文章编号:1004-0609(2007)07-1083-07
Sn-9Zn钎料与内加Cu质点和Cu基体界面生长行为
卫国强1,况 敏2,杨永强1,赵 利2
(1. 华南理工大学 机械工程学院,广州 510641;
2. 广州有色金属研究院 材料表面中心,广州 510651)
摘 要:
在Sn-9Zn无铅钎料中加入Cu金属质点,研究在长时间钎焊条件下钎料/Cu质点、钎料/Cu基体界面金属间化合物(IMCs)的生长行为。结果表明:在钎料/Cu质点和钎料/Cu基体界面处都生成Cu-Zn相(IMCs),其组成为Cu5Zn8+CuZn或Cu5Zn8,而且钎料/质点界面处IMCs的生长速度明显快于钎料/基体处;同时发现,Cu质点的加入大大减小了钎料/Cu基体界面IMCs的厚度。由于Cu质点原位生成Cu-Zn IMCs,消耗了焊点中的Zn,因此Sn-9Zn/Cu接头的可靠性得以提高。
关键词:
中图分类号:TG 454 文献标识码:A
Interfacial growth behavior of Sn-9Zn/additive Cu-particles and Sn-9Zn/Cu-substrate
WEI Guo-qiang1, KUANG Min2, YANG Yong-qiang1, ZHAO Li2
(1. College of Mechanical Engineering, South China University of Technology, Guangzhou 510641, China;
2. Materials Surface Centre, Guangzhou Research Institute of Nonferrous Metals, Guangzhou 510651, China)
Abstract: The growth behavior of interfacial intermetallic compounds (IMCs) of Sn-9Zn/additive Cu-particles and Sn-9Zn additive Cu-substrate was investigated under the condition of extended soldering time. The results show that the Cu-Zn phases, which are composed of Cu5Zn8 and CuZn or Cu5Zn8, are formed at the interfaces of both Sn-9Zn/Cu-particles and Sn-9Zn/Cu-substrate, simultaneously the growth rate of IMCs of Sn-9Zn/Cu-particles is markedly higher than that of Sn-9Zn/Cu-substrate. It is also shown that the addition of Cu-particles in Sn-9Zn solder greatly reduces the thickness of IMCs layer of Sn-9Zn/Cu-substrate. The reliability for Sn-9Zn/Cu joint is improved for the diminished Zn content in soldering joint due to the in-situ formation of IMCs of Sn-9Zn/Cu-particles.
Key words: Sn-9Zn solder; lead-free solder; Cu-particle; intermetallic compound (IMCs)
Sn-38Pb钎料因其优异的性能和低廉的成本在电子组装行业中被广泛使用。但是Pb及其化合物属于有害物质,长期使用会对环境和人类健康造成危害,欧盟等许多国家已禁止进口含Pb电子产品。在寻找传统的Sn-38Pb钎料的替代品的过程中,Sn-9Zn无铅钎料(θm=198 ℃)由于熔点接近Sn-38Pb钎料(θm= 183 ℃),并且具有优异的力学性能及良好的经济性,被认为是Sn-38Pb钎料的潜在替代品之一[1-3]。在对Sn-9Zn钎料的研究中发现,在Sn-9Zn/Cu钎焊接头形成的金属间化合物(IMCs)是Cu-Zn IMCs而不是Cu-Sn IMCs。由于Cu-Zn IMCs的高温不稳定性,在多次再流焊及服役过程中接头界面Cu-Zn IMCs过度生长甚至分解导致接头界面出现孔洞,同时焊点中单质锌的存在将引起电化学腐蚀,降低接头的可靠性[4-7]。
为限制Sn-9Zn/Cu钎焊接头界面Cu-Zn IMCs的生长及降低焊点中单质Zn的含量,可通过合金化方法在Sn-9Zn钎料中加入与Zn的亲合力比Sn大的合金元素,如Cu和Ni等[8-10],以降低Zn的活性来达到以上目的。但Cu或Ni的加入将导致Sn-9Zn钎料的熔点升高,这将丧失Sn-9Zn无铅钎料熔点接近Sn-38Pb钎料的优势。本文作者采用复合钎料[11-17]方法,通过在Sn-9Zn粉状钎料中加入Cu质点,制得Cu质点增强的Sn-9Zn/Cup复合钎料。在钎焊时Cu质点原位生成IMCs,在不升高Sn-9Zn钎料熔点的前提下,降低钎焊接头界面Cu-Zn IMCs的生长速度,从而减小钎料/基体界面IMCs的厚度并降低焊点中单质Zn的含量,以提高焊点的可靠性[18]。为了探讨Cu质点的加入对Cu基体界面IMCs生长的影响,以及Cu质点界面和Cu基体界面IMCs的生长机理,研究Cu质点界面IMCs的生长行为,并与Cu基体界面IMCs的生长行为进行了比较,从理论上解释在Sn-9Zn钎料中加入少量Cu质点就可降低Cu基体界面IMCs厚度的原因。
1 实验
在粒径为45 μm的Sn-9Zn粉状钎料(图1(a))中加入不同比例粒径为8 μm的Cu粉(图1(b)),加入量分别为Sn-9Zn钎料的2%和5%(质量分数,下同),用自
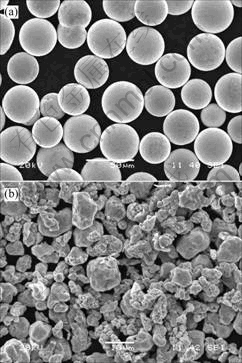
图1 Sn-9Zn粉状钎料和Cu粉的SEM形貌
Fig.1 SEM morphologies of Sn-9Zn powdery solder(a) and Cu-particle(b)
制的活性钎剂加入到按以上比例配制好的钎料中,通过搅拌方法调制成膏,其中钎剂的加入量为复合钎料的12%。为保证粉状钎料和Cu质点的混合均匀,搅拌时间不少于30 min。
Cu基板采用纯度为99.95%的纯铜,尺寸为30 mm×30 mm×0.2 mm。纯铜试样用800号砂纸磨光后,分别在5%(体积分数)的HCl溶液、去离子水和酒精溶液中浸泡并吹干,然后用分配器把复合钎料膏定量的注在试样中央(注入量约为200 mg)。将准备好的试样放入再流焊炉中进行钎焊,钎焊温度为230 ℃,钎焊时间为40 s,出炉空冷。二次再流焊的参数为θr= 230 ℃,tr1=5 min,tr2=10 min。
把钎焊试样在焊点中心处切开,用环氧树脂进行镶嵌,镶样时保证试样和观察面垂直,然后打磨、抛光,最后用腐蚀液(HNO3 5%,HCl 3%,CH3OH 92%,体积分数)进行腐蚀。采用LEICA DMIRM光学显微镜和JSM-5910扫描电镜对组织形貌、界面层厚度进行观察分析,用NORAN能谱分析仪对IMCs成分进行半定量分析。
2 实验结果
图2所示为Sn-9Zn,Sn-9Zn+2%Cu,Sn-9Zn+5%Cu 这3种钎料钎焊后经230 ℃、10 min再流焊的钎料/基体界面的显微形貌。图3所示为在扫描电镜上测量所得的Cu-Zn IMCs的厚度。EDX成分分析表明,钎料/基体IMCs由Cu5Zn8(钎料边)和CuZn(Cu基体边)组成,和文献[5]的结果一致。图4所示为再流焊时间对钎料/基体界面IMCs厚度的影响。
图5所示为Sn-9Zn+2%Cu复合钎料经230 ℃,40 s钎焊后的焊点中Cu质点界面的形貌及元素分布。表1所列为Cu质点从边缘到中心的EDX成分分析结果。从图5及表1可以看出,从质点边缘到中心处相组织分别为Cu5Zn8,CuZn和没有反应的Cu,Cu-Zn IMCs的厚度大约为3.3 μm。
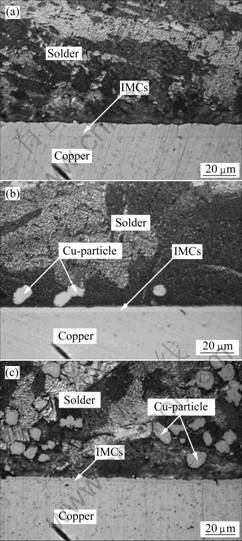
图2 钎料/基体界面的显微形貌
Fig.2 Interfacial morphologies of solder/substrate under reflowing at 230 ℃ for 10 min: (a) Sn-9Zn; (b) Sn-9Zn+2%Cu; (c) Sn-9Zn+5%Cu
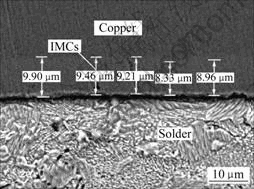
图3 IMCs厚度的测量
Fig. 3 Measurement of thickness of IMCs
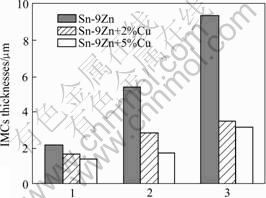
图4 再流焊时间对IMCs厚度的影响
Fig.4 Influence of reflowing time on thickness of IMCs: 1—Soldering 40 s; 2—Reflowing 5 min; 3—Reflowing 10 min
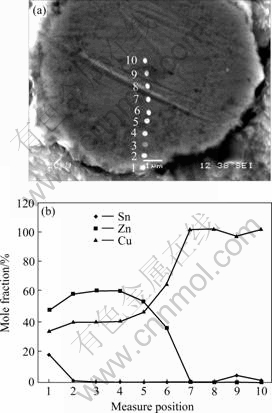
图5 Sn-9Zn+2%Cu/Cu质点界面形貌与元素分布
Fig.5 Sn-9Zn+2%Cu/Cup interface morphology(a) and element distribution(b) under soldering at 230 ℃ for 40 s
图6所示为Sn-9Zn+2%Cu复合钎料经230 ℃,5 min再流焊后焊点中Cu质点界面的形貌及元素分布。表2所列为从Cu质点边缘到中心的EDX成分分析结果。从图6及表2可以看出,由于该质点较小(直径为6.58 μm),Cu已经全部转化为Cu5Zn8相,不存在CuZn相。说明要生成CuZn相,必须要有足够量的Cu供给。
表1 Sn-9Zn+2%Cu钎料230 ℃, 40 s钎焊Cu质点处EDX元素分析
Table 1 EDX element analysis of Sn-9Zn+2%Cu/Cu-particle interface under soldering at 230 ℃ for 40 s
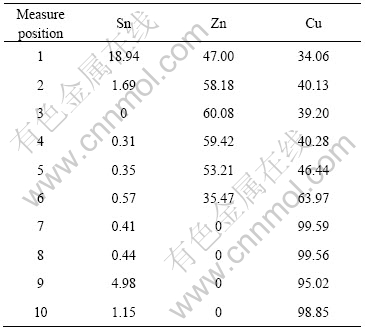
图6 Sn-9Zn+2%Cu/Cu质点形貌及元素分布
Fig.6 Sn-9Zn+2%Cu/Cu-particle interface morphology and element distribution under reflowing at 230 ℃ for 5 min
图7所示为Sn-9Zn+2%Cu复合钎料经230 ℃,10 min再流焊的焊点中Cu质点界面的形貌及元素分布。表3所列为从Cu质点边缘到质点中心的EDX成分分析。从图7和表3可以看出,Cu质点完全转化为Cu-Zn IMCs,Cu质点的外层为Cu5Zn8相,内层为CuZn相,因为质点的直径大约为12 μm,说明Cu-Zn IMCs的厚度超过6 μm。
表2 Sn-9Zn+2%Cu钎料230 ℃, 5 min再流焊Cu质点处EDX元素分析
Table 2 EDX element analysis of Sn-9Zn+2%Cu/Cu-particle interface under reflowing at 230 ℃ for 5 min
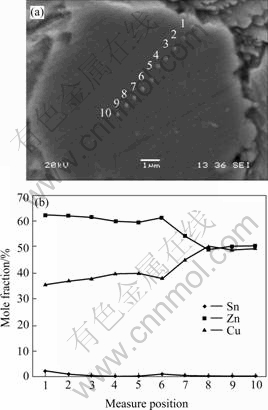
图7 Sn-9Zn+2%Cu/Cu质点形貌及元素分布
Fig.7 Sn-9Zn+2%Cu/Cu-particle interface morphology and element distribution under reflowing at 230 ℃ for 10 min
图8所示为不同再流时间的Cu质点和Cu基体界面处的IMC厚度。可以看出,随着时间的延长,无论是在Cu质点处,还是在Cu基体处,IMC的厚度都增加;在相同的再流焊条件下,Cu质点处的厚度大大超过Cu基体处的厚度。
表3 Sn-9Zn+2%Cu钎料230 ℃, 10 min再流焊Cu质点处EDX元素分析
Table 3 EDX element analysis of Sn-9Zn+2%Cu/Cu-particle interface under reflowing at 230 ℃ for 5 min (mole fraction, %)
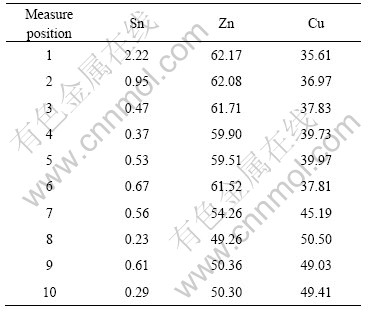
图8 Cu质点和Cu基体界面处IMCs厚度的比较
Fig.8 Comparison of interfacial IMCs thickness between Cu-particle and Cu-substrate: 1—Soldering 40 s; 2—Reflowing 5 min; 3—Reflowing 10 min
3 分析与讨论
在Sn-9Zn粉状钎料中加入少量的Cu质点,就可大大降低钎料/基体界面Cu-Zn IMCs的生长速度,减小界面IMCs的厚度;另一方面,Cu质点原位生成IMCs,降低了焊点中单质Zn的含量,使焊点的耐腐蚀性得以提高;而在焊点中原位生成的IMCs可强化焊点,提高焊点的热疲劳及蠕变性能,从而提高焊点可靠性。
在相同钎焊条件下,Cu质点界面处所形成的Cu-Zn IMCs的厚度大大超过基体界面处所形成的Cu-Zn IMCs的厚度,说明Cu质点“捕获”Zn的能力大于Cu基体。Cu质点的加入对钎料/基体界面IMCs的形成有明显的影响,而钎料/质点界面和钎料/基体界面的IMCs生长行为存在明显的差异。
钎焊时质点、基体界面反应界面示意图如图9所示。当用Sn-9Zn钎料(加质点或不加质点)钎焊Cu时,Cu首先在界面溶解进入液态Sn-9Zn钎料中,在局部位置处达到饱和,由于Zn和Cu的亲合力比Zn和Sn的亲和力大,因此在界面上形成Cu-Zn IMCs,Cu的溶解速率和界面处液态钎料中Zn的浓度分布将影响界面IMCs的生长行为。
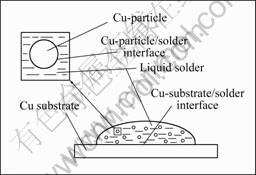
图9 钎焊过程钎料/基体和钎料/质点界面反应示意图
Fig.9 Sketch of interfacial reaction for solder/substrate and solder/particle under soldering
根据固态金属在液态金属中的溶解公式,单位面积固态金属溶解量的表达式为

溶解量的另一表达式为

所消耗的Cu一部分融入液态钎料中,另一部分和Zn反应生成IMCs,因此h可表示为
![]()
式中 w为Cu在液态钎料中的质量分数;w(Cu)为Cu在Cu-Zn IMCs中质量分数;Vc为IMCs的体积;ρc为IMCs的密度。
把式(2)代入式(1)得:
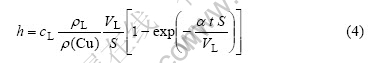
当Cu在液态钎料中达到饱和时,Cu的溶解率只和IMCs的体积变化率有关,因此对式(4)求导数,可得界面IMCs的生长速率为
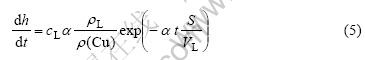
从式(5)可知,界面IMCs的生长速率只和液-固态金属的接触面积、液态钎料的体积及接触时间有关。在本实验条件下Cu质点界面和Cu基体界面在液态Sn-9Zn钎料中所处的状态是不同的,对于单个Cu质点而言,S/VL比值较小(相当于浸沾钎焊),而对于Cu基体而言,S/VL比值较大,从式(5)可以看出,Cu质点/钎料界面处的IMCs的生长速率大于Cu/基体界面处的生长速率,这和实验结果一致。
当在界面处形成的Cu-Zn IMCs变成连续层时,就没有Cu和液态Sn-9Zn钎料的接触,此时Cu-Zn IMCs层的生长就要靠液态钎料中的Zn和质点、基体中的Cu扩散通过IMCs层和IMCs层中的Cu、Zn扩散来满足。扩散过程有新相的生成,属于反应扩散,而反应扩散速度(反应层增厚的速度)取决于化学反应速度和原子扩散速度两个因素。若反应扩散速度由原子扩散控制,则反应层厚度与时间呈抛物线关系;若反应扩散过程由反应速度控制,则反应层厚度与时间呈线性关系。从图4和8可以看出,在本实验条件下,IMCs层的生长主要受化学反应速度控制。
综上所述,由于在钎焊时Cu质点界面、Cu基体界面的溶解速度不同,Cu质点“捕获”钎料中Zn的能力大于Cu基体,在钎料中加少量的Cu粉就能明显减少Cu基体界面IMCs的厚度。少量Cu粉的加入,在钎焊时对钎料的流动性(引起液态钎料的粘度变化)不会产生明显的影响。
4 结论
1) 在Sn-9Zn钎料中加入Cu质点,可大大减低Sn-9Zn钎料/Cu基体界面IMCs的生长速度,从而有效压缩IMCs层的厚度,界面IMCs由Cu-Zn相组成。
2) 钎焊时Sn-9Zn钎料/Cu质点界面Cu的溶解速度明显大于Sn-9Zn钎料/Cu基体界面处的,因此IMCs层的生长速率大于钎料/Cu基体界面的生长速率,导致Cu质点处IMCs的厚度明显大于Cu基体处。
3) 由于Cu质点“捕获”钎料中Zn的能力大于Cu基体,在Sn-9Zn钎料中加入少量的Cu粉,就可显著降低焊点界面IMCs的厚度;Cu质点原位生成IMCs,可减少焊点中单质Zn的含量,从而提高焊点可靠性。
REFERENCES
[1] Laurila T, Vuorinen V, Kivilahti J K. Interfacial reactions between lead-free solders and common base materials[J]. Mater Sci Eng R, 2005, R49: 1-60.
[2] Abtew M, Selvaduray G. Lead-free solders in microelectronics[J]. Mater Sci Eng A, 2000, A27(1): 85-141.
[3] Suganuma K. Advance in lead-free electronics soldering[J]. Current Opinion in Solid State Materials Science, 2001, 5(1): 55-64.
[4] Zeng K, Tu K N. Six cases of reliability of Pb-free solder joints in electronic packaging technology[J].Mater Sci Eng R, 2002, R38(1): 55-105.
[5] Suganuma K, Niihara K. Wetting microstructure between Sn-Zn binary alloys and Cu[J]. Journal of Materials Research, 2002, 13(10): 2859-2865.
[6] Vaynman S, Ghosh G, Fine M E. Some fundamental issues in the use of Zn-containing lead-free solders for electronic packaging [J]. Mater Trans, 2004, 45(3): 630-636.
[7] Huang C W, Lin K L. Interfacial reactions of lead-free Sn-Zn based solders on Cu and plated electroless Ni-P/Au layer under aging[J]. Journal of Materials Research, 2004, 19(12): 3560-3568.
[8] Yu D Q, Xie H P, Wang L. Investigation of interfacial microstructure and wetting property of newly developed Sn-Zn-Cu solders with Cu substrate[J]. Journal of Alloys and Compound, 2004, 385: 119-125.
[9] 谢海平, 于大全, 马海涛, 王 来. Sn-Zn-Cu 无铅钎料的组织、润湿性和力学性能[J]. 中国有色金属学报, 2004, 14(10): 1694-1699.
XIE Hai-ping, YU Da-quan, MA hai-tao, WANG Lai. Microstructure, wettability and mechanical properties of Sn-Zn-Cu lead free solder[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(10): 1694-1699.
[10] Ichitsubo T, Matsubara E, Fujiwara K, Yamaguchi M, Irie H, Kumammoto S, Anada T. Control of compound forming reaction at the interface between SnZn solder and Cu substrate[J]. Journal of Alloys and Compound, 2005, 392: 200-205.
[11] Marshall J L, Calderon. Hard-Particle reinforced composite solders (Part 1): Microcharacterisation[J]. Soldering and Surface mount Technology, 1997, 9(2): 22-28.
[12] Lin D C, Liu S, Guo T M, Wang G X, Srivatsan T S, Petraroli M. An investigation of nanoparticles addition on solidification kinetics and microstructure development of tin/lead solder[J]. Mater Sci Eng A, 2003, A360: 285-292.
[13] Lee J H. Reflow characteristics of Sn-Ag matrix in-situ composite solders[J]. Scripta Materialia, 2000, 42(8): 827-831.
[14] YAN Yan-fu, LIU Jian-ping, SHI Yao-wu, XIA Zhi-dong. Study on Cu particles-enhanced SnPb composite solder[J]. Journal of Electronic Materials, 2004, 33(3): 218-223.
[15] Kumar K M, Kripesh V, Shen L, Tay A A O. Study on the microstructure and mechanical properties of a novel SWCNT-reinforced solder alloy for ultra-fine pitch applications[J]. Thin Solid Films, 2006, 504(1/2): 371-378.
[16] Nai S M L, Wei J, Gupta M. Influence of ceramic Reinforcements on the wettability and mechanical properties of novel lead-free solder composites[J]. Thin Solid Films, 2006, 504(1/2): 401-404.
[17] Lee J G, Guo F, Subramanian K N, Lucas J P. Intermetallic morphology around Ni particles in Sn-3.5Ag solder[J] Soldering and Surface Mount Technology, 2002, 14(2): 11-17.
[18] 卫国强. 一种铜粉增强的锡锌复合钎料及其制备方法[P]. CN1931509, 2006-10-13.
WEI Guo-qiang. A sort of Sn-Zn composite solder reinforced by copper powder and its preparing method: CN, 1931509[P]. 2006-10-13.
收稿日期:2006-09-04;修订日期:2007-05-08
通讯作者:卫国强,高级工程师;电话:020-87114484;传真:020-87114484;E-mail: gqwei@scut.edu.cn


