Trans. Nonferrous Met. Soc. China 22(2012) 2984-2990
Synthesis of (Ca, Mg)-α′-Sialon-AlN-BN powders from boron-rich blast furnace slag by microwave carbothermal reduction-nitridation
JIANG Tao1, 2, 3, XUE Xiang-xin1, 2, 3
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China;
2. Liaoning Key Laboratory for Recycling Science of Metallurgical Resources, Northeastern University, Shenyang 110819, China;
3. Liaoning Key Laboratory for Ecologically Comprehensive Utilization of Boron Resource and Materials, Northeastern University, Shenyang 110819, China
Received 12 April 2012; accepted 3 July 2012
Abstract:
(Ca, Mg)-α′-Sialon-AlN-BN powders were synthesized by the carbothermal reduction and nitridation (CRN) method using boron-rich slag, one of the intermediate products from pyrometallurgy separation of pageit, as the staring material. The influences of synthesis temperature and holding time on the phase composition and microstructure during the microwave CRN were studied by XRD, SEM and EDS. The comparison between two heating techniques, conventional and microwave heating, on the synthesized powder was presented as well. The experimental results revealed that the phase compositions and microstructures of the synthesized products were greatly affected by the synthesis temperature and holding time. With an increase in the synthesis temperature or holding time, the relative amount of α′-Sialon increased and α′-Sialon became the main crystalline phase at 1400 °C for 6 h. The synthesized products also contained AlN, BN and a small amount of β-SiC. Elongated α′-Sialon grains, short rod AlN grains, aggregate nanoscale BN grains were observed in the synthesized powders. The reaction temperature of microwave heating method was reduced by 80 °C, the reaction time was shortened by 2 h, and more elongated α′-Sialon grains with large aspect ratio were observed.
Key words:
boron-rich blast furnace slag; carbothermal reduction-nitridation; microwave; (Ca, Mg)-α′-Sialon-AlN-BN; powders;
1 Introduction
Boron-rich blast furnace slag is one of the major products created during the separation of iron and boron from ludwigite in a blast furnace process. Boron-rich blast furnace slag primarily contains SiO2, Al2O3, MgO, CaO and B2O3 along with a small amount of iron oxide and other materials. Because of the low activity (approximately 50%) and low boron grade (B2O3 content of approximately 12% in mass fraction), it is difficult to use boron-rich blast furnace slag to directly produce boric acid and borax. Thus, its high efficiency utilization is of great importance to the Chinese boron industry.
Based on this background, one innovative process to recycle the boron-rich blast furnace slag in the synthesis of α′-Sialon (MxSi12-(m+n)Al(m+n)OnN16-n) using the carbothermal reduction–nitridation (CRN) method has been proposed. In this process of CRN, SiO2, Al2O3, MgO and CaO in the slag will synthesize (Ca,Mg)- α’-Sialon, at the meantime B2O3 is converted into hexagonal boron nitride, which has a high melting point and thermal conductivity, excellent corrosion and thermal shock resistance, as well as good electric insulativity and lubricity [1,2]. These superior characteristics can improve the properties of the α’-Sialon matrix composites. (Ca,Mg)-α′-Sialon–AlN– BN composite can be used as break ring in the horizontal continuous casting of steel, as a nozzle refractory or in new structural ceramics.
Sialon ceramics (mainly α′-Sialon and β′-Sialon) exhibit great potential in engineering applications because of their excellent mechanical properties, such as high hardness, toughness and strength. Typically, Sialons are prepared by firing powder compacts of Si3N4, AlN, Al2O3 and some oxide sintering additives at high temperature via a reaction sintering method [3-7]. The high manufacturing costs have limited the practical application of Sialon ceramics. Some researchers have turned to the carbothermal reduction and nitridation method to synthesize Sialons powders from a wide range of low-cost precursors. There are reports that α′-Sialon and β′-Sialon powders have been synthesized by heating natural clay powders [8-13] or industrial waste slag [14-16] with carbon in flowing nitrogen. The process requires a long processing time, and hence has obvious disadvantages, such as high-energy consumption. Recently, a novel synthesis method, namely microwave sintering technology, has been extensively explored for the preparation of β′-Sialon, AlN and SiC ceramics powders [17-19]. Compared with most conventional synthesis processes, a microwave sintering has the advantages of quick heating, high thermal efficiency, safety and clean.
At present, there have been no reports about the synthesis of low-cost (Ca, Mg)-α′-Sialon powders from boron-rich blast furnace slag. Therefore, the purpose of this work is to explore the feasibility of synthesizing (Ca,Mg)-α′-Sialon–AlN–BN powders from boron-rich blast furnace slag by the microwave CRN method. The effects of the synthesis temperature and holding time on the phase formation and microstructure evolution during a microwave CRN process were investigated. The comparison between two heating techniques, conventional and microwave heating, on the synthesized powder was presented as well.
2 Experimental
Boron-rich blast furnace slag, silica fume and bauxite chamote were used as the starting materials. Table 1 lists the characteristics of these materials. In addition, carbon black containing 96.7% carbon was used as the reducing agent. The carbon contents in all samples were fixed at 1.2 times the required stoichiometric value.
Table 1 Compositions of starting material powders
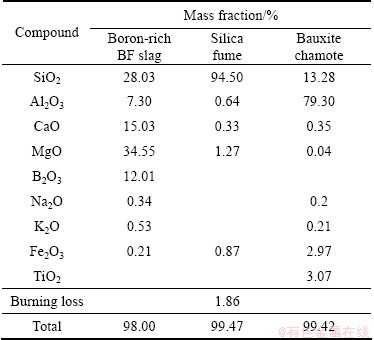
The compositions of all of the samples were designed to nominally lie on the two-dimensional α’ plane. In all of the samples, the m:n was 2:1. Thus, their compositions were nominally located on the tie line between Si3N4 and RO:3AlN (where R=Ca or Mg) in the α’ plane with the formula (Ca, Mg)xSi12-3xAl3xOxN16-x. In this work, the composition with x of 4 was designed.
First, the mixtures were placed in a polyurethane pot and ball-milled in ethanol for 24 h with agate-milling media. After milling, the slurry was dried and mixed again for 4 h. Approximately 1.5 g powder was then uniaxially pressed into a pellet with a diameter of 15 mm. The pellets were then pressureless sintered in a maximum output power 3 kW microwave heating furnace (SYNOTHERM Corporation in Changsha, China) in a nitrogen atmosphere with a N2 flow rate of 1 L/min from 1300 to 1480 °C for 0-8 h. The heating rate of the furnace was set to approximately 15 °C/min below 1000 °C and 6 °C/min above 1000 °C. The residual carbon was finally removed by burning the synthesised powders at 700 °C for 2 h in air.
Phase identification was performed by X-ray diffractometry (XRD, D/MAX-RB) using nickel-filtered Cu Kα radiation. The relative amount of the mainly crystalline phase in the sample was estimated as follows:
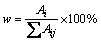 (1)
(1)
where Ai is the relative integral area of the strongest peak of the crystalline phase i, and Aij is the sum of the relative integral area of the strongest peak of all the crystalline phases in the sample.
The morphology of the sample was characterized by scanning electron microscopy (SEM, SSX-550 or S-3400N). The specific microstructural features of synthesised powders were analyzed by energy dispersion spectroscopy (EDS, SSX-550).
3 Results and discussion
3.1 Effect of synthesis temperature on reaction
Figure 1 shows the XRD patterns of the powders synthesized after sintering at 1300–1480 °C for 6 h. Figure 2 presents the relative amount of the crystalline phase. As shown in Fig. 1 and Fig. 2, Mg2SiO4, MgAl2O4, Ca2Al2SiO7 and less SiC were found in the products when the synthesis temperature was 1300 °C. The presence of these products reveals that under these conditions, the Mg2SiO4 in the boron-rich blast furnace slag was not reduced and did not decompose, and solid-phase reactions occurred between SiO2, Al2O3, CaO and MgO to generate compound oxides. At 1300 °C, the reaction products contained amorphous phases because the eutectic temperature in the Ca-Mg-Si-Al system is 1250 °C [20]. Above this temperature, the reaction system generates many liquid phases, which were maintained as amorphous phases during decreasing temperature due to the lower reaction temperature. A thermodynamic analysis indicated that SiC is formed only at higher temperatures under standard thermodynamics condition (pθ=101325 Pa). However, the presence of impurity elements, such as iron, alters the phase equilibria by changing the thermodynamic activity, resulting in the formation of liquid phases at lower temperatures. Because the starting materials in the present work were composed of natural minerals and metallurgical waste slag, which contains many impurities, the formation temperature of the liquid phases decreased, resulting in the generation of SiC at the low temperature of 1300 °C, and its amount increased then decreased with increasing temperature.
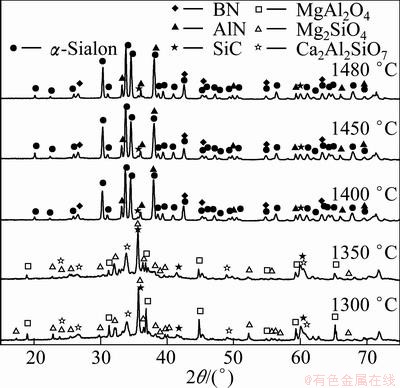
Fig. 1 XRD patterns for samples prepared at different synthesis temperatures
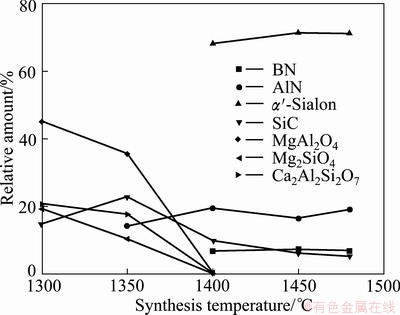
Fig. 2 Variation of phase compositions of samples with synthesis temperature
When the temperature reached 1350 °C, the phase compositions of the synthesized products were the same as those at 1300 °C; however, the amounts of MgAl2O4, Mg2SiO4 and Ca2Al2SiO7 decreased and that of the amorphous phases increased.
At a synthesis temperature of 1400 °C, the amount of α′-Sialon increased rapidly to become the primary crystalline phase. Meantime, other oxides disappeared entirely, which revealed that these oxides were generated as transitional phases in the preparation of a′-Sialon. HEWETT et al [21] also reported that Ca2Al2SiO7 was generated as a transitional phase in the preparation of Ca-α′-Sialon by the reaction sintering method at temperatures between 1200 and 1400 °C, which is the same as that of the present work. And contrasting the diffraction standard card of Ca-α′-Sialon (x=0.8), all the diffraction peaks of α′-Sialon formed were deviated to the direction of big diffraction angles. This indicated that the small amount of Ca2+ and Mg2+ was incorporated into α′-Sialon structure at the moment. In addition, AlN was also produced at 1400 °C. The result revealed that the CRN reaction of Al2O3 happened, yielding AlN, then some of the AlN participated in the synthesis of a′-Sialon. Based on the phase relationship of the Ca-Si-Al-O-N system [20], the upper limit of Ca-a’-Sialon on the link Si3N4-CaO:3AlN is at x value of 1.4, beyond which there is a multi-phase region, composed of α’-Sialon, a liquid phase and AlN polytypoids or AlN. Moreover, the partial SiO2 in the initial materials was reduced to SiO gas and removed from the reaction system with the flowing N2, which resulted in an excess of Al2O3 in the starting materials. Subsequently, the Al2O3 surplus was converted to AlN in the CRN process. The typical diffraction peak of h-BN occurred at the temperature of 1400 °C, and did not change significantly through a temperature of 1480 °C, which was the maximal temperature used in the present work. YOON and JHA [22] reported that the optimal synthesis temperature of h-BN was between 1300 and 1400 °C, using B2O3 and carbon mixtures as the starting materials, which is consistent with the present work.
When the synthesis temperature was increased to 1450 °C, the amount of α’-Sialon increased gradually, and that of AlN decreased. At this time, the microwave CRN reaction proceeded completely. At 1480 °C, the amount of α’-Sialon decreased, but that of AlN increased at the same time, suggesting that α′-Sialon had decomposed at this temperature.
Figure 3 shows the SEM micrographs of the powders that were synthesized at temperatures ranging from 1300 to 1480 °C for 6 h. Figure 4 shows the EDS spectra of the characteristic grains in the synthesized powders. As shown in Fig. 3 and Fig. 4, the grains in the powders synthesized at 1300 °C and 1350 °C were flaky and irregular and formed large agglomerates. This suggested that the reaction system generated many liquid phases, which promoted the formation of aggregates. A large quantity of elongated α’-Sialon with higher aspect ratio increased rapidly with the increase of synthesis temperature, and displayed obvious crystallization orientation, which indicated that the microwave field could promote preferred orientation of the α’-Sialon grains. The AlN grains were in the form of short rods, and agglomerates of nano-sized grains that are thought to be BN grains were observed as well.
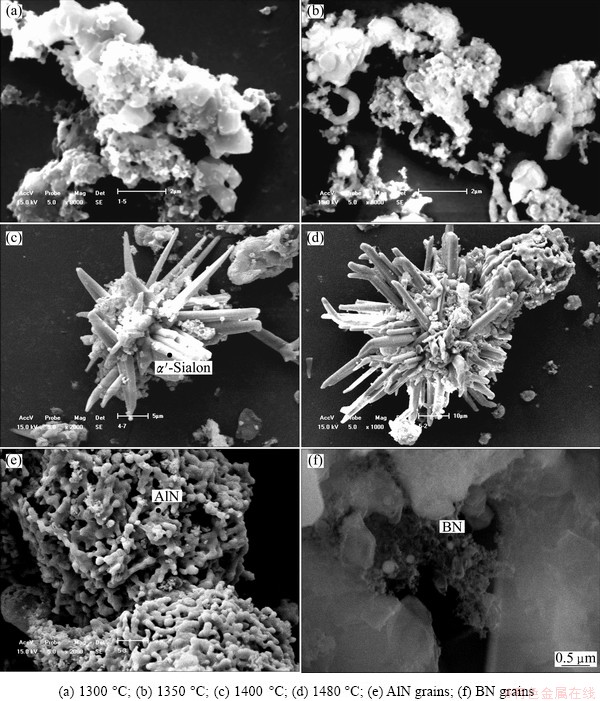
Fig. 3 SEM images of synthesized powders and characteristic grains at different temperatures
As shown in Fig. 4(a), both Ca2+ and Mg2+ were incorporated into the α′-Sialon structure, but the Ca2+ was present to a greater extent than the Mg2+ in the lattice. In this work, the compounds in the synthesized products that include Mg were not observed by the XRD characterization, even though the amount of MgO in the boron-rich blast furnace slag was very high. This lack of Mg detection suggests that much of the magnesium oxide may have been reduced and lost during the carbothermal reduction process. The results of some studies noticed the volatile phenomenon during the CRN process of MgO at higher temperature, which resulted in the loss of magnesium [23-25].
3.2 Effect of holding time on reaction
The phase assembly of the sample as a function of the holding time at 1400 °C is shown in Fig. 5. As shown in this figure, the phases of the products were composed of MgAl2O4, Mg2SiO4 and SiC at 1400 °C immediately. With prolonging holding time to 1 h, Mg2SiO4 vanished and Ca2Al2SiO7 and little β′-Sialon occurred, which showed that the reduction-nitridation reactions have proceeded at this time. After 2 h, AlN was formed. With a holding time of 4 h, MgAl2O4 and Ca2Al2SiO7 vanished entirely, the amount of AlN and β′-Sialon decreased continuously, and α′-Sialon was generated. The amount of α′-Sialon increased with an increase in the holding time and reached its maximum with a holding time of 6 h. Subsequently, α′-Sialon content decreased and AlN content increased slightly. BN was formed after 4 h, and its content did not changed nearly.
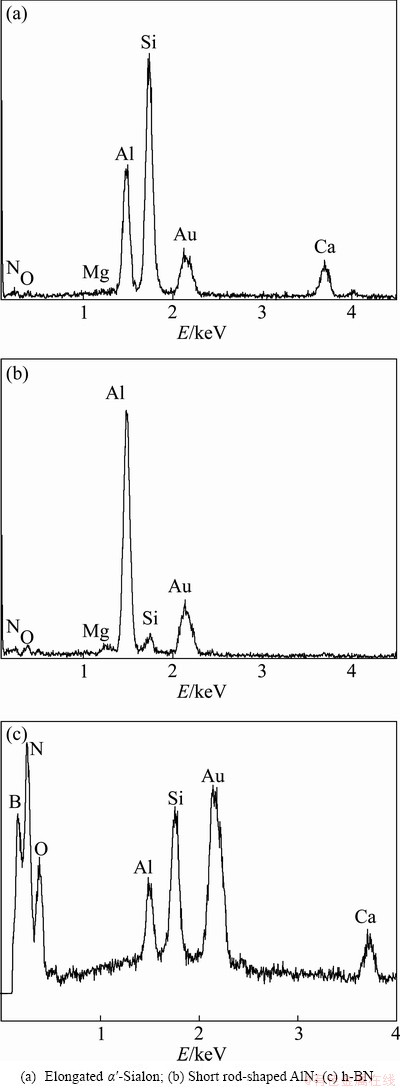
Fig. 4 EDS spectra of characteristic grains
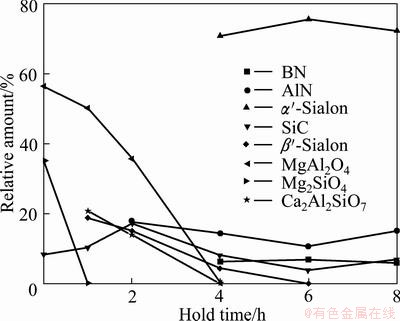
Fig. 5 Variation of phase compositions of reaction products with holding time at 1400 °C
3.3 Comparison between conventional and microwave heating
The XRD pattern of the (Ca,Mg)-α′-Sialon- AlN-BN powders that were synthesized by conventional heating method at 1480 °C for 8 h is shown in Fig. 6. As shown in Fig. 6, the synthesized products contain basically the same phase compositions by conventional and microwave heating, both of which were mainly composed of α′-Sialon, AlN, BN and a small amount of SiC.
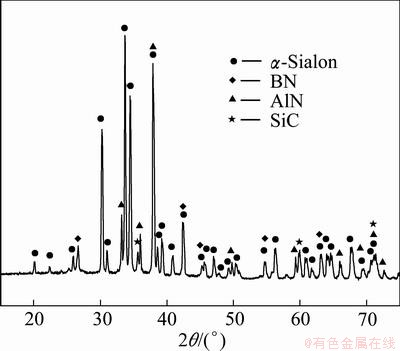
Fig. 6 XRD pattern of powder synthesized by resistance heating
Figure 7 shows SEM images of the powders synthesized by conventional heating method at 1480 °C for 8 h. As seen in Fig. 7, elongated and equiaxed α′-Sialon grains were observed in synthesized powders, but elongated α′-Sialon grains did not crystallized completely and the quantity and aspect ratio of the grains were less than microwave synthesize powders.
The optimal parameters for powders synthesized by microwave sintering and conventional sintering are shown in Fig. 8. As shown in Fig. 8, the reaction temperature of microwave heating method was reduced by 80 °C and the reaction time was shortened by 2 h. The results indicated that the microwave sintering method can obviously reduce energy consumptions.

Fig. 7 SEM image of powder synthesized by resistance heating
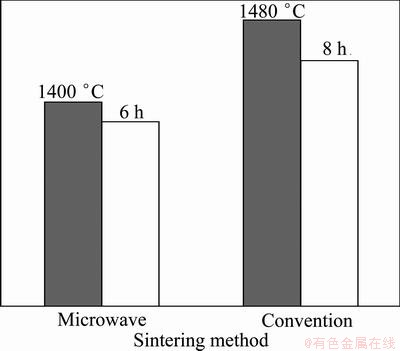
Fig. 8 Microwave sintering parameters vs conventional sintering parameters of powders
4 Conclusions
The synthesis of (Ca,Mg)-α’-Sialon-AlN-BN powders by the microwave CRN method using boron-rich blast furnace slag as a starting material was greatly affected by the process conditions. With an increase in the synthesis temperature or holding time, the CRN reactions were promoted, and the production of α’-Sialon increased. The optimal conditions for powder synthesis were a temperature of 1400 °C and a holding time of 6 h, under which the crystalline phases of the products included α’-Sialon, AlN, BN and a small amount of SiC. A large number of elongated α’-Sialon grains were produced at higher temperatures. AlN grains were in the form of short rods or had an irregular morphology. Some nano-sized h-BN grains were also observed in synthesized powders. The reaction temperature of microwave heating method was reduced by 80 °C and the reaction time was shortened by 2 h compared with conventional heating method, and more elongated α′-Sialon grains with large aspect ratio were observed.
References
[1] WIDEMAN T, SNEDDON G. Dipentylamine-modified polyborazylene: A new, melt-spinnable polymeric precursor to boron nitride ceramic fibers [J]. Chemical Materials, 1996, 8(1): 3-5.
[2] TOURY B, MIELE P, COMU D, VINCENT H, BOUIX J. Boron nitride fibers prepared from symmetric and asymmetric alkylaminoborazines [J]. Advanced Functional Materials, 2002, 12(3): 228-234.
[3] WOOD C A, ZHAO H, CHENG Y B. Microstructural development of calcium α′-Sialon ceramics with elongated grains [J]. Journal of American Ceramic Society, 1999, 82(2): 421-428.
[4] WANG P L, ZHANG C, SUN W Y, YAN D S. Characteristics of Ca-α′-Sialon—Phase formation, microstructure and mechanical properties [J]. Journal of the European Ceramic Society, 1999, 19(5): 553-560.
[5] van RUTTEN J W T, HINTZEN H T, METSELAAR R. Phase formation of Ca-α-Sialon by reaction sintering [J]. Journal of the European Ceramic Society, 1996, 16(9): 995-999.
[6] SHEN Z J, NYGREN M. On the extension of the α′-Sialon phase area in yttrium and rare-earth doped systems [J]. Journal of the European Ceramic Society, 1997, 17(13): 1639-1645.
[7] BARTEK A,  T, HERBERTSSON H, JOHANSSON T. Yttrium α′-Sialon ceramics by hot isostatic pressing and post-hot isostatic pressing [J]. Journal of American Ceramic Society, 1992, 75(2): 432-439.
T, HERBERTSSON H, JOHANSSON T. Yttrium α′-Sialon ceramics by hot isostatic pressing and post-hot isostatic pressing [J]. Journal of American Ceramic Society, 1992, 75(2): 432-439.
[8] ZHANG C, KOMEYA K, TATAMI J, MEGURO T, CHENG Y B. Synthesis of Mg-α-Sialon powders from talc and halloysite clay minerals [J]. Journal of the European Ceramic Society, 2000, 20(11): 1809-1814.
[9] ANTSIFEROV V N, GILEV V G, BEKKER V Y, FILIMONOVA I V. A study of Sialon synthesis from Kaolin by carbothermal reduction and simultaneous nitriding [J]. Refractories and Industrial Ceramics, 2000, 41(9-10): 338-344.
[10] SUVOROV S A, DOLGUSHEV I V, ZABOLOTSKII A V. Rapid synthesis of finely dispersed Sialon powder by carbothermal nitridation of Kaolin [J]. Refractories and Industrial Ceramics, 2002, 43(3-4): 113-116.
[11] PANDA P K, MARIAPPAN L, KANNAN T S. Carbothermal reduction of kaolinite under nitrogen atmosphere [J]. Ceramics International, 2000, 26(5): 455-461.
[12] GILEV V G. IR spectra and structure of Si-Al-O-N phase prepared by carbothermal reduction of Kaolin in nitriding atmosphere [J]. Inorganic Materials, 2001, 37(10): 1041-1045.
[13] HRABE Z, KOMARNENI S, MALLA P, SRIKANTH V, ROY R. Pillared montmorillonite clay as a raw material for the synthesis of β-Sialon [J]. Journal of Materials Science, 1992, 27(17): 4614-4618.
[14] CHEN W W, WANG P L, CHEN D Y, ZHANG B L , JIANG J X , CHENG Y B, YAN D S. Synthesis of (Ca, Mg)-α-Sialon from slag by self-propagating high-temperature synthesis [J]. Journal of Materials Chemistry, 2002, 12(4): 1199-1202.
[15] LIU Ke-ming, WANG Fu-ming, LI Wen-cao, XIE Li-jun, GUO Yu-yan. Synthesis of Ca-α-Sialon-SiC powders from blast furnace slag and optimization of its synthesis process [J]. Journal of University of Science and Technology Beijing, 2001, 23(5): 404-408. (in Chinese)
[16] JIANG T, XUE X X, DUAN P N, LIU X, ZHANG S H, LIU R. Carbothermal reduction–nitridation of titania-bearing blast furnace slag [J]. Ceramics International, 2008, 34(7): 1643-1651.
[17] FU Fang, JIA Xiao-lin, ZHANG Hai-jun. Synthesis of β-Sialon ultrafine powder by the sol-gel and microwave carbothermal reduction nitridation methods [J]. Journal of Chinese Ceramic Society, 2007, 35(3): 317-321. (in Chinese)
[18] XIAO Jin, ZHOU Feng, CHEN Yan-bin. Preparation of AlN powder by microwave carbonthermal reduction [J]. Journal of Inorganic Materials, 2007, 24(4): 755-758. (in Chinese)
[19] WANG Fu, WANG Qiang, CAO Wen-bin, SUN Jia-lin. Microwave synthesis of SiC powders with coke and quartzite [J]. Journal of Materials Engineering, 2009, 7(1): 32-35. (in Chinese)
[20] ROBBINS C R, MCMUEDIE H F, LEVIN E M. Phase diagrams for ceramists [M]. Columbus, OH: American Ceramic Society, 1964.
[21] HEWETT C L, CHENG Y B, MUDDLE B C. Phase relationships and related microstructural observations in the Ca-Si-Al-O-N system [J]. Journal of American Ceramic Society, 1998, 81(7): 1781-1788.
[22] YOON S J, JHA A. Vapour-phase reduction and the synthesis of boron-based ceramic phases, part II, the synthesis of hexagonal boron nitride phase [J]. Journal of Materials Science, 1996, 31(9): 2265-2277.
[23] ARIK H, SARITAS S,  M. Production of Si3N4 by carbothermal reduction and nitridation of sepiolite [J]. Journal of Materials Science, 1999, 34(4): 835-842.
M. Production of Si3N4 by carbothermal reduction and nitridation of sepiolite [J]. Journal of Materials Science, 1999, 34(4): 835-842.
[24] HUANG Zhen-qi, DAI Heng, LIU Hao-liang, YANG Zu-pan. Phenomenon of reduction and volatilization of MgO at high temperatures [J]. Journal of Northeastern University, 2002, 23 (4): 355-358. (in Chinese)
[25] LIU Ran, XUE Xiang-xin, JIANG Tao, WANG Xing-juan. Volatilization of MgO from ludwigite in carbothermal reduction process [J]. Journal of Northeastern University, 2007, 28 (2): 233-236. (in Chinese).
微波还原氮化富硼渣合成(Ca, Mg)-α′-Sialon-AlN-BN复合粉体
姜 涛1,2,3,薛向欣1,2,3
1. 东北大学 材料与冶金学院,沈阳 110819;
2. 东北大学 辽宁省冶金资源循环科学重点实验室,沈阳 110819;
3. 东北大学 辽宁省高校硼资源生态化综合利用技术与硼材料重点实验室,沈阳 110819
摘 要:以硼铁矿高炉铁硼分离后的产物富硼渣为主要原料,在微波场下采用碳热还原氮化法合成了(Ca,Mg)-α′-Sialon-AlN-BN复合粉体。利用X射线衍射、扫描电镜和能谱分析手段研究了合成温度和恒温时间对合成粉体相组成和显微形貌的影响,并与传统加热方式合成的粉体进行了对比。结果表明:在微波场下,合成温度和恒温时间对产物相组成影响显著。随着合成温度的升高或恒温时间的延长,产物中α′-Sialon相对含量逐渐增加,并在1400 °C、恒温6 h时成为产物主晶相。此时,产物中还有AlN、BN及少量β-SiC。合成粉体中α′-Sialon晶粒多呈长柱状,AlN晶粒呈短棒状,BN晶粒多为纳米级并彼此发生团聚。与传统加热方式的最佳合成条件相比,微波场下粉体的合成温度降低了80 °C,恒温时间缩短了2 h,产物中长柱状α′-Sialon晶粒数量明显增多,且具有更大的长径比。
关键词:富硼渣;碳热还原氮化;微波;(Ca,Mg)-α′-Sialon-AlN-BN;粉体
(Edited by YANG Hua)
Foundation item: Project (2006AA06Z368) supported by High-tech Research and Development Programs of China; Project (N100402007) supported by the Fundamental Research Funds for the Central Universities in China
Corresponding author: JIANG Tao; Tel: +86-13998229100; E-mail: jiangt@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)61560-4
Abstract: (Ca, Mg)-α′-Sialon-AlN-BN powders were synthesized by the carbothermal reduction and nitridation (CRN) method using boron-rich slag, one of the intermediate products from pyrometallurgy separation of pageit, as the staring material. The influences of synthesis temperature and holding time on the phase composition and microstructure during the microwave CRN were studied by XRD, SEM and EDS. The comparison between two heating techniques, conventional and microwave heating, on the synthesized powder was presented as well. The experimental results revealed that the phase compositions and microstructures of the synthesized products were greatly affected by the synthesis temperature and holding time. With an increase in the synthesis temperature or holding time, the relative amount of α′-Sialon increased and α′-Sialon became the main crystalline phase at 1400 °C for 6 h. The synthesized products also contained AlN, BN and a small amount of β-SiC. Elongated α′-Sialon grains, short rod AlN grains, aggregate nanoscale BN grains were observed in the synthesized powders. The reaction temperature of microwave heating method was reduced by 80 °C, the reaction time was shortened by 2 h, and more elongated α′-Sialon grains with large aspect ratio were observed.


