ℃,反应过程中快速取样3 mL,过滤后检测溶液中硒离子浓度,为研究催化还原过程中硒还原产物的变化情况,采用上述相同的实验条件,反应过程中取10 mL溶液,过滤后固体干燥后送XRD与SEM检测。
硒离子浓度是通过电感耦合等离子体发射光谱仪(ICP-OES,SPECTRO,SPECTROBLUE FMX26)检测,配置溶液中硒离子价态是通过离子色谱仪(IC,METROHM,861 Advanced Compact IC)检测,对比标准六价硒溶液(国药集团)得出结果的,固体产物的特性是采用扫描电镜(SEM,FEI ESEM,Quanta 200)配合X射线衍射(XRD,Rigaku,TTRAX-3)来确定的,配置溶液的酸碱度采用人工滴定的方法测得,反应过程中检测溶液电位的仪器是辰华CHI-604D电化学工作站,参比电极为汞/硫酸亚汞电极(雷磁),工作电极为铂电极(雷磁)。
2 结果与讨论
2.1 酸度和温度影响
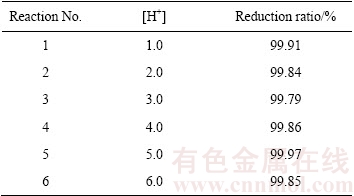
本实验中首先研究了溶液酸度对Se (VI)溶液催化还原的影响,溶液酸度及硒还原率如表1所列。实验结果表明,溶液酸度对催化还原影响较小,硒还原率最低为99.79%。
表1 不同酸度溶液中硒还原率
Table 1 Selenium reduction ratio in solutions with different acid
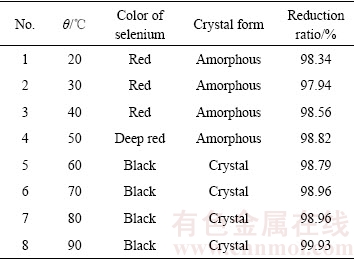
实验温度对反应的影响如表2所列。由表2可知,反应水浴温度对于催化还原产物的形态影响较大。随着水浴温度上升,还原产物的颜色逐渐加深,20 ℃水浴时反应产物为血红色;当温度上升至50 ℃后,产物颜色加深至深红色;当温度到达60 ℃后,还原产物完全转变为黑色。经过X射线衍射检测结果显示在水浴温度为20~50 ℃条件下,反应产物均为非晶体,均未见晶型衍射峰,而水浴温度为60 ℃以上的反应产物均为三方偏方(Trigonal-Trapezohedral)晶型的黑硒单质。
对不同酸度、温度的Se(VI)溶液开展催化还原研究,溶液电位变化如图1所示。由图1可知,溶液电势变化总体呈现3个阶段:初始平台期、还原反应下降期和末尾稳定期。
表2 不同反应温度硒还原率及还原产物情况
Table 2 Selenium reduction ratio and morphology of reduction products in different temperatures
结合反应式(2)及能斯特方程的分析可知:当二氧化硫溶解后,溶液电位为φ=φΘ+RT/{nF ln([SO42-]/ [SO2])}正比于硫酸根离子浓度,因此,在溶液电位段初始平台期,相同温度条件下,酸度较高的溶液电位较高。
在溶液电位下降期,相同反应温度不同酸度的溶液电位下降情况相似。在0和25 ℃反应中,溶液电位匀速下降至最低。而在60和90 ℃反应中,溶液电位下降速度出现快-慢-快的变化且反应温度越高电位变化越明显。在下降速度较慢的“平台”时间,还原产物明显地发生由红硒向黑硒的形态转变。
电位进入末尾稳定期后,还原产物颜色不再变化。此时电位下降主要是因为通入的二氧化硫继续溶解,将溶液电位拉低至接近其标准还原电位[39](φΘ=0.33 V (vs Hg/Hg2SO4))。
由溶液酸度-反应温度系列实验发现,高温反应中还原产物并非始终是黑色,是在通入二氧化硫反应初期产生的红色产物继续转化为黑色的,对此现象,进一步研究催化还原历程中硒单质变化。
2.2 反应历程中硒浓度变化
采用间歇取样检测硒离子浓度与固体产物形态变化的方法研究了催化还原过程中黑硒单质形成机理。实验过程中溶液电位进入下降期后出现还原产物由红硒到黑硒的转变,绘制反应电位-时间与浓度-时间图,为方便讨论,将此阶段电位与硒浓度变化区域放大,并划分为4个区域,如图2所示。
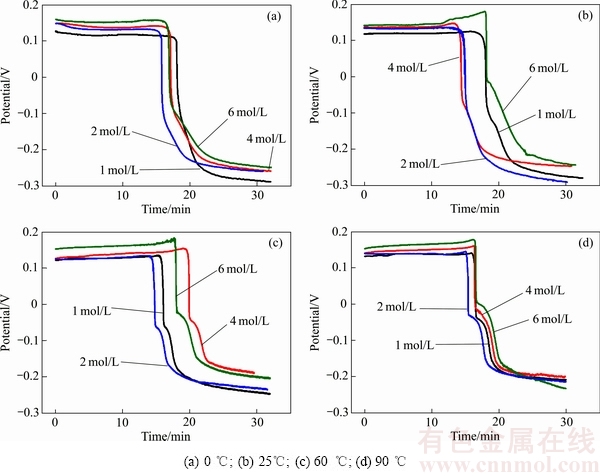
图1 不同反应温度条件下不同酸度溶液的电位变化图
Fig. 1 Potential change curves of solutions with various H+ concentrations at different reaction temperatures
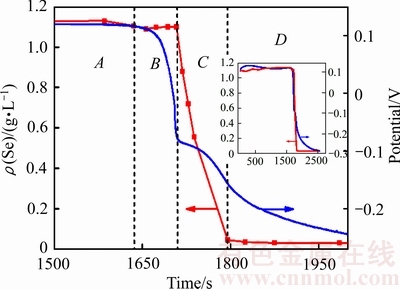
图2 溶液电位及硒浓度随时间变化图
Fig. 2 Solution potential and Se concentration change during the reaction
图2中A区域为溶液电位初始平台期末段,部分硒开始被还原为单质,溶液转变为淡红色;B区域为溶液电位第一次快速下降区域,区域溶液颜色逐步加深至浅红色。溶液中硒离子浓度小幅下降后平稳上升,说明此阶段还原产生的硒单质在逐渐增多的同时会发生反溶解的现象。C区域电位下降速度变慢,产生一个明显的电位“平台”,此时溶液中红硒生成量增多,硒离子浓度快速下降,溶液颜色加深为血红色。到C区域中间阶段时,溶液中出现团聚的黑色硒单质颗粒,溶液颜色由血红色转变为深红色,在C区域的末段,溶液颜色突然变为无色,还原产生的红硒全部转化为黑硒,硒离子浓度达到最低点,此时硒还原率达到98.79%。
据此实验现象认为:溶液中产生的黑硒并非由红硒在高温条件下晶型重排产生,而是发生了:生成红硒-红硒反溶解-黑硒再生成的过程。为验证这种硒单质转变机理,从还原产物晶型及微观形貌角度继续深入研究。重复实验并将过滤产物进行XRD与SEM检测,实验电位变化如图3所示,取样点名称及取样时间如图3中标注,对应样品检测SEM与XRD结果如图4和5所示。
由还原产物电镜检测可以看出,硒单质为形貌不规则颗粒。图4(a)显示:当溶液电位开始下降时,首先出现的红硒颗粒形貌不均一,以团聚后的二次颗粒为主;图4(b)中颗粒形貌没有出现显著变化,颗粒的尺寸有明显的增长;图4(c)所示为电位下降过程中平台拐点取样产物,此时溶液为浅红色,颗粒明显的团聚更为紧密,整体尺寸增大;图4(d)所示为电位“平台”初期时产物取样图,此时溶液中出现黑硒,溶液颜色转变为深红色。固体颗粒微观形貌发生明显改变,由原来生成的紧密团聚产物变为疏松的珊瑚状颗粒团聚体,这说明电位再次快速下降时的还原产物不是前一阶段还原产物的简单转化,而是前一阶段产物“破坏”后的重组再生;图4(e)所示为电位“平台”中间点,溶液颜色由红色变为无色透明,还原产物完全转变为黑硒,电镜检测显示此时取样的颗粒同样为珊瑚状;图4(f)所示为溶液电位再次快速下降点,产物微观形貌增长为致密的珊瑚状;最终,还原产物生长为形貌不规则的团聚颗粒,尺寸为1~3 μm之间,如图4(g)所示。
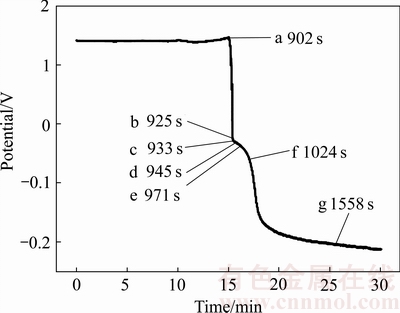
图3 重复实验电位及取样时间图
Fig. 3 Solution potential change and sampling time of repeat research
图5所示为反应产物的XRD谱。由图5可以看出,a~c取样产物主要以红硒为主,无晶型的红硒晶包从a到c逐渐凸显,其中c曲线在晶包中出现部分斜方黑硒的晶峰,说明c取样点中有一部分硒单质以黑硒的形式存在;由于取样产物较少且难以与滤纸分离,d~f取样产物衍射峰有黑硒的峰和滤纸的纤维素的峰同时存在。d~f变化过程中,硒衍射峰在逐渐变得更为尖锐。g为最终产物黑硒的XRD检测结果,为标准的三方偏方(Trigonal-Trapezohedral)晶型黑硒(JCPDS data. ID nos. 42-1425 (Se))。
2.3 催化还原机理讨论
在催化还原过程中,不同水浴温度反应产生的硒单质有较大的形态改变,虽然还原过程中硒颜色转变早有报道[40],但本文作者得出的催化还原过程硒形态改变机理与以往研究的结论不同[41-43]。本实验中发现六价硒催化还原过程会出现一个硒离子浓度稳定区(见图1(b))和电位稳定区(见图1(c)),这两个区域的先后出现改变了硒还原产物的晶型及形貌,这一现象尚未见相关研究报道。
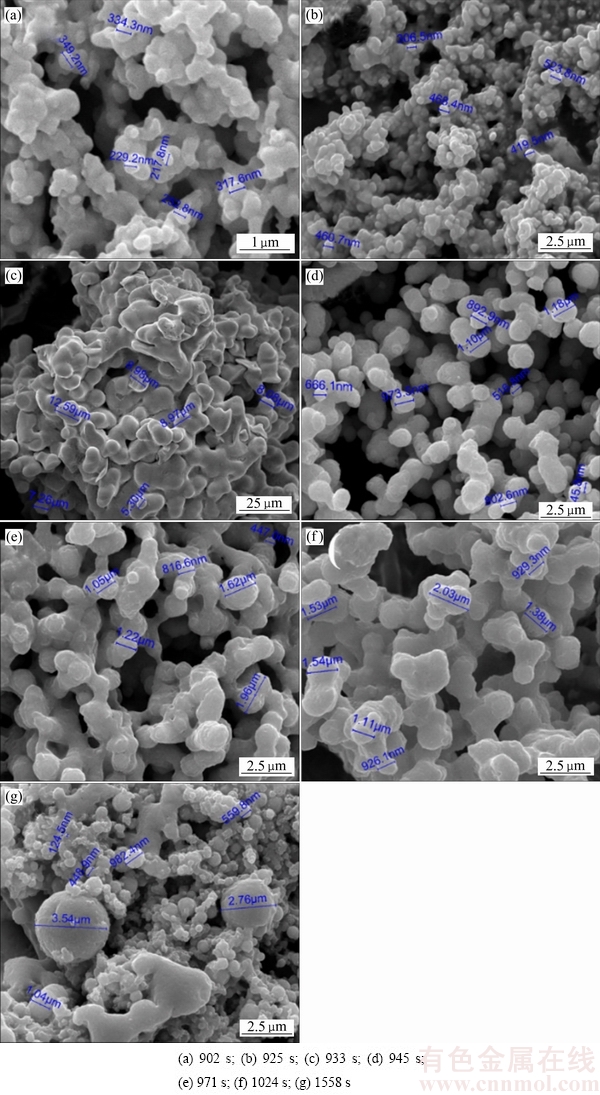
图4 不同反应阶段固体产物的 SEM像
Fig. 4 SEM images of reduction products
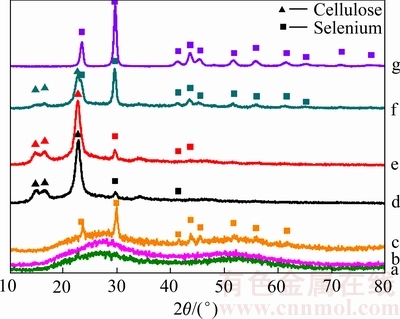
图5 固体产物的XRD谱
Fig. 5 XRD patterns of reduction products
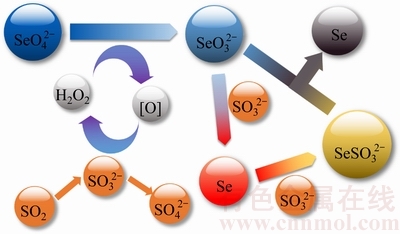
图6 催化还原机理示意图
Fig. 6 Schematic diagram of catalytic reduction mechanism
对此实验现象,认为催化还原机理如下:首先Se(Ⅵ)被双氧水催化还原为Se(Ⅳ)(见式(4)),Se(Ⅳ)被二氧化硫直接还原为红硒单质(见式(5)),红硒单质在低温条件下稳定性较高,不与二氧化硫继续反应;在高温条件下,红硒会被二氧化硫进一步还原为Se(Ⅱ)(见式(6)),使得溶液中不断产生的红硒单质反溶解,宏观上显示为硒离子浓度稳定区(见图2中B区),此后通入的二氧化硫全部用于过还原红硒单质,宏观上显示为溶液电位稳定区域(见图2中C区),当过还原产生的Se(-Ⅱ)达到一定浓度后,开始与溶液中Se(Ⅳ)发生归中反应生成黑硒单质沉淀析出(见式(7)),此时溶液中硒离子浓度才快速下降,当溶液中红硒全部被过还原后,溶液电位再次开始下降并下降至最低点。
反应过程中可能的化学方程式如下:
SeO42- +SO2+H2O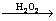 SeO32-+SO42-+2H+ (4)
SeO32-+SO42-+2H+ (4)
SeO32-+2SO2+H2O→Se(红)+2SO42-+2H+ (5)
Se(红) +SO2+H2O SeSO32-+2H+ (6)
SeSO32-+2H+ (6)
SeSO32-+ SeO32-+2H+→Se(红)+2SO42-+H2O (7)
3 结论
1) 发现了双氧水催化二氧化硫还原六价硒的新方法,研究了还原产物形成机理。通过对催化还原溶液酸度及还原温度的研究发现:溶液酸度及水浴温度不影响六价硒的还原率,在本实验条件下,硒还原率均达到99%以上。进一步研究结果显示,在温度低于60 ℃的反应中六价硒离子还原产物为无晶型的红硒单质,在温度高于60 ℃的反应中六价硒离子还原产物为三方偏方晶型的黑硒单质。
2) 设计实验研究了高温还原过程的硒离子浓度、溶液电位变化、还原产物微观形貌等内容后认为,双氧水催化二氧化硫还原六价硒过程是:首先通过双氧水的催化作用将六价硒离子还原为四价硒离子,随后四价硒离子被二氧化硫还原为无晶型的红硒单质,在温度小于60 ℃反应条件下红硒单质为最终还原产物,而反应温度高于60 ℃时红硒单质会被二氧化硫过还原为硒代硫酸根离子,硒代硫酸根离子会与溶液中亚硒酸根离子发生归中反应生成黑硒单质。
REFERENCES
[1] 姚凤仪, 郭德威. 无机化学丛书(第五卷)[M]. 北京: 科学出版社, 1998.
YAO Feng-yi, GUO De-wei. Inorganic chemistry (Vol. 5)[M]. Beijing: Science Press, 1998.
[2] 谭柱中, 梅光贵, 李维健. 锰冶金学[M]. 长沙: 中南大学出版社, 2004: 345.
TAN Zhu-zhong, MEI Guang-gui, LI Wei-jian. Metallurgy of manganese[M]. Changsha: Central South University Press, 2004: 345.
[3] WALLIN E, MALM U, JARMAR T, EDOFF O L, STOLT L. World record Cu(In,Ga)Se2 based thin film sub module with 17.4% efficiency[J]. Progress in Photovoltaics: Research and Applications, 2012, 20(7): 851-854.
[4] JACKSON P, HARISKOS D, LOTTER E, PAETEL S, WUERZ R, MENNER R, POWALLA M. New world record efficiency for Cu(In,Ga)Se2 thin-film solar cells beyond 20%[J]. Progress in Photovoltaics: Research and Applications, 2011, 19(7): 894-897.
[5] 翟秀静, 稀散金属[M] 北京: 中国科学技术大学出版社, 2009: 237.
ZAI Xiu-jing. Scattered metals[M]. Beijing: Press of University of Science and Technology of China, 2009: 237.
[6] ANDERSON C S. Selenium and Tellurium. 2013 Minerals Yearbook[R]. US Geological Survey, 2015.
[7] 李静贤, 刘家军. 硒矿资源研究现状[J]. 资源与产业, 2014, 16(2): 90-97.
LI Jing-xian, LIU Jia-jun. Research status of selenium mineral resources[J]. Resources and Industry, 2014, 16(2): 90-97.
[8] 李 栋, 徐润泽, 许志鹏, 郭学益. 硒资源及其提取技术研究进展[J]. 有色金属科学与工程, 2015, 6(1): 18-23.
LI Dong, XU Run-ze, XU Zhi-peng, GUO Xue-yi. Research progress of selenium resources and its extraction technology[J]. Nonferrous Metals Science and Engineering, 2015, 6(1): 18-23.
[9] LIU W, YANG T, ZHANG D, CHEN L, LIU Y. Pretreatment of copper anode slime with alkaline pressure oxidative leaching[J]. International Journal of Mineral Processing, 2014, 128: 48-54.
[10] 周令冶, 陈少纯. 稀散金属提取冶金[M]. 北京: 冶金工业出版社, 2008.
ZHOU Ling-ye, CHENG Shao-chun. Dilute metal extraction[M]. Beijing: Metallurgical Industry Press, 2008: 308.
[11] 马亚赟, 郑雅杰, 丁光月, 王俊文, 董俊斐, 张福元. 卤素离子催化作用下SO2还原沉金后液及其热力学特征[J]. 中国有色金属学报, 2016, 26(4): 901-907.
MA Ya-zan, ZHENG Ya-jie, DING Guang-yue, WANG Jun-wen, DONG Fei-ji, ZHANG Yuan-fu. Precipitated gold solution reduced by SO2 under halogen ion composite catalyst and its thermodynamic characteristics[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(4): 901-907.
[12] 马玉天, 龚竹青, 陈文汨, 李宏煦, 阳征会, 黄 坚. 从硫酸溶液中还原制取金属碲粉[J]. 中国有色金属学报, 2006, 16(1): 189-194.
MA Yu-tian, GONG Zhu-qing, CHEN Wen-mi, LI Hong-xu, YANG Zheng-hui, HUANG Jian. Preparation of powdered- tellurium by reduction from sulfuric acid solution[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(1): 189-194
[13] 张福元, 郑雅杰, 孙召明, 马亚赟, 董俊斐. 采用亚硫酸钠还原法从沉金后液中回收稀贵金属[J]. 中国有色金属学报, 2015, 25(8): 2293-2299.
ZHANG Yuan-fu, ZHENG Ya-jie, SUN Zhao-ming, MA Ya-bing, DONG Fei-ji. Recovery of rare and precious metals from precipitated gold solution by Na2SO3 reduction[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(8): 2293-2299.
[14] SUBRAMANIAN K N, ILLIS A, NISSEN N C. Recovery of selenium: US Patent 4163046[P]. 1979-07-31.
[15] REUSSER ROBERT E. Removal of selenium from uranium leach liquors: US Patent 3239307[P]. 1966-03-08.
[16] KOSHIKUMO F, MURATA W, AKIYUKI O O Y A, IMABAYASHI S I. Acceleration of electroreduction reaction of water-soluble selenium compounds in the presence of methyl viologen[J]. Electrochemistry, 2013, 81(5): 350-352.
[17] TAN T T Y, YIP C K, BEYDOUN D, AMAL R. Effects of nano-Ag particles loading on TiO 2 photocatalytic reduction of selenate ions[J]. Chemical Engineering Journal, 2003, 95(1): 179-186.
[18] PEAK D, SPARKS D L. Mechanisms of selenate adsorption on iron oxides and hydroxides[J]. Environmental Science & Technology, 2002, 36(7): 1460-1466.
[19] BAEK K, KASEM N, CIBLAK A, VESPER D, PADILLA I, ALSHAWABKEH A N. Electrochemical removal of selenate from aqueous solutions[J]. Chemical Engineering Journal, 2013, 215(15): 678-684.
[20] 胡 琴, 吴 展. 从铜阳极泥处理分铜后液中回收硒和碲[J]. 有色金属工程, 2014, 4(4):41-43.
HU Qin, WU Zhan. Recovery of Se and Te from copper anode slime treatment[J]. Nonferrous Metal Engineering, 2014, 4(4): 41-43.
[21] 江用彬, 季宏兵, 李甜甜, 王丽新. 环境硒污染的植物修复研究进展[J]. 矿物岩石地球化学通报, 2007, 26(1): 98-104.
JIANG Yong-bin, LI Hong-bing, LI Tian-tian. Research progress on phytoremediation of selenium contaminated environment[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2007, 26(1): 98-104.
[22] 田贺忠, 曲益萍, 王 艳, 程 轲, 潘 迪. 中国燃煤大气硒排放及其污染控制[J]. 中国电力, 2009, 42(8): 53-57.
TIAN He-zhong, QU Yi-ping, WANG Yan, CHEN Ke, PAN Di. Atmospheric selenium emission from coal combustion in China and its pollution control[J]. Electric Power, 2009, 42(8): 53-57.
[23] ROWE C L, HOPKINS W A, CONGDON J D. Ecotoxicological implications of aquatic disposal of coal combustion residues in the United States: A review[J]. Environmental Monitoring and Assessment, 2002, 80(3): 207-276.
[24] TIAN H, WANG Y, XUE Z, QU Y, CHAI F, HAO J. Atmospheric emissions estimation of Hg, As, and Se from coal-fired power plants in China, 2007[J]. Science of the Total Environment, 2011, 409(16): 3078-3081.
[25] SENIOR C, van OTTEN B, WENDT J O, SAROFIM A. Modeling the behavior of selenium in pulverized-coal combustion systems[J]. Combustion and Flame, 2010, 157(11): 2095-2105.
[26] AKIHO H, ITO S, MATSUDA H, YOSHIOKA T. Elucidation of the mechanism of reaction between S2O82–, selenite and Mn2+ in aqueous solution and limestone-gypsum FGD liquor[J]. Environmental Science & Technology, 2013, 47(19): 11311-11317.
[27]  B J, SELLECK R E. Reverse osmosis treatment of multicomponent electrolyte solutions[J]. Journal of Membrane Science, 1992, 72(3): 211-229.
B J, SELLECK R E. Reverse osmosis treatment of multicomponent electrolyte solutions[J]. Journal of Membrane Science, 1992, 72(3): 211-229.
[28] MAVROV V, STAMENOV S, TODOROVA E, CHMIEL H, ERWE T. New hybrid electrocoagulation membrane process for removing selenium from industrial wastewater[J]. Desalination, 2006, 201(1): 290-296.
[29] JAIN R, MATASSA S, SINGH S, van HULLEBUSCH E D, ESPOSITO G, LENS P N. Reduction of selenite to elemental selenium nanoparticles by activated sludge[J]. Environmental Science and Pollution Research, 2016, 23(2): 1193-1202.
[30] KAMARAJ R, VASUDEVAN S. Decontamination of selenate from aqueous solution by oxidized multi-walled carbon nanotubes[J]. Powder Technology, 2015, 274: 268-275.
[31] LING L, PAN B, ZHANG W. Removal of selenium from water with nanoscale zero-valent iron: mechanisms of intraparticle reduction of Se(Ⅳ)[J]. Water Research, 2015, 71: 274-281.
[32] ZHOU J, SU Y, ZHANG J. Distribution of OH bond to metal-oxide in Mg3-xCaxFe-layered double hydroxide (x=0-1.5): Its role in adsorption of selenate and chromate[J]. Chemical Engineering Journal, 2015, 262: 383-389.
[33] CONLEY J M, FUNK D H, HESTERBERG D H, HSU L C, KAN J, LIU Y T, BUCHWALTER D B. Bioconcentration and biotransformation of selenite versus selenate exposed periphyton and subsequent toxicity to the mayfly Centroptilum triangulifer[J]. Environmental Science & Technology, 2013, 47(14): 7965-7973.
[34] SONSTEGARD J, HARWOOD J, PICKETT T. Full scale implementation of GE ABmet biological technology for the removal of selenium from FGD wastwarners[C]// Proceedings of 68th International Water Conference. Pittsbursh, PA: Engineer’s Society of Western Pennsylvania, 2007, 2: 580.
[35] 周瑞兴. 连续逆流离子交换法回收废水中硒的过程模拟和优化[D]. 广州: 华南理工大学, 2012.
ZHOU Rui-xing. Process simulation and optimization of recovery of selenium from wastewater by continuous countercurrent ion exchange[D]. Guangzhou: South China University of Technology, 2012.
[36] DEAN J A. 兰氏化学手册[M]. 魏俊发, 译. 北京: 科学出版社, 2003.
DEAN J A. Lange’s handbook of chemistry[M]. WEI Jun-fa, transl. Beijing: Science Press, 2003.
[37] 杨显万. 高温水溶液热力学数据计算手册[M]. 北京: 冶金工业出版社, 1983.
YANG Xian-wan. Handbook of thermodynamic data calculation for high temperature aqueous solution[M]. Beijing: Metallurgical Industry Press, 1983.
[38] 叶大伦. 实用无机物热力学数据手册 [M]. 2版. 北京: 冶金工业出版社, 2002.
YE Da-lun. Handbook of thermodynamic data for practical inorganic materials[M]. 2nd ed. Beijing: Metallurgical Industry Press, 2002: 302-305.
[39] 朱元保, 沈子琛, 张传福, 黄德培. 电化学数据手册[M]. 长沙: 湖南科学技术出版社, 1985.
ZHU Yuan-bao, SHENG Zi-chun, ZHANG Chuan-fu, HUANG De-pei. Handbook of electroChemical data[M]. Hunan: Hunan Science and Technology Press, 1985.
[40] LINGANE J J, NIEDRACH L W. Polarography of selenium and tellurium. I. The-2 states[J]. Journal of the American Chemical Society, 1948, 70(12): 4115-4120.
[41] 刘 东, 张瀛洲. 旋转环盘电极法研究亚硒酸的电化学还原机理[J]. 厦门大学学报(自然科学版), 1989, 28(5): 495-499.
LIU Dong, ZHANG Ying-zhou. Electrochemical reduction mechanism of rotating ring disk electrode method research of selenite[J]. Journal of Xiamen University (Natural Science), 1989, 28(5): 495-499.
[42] 郭东红, 张飞宇, 卢凤丽. 旋转环-盘电极法研究磷酸溶液中亚硒酸的电化学还原机理[J]. 河南大学学报(自然科学版), 1999, 29(1): 33-38.
GUO Dong-hong, ZHANG Fei-yu, LU Feng-li. Rotating ring disk electrode by electrochemical study of phosphate reduction mechanism of selenious acid solution[J]. Journal of Henan University (Natural Science), 1999, 29(1): 33-38.
Hydrogen peroxide catalytic reduction of selenate by sulfur dioxide and formation mechanism of selenium
GUO Xue-yi, XU Run-ze, TIAN Qin-hua, LI Dong
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: In the present paper, the hydrogen peroxide catalytic reduction of selenate by sulfur dioxide was reported for the first time, by which the reduction rates of selenate reached 99.8%, proving a new way to recycle Se6+. A series of experiments were carried out by monitoring the change of solution potential, selenium concentration, the change of morphology and crystal. In addition, intermittent sampling during the reaction process was carried out to analyze the morphology transformation mechanism of catalytic reduction process. It was found that selenate could be directly catalytic reduced into red selenium below 60 ℃. The trigonal crystal selenium was formed by reaction between Se2- and Se4+ when reaction temperature was above 60 ℃, where the Se2- was produced by further reduction of red selenium.
Key words: hexavalent selenium; catalyze reduction; sulfur dioxide; hydrogen peroxide
Foundation item: Project(51234009) supported by the National Natural Science Foundation of China; Project (2014DFA90520) supported by the National Science and Technology Cooperation of China
Received date: 2017-02-21; Accepted date: 2017-07-02
Corresponding author: LI Dong; Tel: +86-731-88876089; E-mail: yejin013_18@163.com
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51234009);国家国际科技合作专项项目(2014DFA90520)
收稿日期:2017-02-21;修订日期:2017-07-02
通信作者:李 栋,讲师,博士;电话:0731-88876089;E-mail: yejin013_18@163.com
摘 要:研究采用双氧水催化二氧化硫还原六价硒获得单质硒的新方法。通过对催化还原过程中的溶液电位、离子浓度、产物形貌及晶型变化规律的研究,揭示催化还原过程中硒单质晶型转变机理。结果表明:在反应温度小于60 ℃时硒还原率可达到98%以上,六价硒还原产物为无晶型红硒单质;反应温度高于60 ℃时还原产物为斜方晶型的黑硒单质,其还原反应历程为SeO42-还原产生红硒,红硒进一步被二氧化硫过还原为Se2-离子,Se2-离子与还原中间产物SeO32-发生归中反应最终生成斜方晶型的黑硒单质。
[1] 姚凤仪, 郭德威. 无机化学丛书(第五卷)[M]. 北京: 科学出版社, 1998.
YAO Feng-yi, GUO De-wei. Inorganic chemistry (Vol. 5)[M]. Beijing: Science Press, 1998.
[2] 谭柱中, 梅光贵, 李维健. 锰冶金学[M]. 长沙: 中南大学出版社, 2004: 345.
[5] 翟秀静, 稀散金属[M] 北京: 中国科学技术大学出版社, 2009: 237.
[6] ANDERSON C S. Selenium and Tellurium. 2013 Minerals Yearbook[R]. US Geological Survey, 2015.
[7] 李静贤, 刘家军. 硒矿资源研究现状[J]. 资源与产业, 2014, 16(2): 90-97.
[8] 李 栋, 徐润泽, 许志鹏, 郭学益. 硒资源及其提取技术研究进展[J]. 有色金属科学与工程, 2015, 6(1): 18-23.
[10] 周令冶, 陈少纯. 稀散金属提取冶金[M]. 北京: 冶金工业出版社, 2008.
[11] 马亚赟, 郑雅杰, 丁光月, 王俊文, 董俊斐, 张福元. 卤素离子催化作用下SO2还原沉金后液及其热力学特征[J]. 中国有色金属学报, 2016, 26(4): 901-907.
[12] 马玉天, 龚竹青, 陈文汨, 李宏煦, 阳征会, 黄 坚. 从硫酸溶液中还原制取金属碲粉[J]. 中国有色金属学报, 2006, 16(1): 189-194.
[13] 张福元, 郑雅杰, 孙召明, 马亚赟, 董俊斐. 采用亚硫酸钠还原法从沉金后液中回收稀贵金属[J]. 中国有色金属学报, 2015, 25(8): 2293-2299.
[14] SUBRAMANIAN K N, ILLIS A, NISSEN N C. Recovery of selenium: US Patent 4163046[P]. 1979-07-31.
[20] 胡 琴, 吴 展. 从铜阳极泥处理分铜后液中回收硒和碲[J]. 有色金属工程, 2014, 4(4):41-43.
[21] 江用彬, 季宏兵, 李甜甜, 王丽新. 环境硒污染的植物修复研究进展[J]. 矿物岩石地球化学通报, 2007, 26(1): 98-104.
[22] 田贺忠, 曲益萍, 王 艳, 程 轲, 潘 迪. 中国燃煤大气硒排放及其污染控制[J]. 中国电力, 2009, 42(8): 53-57.
[35] 周瑞兴. 连续逆流离子交换法回收废水中硒的过程模拟和优化[D]. 广州: 华南理工大学, 2012.
[36] DEAN J A. 兰氏化学手册[M]. 魏俊发, 译. 北京: 科学出版社, 2003.
DEAN J A. Lange’s handbook of chemistry[M]. WEI Jun-fa, transl. Beijing: Science Press, 2003.
[37] 杨显万. 高温水溶液热力学数据计算手册[M]. 北京: 冶金工业出版社, 1983.
[38] 叶大伦. 实用无机物热力学数据手册 [M]. 2版. 北京: 冶金工业出版社, 2002.
[39] 朱元保, 沈子琛, 张传福, 黄德培. 电化学数据手册[M]. 长沙: 湖南科学技术出版社, 1985.
[41] 刘 东, 张瀛洲. 旋转环盘电极法研究亚硒酸的电化学还原机理[J]. 厦门大学学报(自然科学版), 1989, 28(5): 495-499.
[42] 郭东红, 张飞宇, 卢凤丽. 旋转环-盘电极法研究磷酸溶液中亚硒酸的电化学还原机理[J]. 河南大学学报(自然科学版), 1999, 29(1): 33-38.


