
Influence of AlF3 and hydrothermal conditions on morphologies of α-Al2O3
FU Gao-feng(付高峰)1, WANG Jing(王 晶)2, KANG Jian(康 健)2
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China;
2. College of Materials Science and Engineering, Dalian Jiaotong University, Dalian 116028, China
Received 10 September 2007; accepted 12 April 2008
Abstract:
Homogeneous α-Al2O3 platelets were synthesized by introducing AlF3 to alumina precursor. The effects of AlF3 additive on the phase transformation and morphology of the prepared α-Al2O3 platelets were investigated. The results show that a single phase of α-Al2O3 with an average particle size of 8 mm can be obtained at 900 ℃ with 2% AlF3 additive. The transformation temperature decreasing is attributed to introduction of Al3+ vacancy and to the formation of intermediate compound of AlOF, which is considered to accelerate the mass transportation from transitional Al2O3 to α-Al2O3. AlF3 concentration and hydrothermal temperature can also affect the morphology of α-Al2O3. When hydrothermal temperature is 120 ℃, the morphology of α-Al2O3 transforms from irregular to flat hexangular platelet with increasing AlF3 concentration. As hydrothermal temperature increases, the morphology of α-Al2O3 with 2% AlF3 additive changes from polyhedron to hexangular platelet and then to vermicular.
Key words:
α-Al2O3 platelets; AlF3; hydrothermal treatment; morphology;
1 Introduction
Alumina is one of the most important oxides and has been intensively studied over a long period because of its widely potential applications in ceramic, glass, functional materials, adsorbents, catalysts and catalyst supports etc[1-3]. Alumina exists in a number of metastable transition phases as well as the thermodynamically stable α-Al2O3 or corundum.
As for the wet chemical methods, it is known that almost all those salt-derived aluminum hydroxides dehydrate to form g-Al2O3. The transformation proceeds through the following sequence, g—δ—θ—α[4]. The g-Al2O3 is a metastable defect spinel with the oxygen atoms in cubic packing and aluminum in both tetrahedral and octahedral coordinations. The δ and θ forms of alumina are also metastable defect spinels, making the rearrangement process from g-Al2O3 to θ-Al2O3 of relatively low energy[5-7]. However, the transformation from θ-Al2O3 to α-Al2O3 involves a significant change in the oxygen sublattice from cubic peaking to hexagonal close packing and generally requires temperatures above 1 200 ℃ for complete conversion to the thermodynami- cally stable corundum phase. The gained α-Al2O3 with hard agglomeration or vermicular morphology makes sintering to full density even more difficult due to the formation of large pores that are often entrapped within grains.
Thus, lowering the phase transformation temperatures is the target for preparing α-Al2O3. Some studies have already been carried out on the phase transformation of alumina. For example, some papers dealt with the mechanisms of phase transformation and the transformation mechanism of γ- to α-Al2O3, involving the conversion of cubic close packing of oxygen ions into stable hexagonal close packing[1,8-9]. While others reported the methods for enhancing the kinetic rate of phase transformation[10]. It is known that the temperatures of γ- to α-Al2O3 transition can be influenced by some cation additives. For example, the addition of transition metals such as Fe2+, Cr3+ and Ti4+ has been reported to accelerate the γ- to α-Al2O3 phase transformation, whereas Cs+, Ba2+, La3+, Si4+ additives are well known to retard it[11-14].
In this work, fluoride aluminum was introduced to the alumina precursor synthesized by aluminum alkoxide hydrolysis and hydrothermal treatment methods. The effects of AlF3 additive on the phase transformation and the morphologies of the prepared α-Al2O3 particles are investigated.
2 Experimental
Solution A was prepared by adding aluminum isopropoxide to certain content of analytical grade isopropanol (A.R., Dalian Inorganic Chemical Co., China). Certain amount of AlF3 (C.P., Shanghai Reagent Co., China) was added to the mixture solution of deionized water and isopropanol to make slurry B. The slurry B was dispersed by ultrasonic for 10 min and then mixed with solution A at 65 ℃ under rapid stirring and refluxing for 2 h. The resulting product was transferred into a Teflon-lined stainless autoclave to fill 80% of the total volume. The autoclave was sealed and maintained at certain temperature for 24 h. The autoclave was then allowed to cool naturally to room temperature and a white precipitated powder was obtained. The powder was filtered and washed several times with absolute ethanol and distilled water, and then dried in vacuum at 60 ℃ for 12 h. Al2O3 particles were obtained by annealing the as-synthesized powders in air at different temperatures for 3 h.
The crystal structure of the product was examined by X-ray diffraction (XRD, Cu Kα radiation, D/max-rB, Japan). An overview of the sample morphology was checked with a scanning electron microscope (SEM, JSM-6700F, JEOL, Japan).
3 Results and discussion
3.1 Phase transformation of Al2O3
Fig.1 shows the XRD patterns of the gained powders from the precursor without additive after hydrothermal treatment at 120 ℃ and calcination at different temperatures. Diffraction peaks corresponding to g-Al2O3, δ-Al2O3 and θ-Al2O3 are found for the sample calcined at 900 ℃. No significant change in the structure is found after calcination at temperature up to 1 000 ℃. The amount of α-Al2O3 increases drastically when the calcination temperature increases up to 1 100 ℃. However, a small amount of θ-Al2O3 remains.
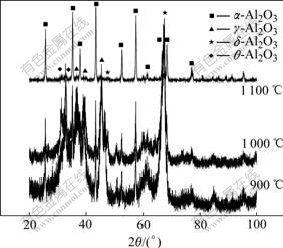
Fig.1 XRD patterns of calcined powders from precursor without additive
Fig.2 shows the XRD patterns of the gained powders from precursor with 2% AlF3 additive after hydrothermal treatment at 120 ℃ for 24 h and calcination from 550 to 1 200 ℃. It can be seen that g- Al2O3 forms for the sample calcined at 550 ℃ and 800 ℃. The phase transformation of g-Al2O3 to α-Al2O3 is completed at 900 ℃ and no significant change is found after the calcination at temperature up to 1 200 ℃.
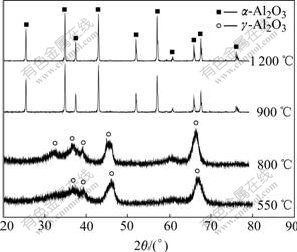
Fig.2 XRD patterns of Al2O3 precursor with AlF3 calcined at different temperatures
Generally, the transformation from metastable transition phases to α-Al2O3 involves a significant change in the oxygen sublattice and usually a temperature above 1 200 ℃ is required for complete conversion to the thermodynamically stable corundum phase. It can be seen from Fig.2 that the addition of AlF3 can enhance the kinetic rate of the phase transformation form g-Al2O3 to α-Al2O3 and reduce the transformation temperature of α-Al2O3 phase. During the heat treatment, the existence of AlF3 destroys the well-regulated atomic packing configuration and introduces Al3+ vacancy. When AlF3 is introduced to alumina precursor, two defect reactions may take place as follows:
![]() (1)
(1)
![]() (2)
(2)
The vacancies and interstitial atoms can be formed through these reactions. There are no significant distinctions between F- radius and O2- radius, so F- can not take interstitial sites. Comparatively, the formation energy of vacancy is lower than that of interstitial atoms. Thus, the defect can give priority to Al3+ vacancies. Al3+ vacancies accelerate Al3+ diffusion and reduce the transformation temperature of g-Al2O3 to α-Al2O3.
In addition, some intermediate compounds can be formed in the case of the phase transformation. They can also accelerate the mass transportation from transitional phase to α-Al2O3. It is known that γ-Al2O3 is highly active and has large surface area. Thus, it is strongly hygroscopic to adsorb H2O in the air. During the heat treatment, AlF3 can take the following reversible reaction with H2O adsorbed[15]:
2AlF3+3H2O![]() Al2O3+6HF (3)
Al2O3+6HF (3)
And the positive reaction may be performed through the secondary reactions as follows.
1) The gaseous intermediate compound, AlOF, can be synthesized by the following reaction.
AlF3+H2O=AlOF+2HF (4)
The gaseous intermediate compound, AlOF, can play the role of gas transmission and accelerate atomic transference velocity to enhance the phase transformation. In this work, although it is quite difficult to define the composition of the intermediate compound, it can ultimately transform into alumina:
3AlOF=Al2O3+AlF3 (5)
2AlOF+H2O=Al2O3+2HF (6)
2) The intermediate compound, Al2O, can be synthesized as:
2AlF3+3H2O=Al2O+6HF+O2 (7)
Finally, Al2O takes part in the following reactions and transforms into alumina:
3Al2O=Al2O3+4Al (8)
Al2O+2H2O=Al2O3+2H2 (9)
Similarly, the intermediate compound, Al2O, can enhance the phase transformation by accelerating atomic transference velocity.
3.2 Morphology of α-Al2O3
The morphologies of the gained powders from precursor with 2% AlF3 additive after hydrothermal treatment at 120 ℃ for 24 h and calcination from 800 to 1 200 ℃ were examined by SEM. Fig.3 shows the SEM micrographs of the obtained products. The microstruc- ture of the sample calcined at 800 ℃ is floccule and the full-grown crystal is not seen. The alpha phase does not appear, which is convinced by XRD pattern at 800 ℃ as shown in Fig.2. When the calcinating temperature is raised to 900, 1 000 and 1 200 ℃, respectively, the morphologies of those particles are plate-like and there is no remarkable change in particle size. It is shown that once alpha phase is obtained, the effects of temperature on morphology and size are rather feeble.

Fig.3 SEM micrographs of samples treated at different temperatures: (a) 800 ℃; (b) 900 ℃; (c) 1 000 ℃; (d) 1 200 ℃
Fig.4 shows the SEM micrographs of the gained α-Al2O3 powders from precursors with different AlF3 concentration calcined at 1 200 ℃. It can be seen thatα-Al2O3 passes through the morphology transformation of irregular platelet to flat hexangular platelet as AlF3 concentration is increased.
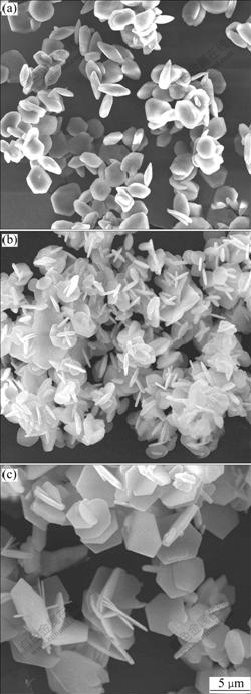
Fig.4 Effect of AlF3 concentration on morphology of α-Al2O3: (a) 2%AlF3; (b) 10%AlF3; (c) 20%AlF3
α-Al2O3 belongs to rhombohedral crystal system and the space group is ![]() The existence of AlF3 additive causes different growth rate along the respective crystal axis and finally has effects on the morphology of α-Al2O3. In view of crystal growth, the microstructure is determined by crystal growth rate. The crystal plane with larger growth rate will gradually disappear and while the crystal plane with smaller growth rate will be maintained. The shape of the crystal plane is relevant to the crystal plane with larger growth rate. The unsaturated surface tension existed on the surface of solid can adsorb AlF3 around. And the adsorption capacity varies with the anisotropic surface tension. Usually, the surface tension of crystal plane with close-packed atom is smaller. In α-Al2O3 unit cell, (0001) and its symmetrical crystal plane takes with close-packed atom. They have weaker capacity to adsorb gaseous molecule and accordingly there are less atoms transferred by gas transmission, thus (0001) and its symmetrical crystal plane are maintained because of the lower growth rate along the crystal axis. Generally, the crystal planes with lower growth rate have more perfect crystal structure, so (0001) and its symmetrical crystal plane are much more smooth than others with excessive crystal defects caused by rapid growth. Then the crystal planes with larger growth rate will gradually disappear and transform to final platelet morphology.
The existence of AlF3 additive causes different growth rate along the respective crystal axis and finally has effects on the morphology of α-Al2O3. In view of crystal growth, the microstructure is determined by crystal growth rate. The crystal plane with larger growth rate will gradually disappear and while the crystal plane with smaller growth rate will be maintained. The shape of the crystal plane is relevant to the crystal plane with larger growth rate. The unsaturated surface tension existed on the surface of solid can adsorb AlF3 around. And the adsorption capacity varies with the anisotropic surface tension. Usually, the surface tension of crystal plane with close-packed atom is smaller. In α-Al2O3 unit cell, (0001) and its symmetrical crystal plane takes with close-packed atom. They have weaker capacity to adsorb gaseous molecule and accordingly there are less atoms transferred by gas transmission, thus (0001) and its symmetrical crystal plane are maintained because of the lower growth rate along the crystal axis. Generally, the crystal planes with lower growth rate have more perfect crystal structure, so (0001) and its symmetrical crystal plane are much more smooth than others with excessive crystal defects caused by rapid growth. Then the crystal planes with larger growth rate will gradually disappear and transform to final platelet morphology.
SEM micrographs of α-Al2O3 synthesized at different hydrothermal temperatures for 24 h and calcined at 1 200 ℃ for 3 h are shown in Fig.5. The sample via 120 ℃ hydrothermal treatment shows the polyhedron morphology, and plate-like morphology can be obtained when the hydrothermal temperature is increased up to 160 ℃. Nevertheless, α-Al2O3 passes through the morphology transformation of platelet to vermiform morphology with increasing the hydrothermal temperature to 180 ℃. And with increasing the hydrothermal temperature up to 240 ℃, the α-Al2O3 powder obtained is more seriously aggregated and the powders connects with each other.
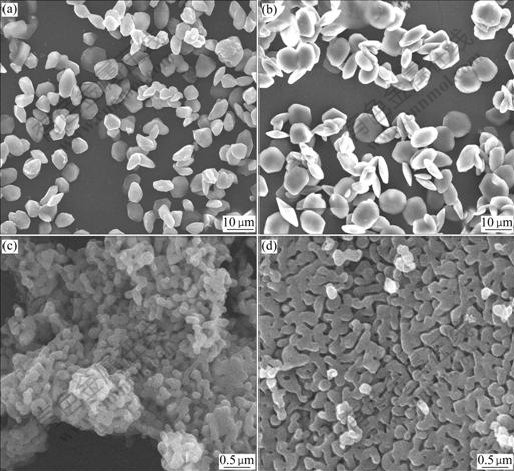
Fig.5 SEM micrographs of samples heat treated at different hydrothermal temperatures: (a) 120 ℃; (b) 160 ℃; (c) 180 ℃; (d) 240 ℃
The microscopic mechanism of the crystal growth is essentially the dynamic balance between adsorption and desorption of the growth unit from the microscopic kinetics. When the adsorption rate of the growth unit is larger than the desorption rate, the crystal represents to grow in favor of the formation of α-Al2O3 platelets. However, the adsorption rate will be lower than the desorption rate with increasing hydrothermal temperature up to the critical value, in which the formation of α-Al2O3 platelets is restrained. When the alumina precursor with AlF3 additive is treated at 240 ℃ under high pressure, the existence of AlF3 can make the growth units irregularly syncretize and superpose mutually on the same crystal plane and finally the seriously aggregated microstructure can be formed with the crystal growth. It is well known that AlF3 presents in a supersaturated mother phase and it can significantly change the nucleation of crystals, the growth and aggregation of nuclei and so on. But the effect of AlF3 additive varying with the different hydrothermal temperature can result in distinct crystal growth habit during calcinations and then influence the formation of α-Al2O3 platelets.
4 Conclusions
1) In hydrothermal process, the transformation temperature of γ-Al2O3 to α-Al2O3 can be lowered by 300 ℃ through the addition of 2% AlF3 and the well-dispersed α-Al2O3 powders with an average particle size of 8 mm can be obtained.
2) Once alpha phase appears, the effects of temperature on particle morphology and size are quite weak. As AlF3 concentration is increased, the morphology of α-Al2O3 powders could be transformed from irregular platelet to flat hexangular platelet.
3) The plate-like morphology can be transformed into vermiform morphology when the hydrothermal temperature is increased to above 180 ℃ and the α-Al2O3 particles synthesized after 240 ℃ hydrothermal treatment are seriously aggregated.
References
[1] TARTAJ P, TARTAJ J. Preparation, characterization and sintering behavior of spherical iron oxide doped alumina particles [J]. Acta Materialia, 2002, 50: 5-12.
[2] DONG Yan, JIANG Jian-qing, LIU Gang, LIANG Chao, SUN Ming-tao. Preparation of alumina as a raw material of phosphor and controlling of its particle size and shape [J]. Journal of the Chinese Ceramic Society, 2004, 32(4): 393-397. (in Chinese)
[3] ZHOU Zhen-jun, YANG Zheng-fang, YUAN Qi-ming. Preparation of small sized α-alumina platelet by sol-gel method [J]. Bulletin of the Chinese Ceramic Society, 2003(4): 11-15. (in Chinese)
[4] SHEPPARD L M. Enhancing performance of ceramic composites [J]. J Am Ceram Soc, 1992, 71: 617-631.
[5] BOWEN P, SHENG J, JONGEN N. Particle size distribution measurement of anisotropic-particles cylinders and platelets-practical examples [J]. Powder Technology, 2002, 128: 256-261.
[6] LI Jiang, PAN Yu-bai, XIANG Chang-shu. Low temperature synthesis of ultrafine α-Al2O3 powder by a simple aqueous sol-gel process [J]. Ceramic International, 2006, 32(5): 587-591.
[7] ZHU H Y, GAO X P, SONG D Y, RINGER SIMON P, XI Y X, FROST RAY L. Manipulating the size and morphology of aluminum hydrous oxide nanoparticles by soft-chemistry approaches [J]. Microporous and Mesoporous Materials, 2005, 85: 226-233.
[8] QU Li-hong, HE Chang-qing, YANG Yue, HE Yan-li, LIU Zhong-min. Hydrothermal synthesis of alumina nanotubes templated by anionic surfactant [J]. Materials Letters, 2005, 59: 4034-4037.
[9] XIE Zhi-peng, LU Ji-wei, GAO Li-chun, XU Li-hua, WANG Xi-dong. Influence of different seeds on transformation of aluminum hydroxides and morphology of alumina grains by hot pressing [J]. Materials & Design, 2003, 24: 209-2l4.
[10] WU Yi-quan, ZHANG Yu-feng, HUANG Xiao-xian, GUO Jing-kun. Preparation of platelike nano alpha alumina particles [J]. Ceramics International, 2001, 27: 265-268.
[11] WU Yi-quan, ZHANG Yu-feng, PEZZOTTI G. Influence of AlF and ZnF on the phase transformation of gamma to alpha alumina [J]. Materials Letters, 2002, 52: 366-369.
[12] MACEDO M I F, BERTRAN C A, OSAWA C C. Kinetics of the γ- α-alumina phase transformation by quantitative X-ray diffraction [J]. Journal of Materials Science, 2007, 42(8): 2830-2836.
[13] LOPEZ V A, REYES B J L, SONG S, HERRERA U R. Temperature effect on the zeta potential and fluoride adsorption at the α-Al2O3/ aqueous solution interface [J]. Journal of Colloid and Interface Science, 2006, 298(1): 1-5.
[14] LI Jiang, WU Yu-song, PAN Yu-bai, LIU Wen-bing, GUO Jing-kun. Influence of fluorides on phase transition of α-Al2O3 formation [J]. Ceramics International, 2007, 33(6): 919-923.
[15] YAMAI I, SAITO H. Vapor phase growth of alumina whiskers by hydrolysis of alumina fluoride [J]. Journal of Crystal Growth, 1978, 45: 511-516.
Corresponding author: WANG Jing; Tel: +86-411-84107583; E-mail: wangjing@djtu.edu.cn


