
Crystallization kinetic characteristics of oxygen-induced I-phase and Zr2Cu phase in Zr65Cu27.5Al7.5 glassy alloy
HE Lin(贺 林), HAN Zhen-hua(韩振华), JIANG Feng(江 峰), SUN Jun(孙 军)
State Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University, Xi’an 710049, China
Received 23 July 2007; accepted 13 November 2007
Abstract:Based on the construction of TTT diagrams by isothermal DSC measurements, the thermal stabilities of Zr65Cu27.5Al7.5 glassy alloys containing 0.68% and 0.06% [O] (molar fraction) were compared. The changing tendencies of the thermal stabilities reflected in the TTT-diagrams were validated by XRD analyses and TEM observations. The crystallization kinetic characteristics of oxygen-induced I-phase and Zr2Cu phase in Zr65Cu27.5Al7.5 glassy alloy were discussed. It is found that oxygen promotes the precipitation of I-phase and retards the formation of Zr2Cu phase. If the pre-crystallization event for oxygen-induced I-phase is permitted and the main-crystallization event for Zr2Cu phase is taken as the thermal stability criterion, the alloy with higher oxygen content has longer onset time for crystallization within a rather large supercooling temperature range. The possibility for the preparation of Zr65Cu27.5Al7.5 glassy alloy-based composite containing oxygen-induced I-phase was also forecasted.
Key words:
metallic glasses; thermodynamic properties; phase transition; oxygen impurity;
1 Introduction
In Zr-based bulk metallic glasses(BMGs), oxygen is an unavoidable impurity element, which can come from raw materials, fabricating atmosphere or crucible used because of the very strong affinity of Zr—O[1-2]. It has been well known that the glass forming ability(GFA) of Zr-based BMGs strongly depends on their oxygen contents[3-4]. Expensive raw materials with very low oxygen content need to be used for the alloying of Zr-based BMGs, which makes their fabrication very costly. However, there was evidence that a certain amount of oxygen was necessary for achieving ultrafine-grained microstructure upon crystallization of Zr-based BMG precursors[5]. The phenomenon was related with the formation of oxygen-induced phases, such as icosahedral quasicrystalline phase (I-phase). The preparation of as-crystallized Zr-based BMGs with ultrafine-grained microstructure was expected to be an effective way to improve their mechanical properties [6-7]. Compared with the I-phase induced by noble elements[7], oxygen-induced I-phase is more attractive for potential engineering applications.
Recently, powder consolidation by hot-pressing(HP) sintering process was reminded again for Zr-based BMGs[8]. For the HP sintering process, only glassy alloy powders with particle size usually less than 100 μm need to be prepared by appropriate atomization method[2]. As a precondition, the information about how oxygen affects the thermal stabilities of Zr-based glassy alloys needs to be obtained. Moreover, the allowance of oxygen contents in Zr-based BMGs may also provide an opportunity for the utilization of oxygen-induced phases, such as oxygen-induced I-phase, to improve the mechanical properties.
The influence of oxygen on the TTT diagram of Zr65Cu27.5Al7.5 alloy was presented in this work. The crystallization kinetic characteristics of oxygen-induced I-phase and bct-Zr2Cu phase in the glassy alloy were analyzed. Considered the powder consolidation by HP process, the possibility of utilizing oxygen-induced I-phase in the Zr-based glassy alloy was discussed.
2 Experimental
Zr65Cu27.5Al7.5 alloy was used as the base alloy in the current study. MURTY et al[9] reported the formation of oxygen-induced I-phase in the ternary alloy as its oxygen concentration was higher than the critical level of 0.4% (molar fraction). High purity crystal Zr containing about 0.05% [O] and sponge Zr with about 0.5% [O] (molar fraction) were used respectively for alloy preparation. Cu and Al used have purities of 99.99%(mass fraction). ZrO2 with high purity was employed to further increase the oxygen content in the alloy made of sponge Zr. Master alloys were completely melted more than four times under a Ti-gettered argon atmosphere, and melts were electromagnetically stirred during the melting process to make oxygen in the melts distribute homogeneously. Oxygen concentration was measured by hot extraction using a LECO RO-416D2 analyzer. The master alloy made of high purity Zr containing 0.06% [O] (molar fraction) was designated as Alloy L; the master alloy made of sponge Zr and stoichiometric ZrO2 addition containing 0.68% [O] (molar fraction) was designated as Alloy UH. From the alloy ingots, ribbons with a cross section of about 3.5 mm×0.05 mm were prepared by a single-roller melt-spinning method (rotation speed 26.2 m/s) in an Ar atmosphere.
The original microstructures of two melt-spun alloys were checked by X-ray diffractometry(XRD) using a D/MAX 2400 diffractometer (Cu Ka) and by transmission electron microscopy(TEM) with JEOL JEM-200CX microscope. The thermal properties and crystallization behavior were examined by differential scanning calorimetry(DSC) using a SETARAM LabsysTM TG DSC calorimeter in an argon atmosphere. For continuously-heating mode, the heating rate of 0.167 K/s was chosen. For isothermal mode, samples were rapidly heated to the designed temperature at a constant heating rate of 0.67 K/s. The TTT-diagrams of two alloys were constructed by the isothermal DSC measurements. In order to check the constructed TTT-diagrams and investigate the primary phases obtained, melt-spun alloys were sealed in quarts tubes under vacuum (base pressure 10-4 Pa) and put into a pre-heated tube furnace for isothermal annealing treatments. The temperature fluctuation during annealing treatments was less than 0.5 K. Changes in the microstructures of the melt-spun glassy alloys after isothermal annealing were examined by XRD analyses and TEM observations.
3 Results
The XRD patterns of two ribbons, L and UH, were all characterized by broad maxima characteristic for amorphous materials (Fig.1(a)). The broad diffraction maxima moved towards lower diffraction angle with increasing oxygen content, which might correspond to the increases of local inter-atomic distances caused by the addition of oxygen atoms[10]. HAJLAOUI et al[11] found that the ultrafine-grained crystals with the dimension of several nanometers in an amorphous matrix could not be detected by X-ray diffractometry. The microstructures of two ribbons were further examined by TEM observation. Only featureless contrast was observed over the entire bright-field SEM image (Fig.1(b)) and the corresponding SED pattern (inserted in Fig.1(b)) consisted only halo rings with no sharp diffraction rings or spotty diffraction patterns. Thus, the ribbons were not only ‘X-ray amorphous’ but also ‘electron-diffraction amorphous’.
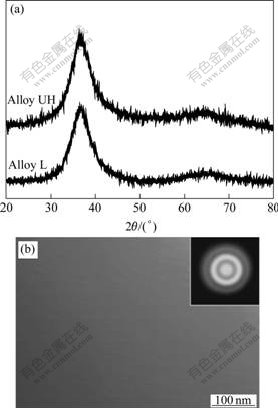
Fig.1 XRD patterns of as-spun Alloy L and UH ribbons (a), and bright-field TEM image of Alloy UH and corresponding SED pattern (inserted) (b)
Fig.2 shows the continuously-heating DSC curves of two ribbons at the heating rate of 0.167 K/s. It can be seen that Alloy L crystallized in a single-stage with an exothermic peak at 725 K and a large supercooled liquid regime (ΔTX=84 K), which is comparable to that reported by MURTY et al[12] for the same alloy with 0.14%[O] at the same heating rate. For Alloy UH, a pre-crystallization peak at 708 K appeared before the main-crystallization event at 728 K, and oxygen obviously reduced the onset temperature for crystallization, TX, and raised Tg, which made the ΔTX value decrease from 84 K to 59 K. It is worth to note that the temperature for the main-crystallization peak, Tmp, of Alloy UH was higher than that of Alloy L. Oxygen did not accelerate the crystallization event corresponding to the main-crystallization peak.
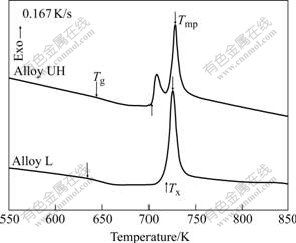
Fig.2 Continuously-heating DSC curves of alloys with different oxygen contents at 0.167 K/s
In order to get the ‘panoramic effects’ of oxygen on the thermal stability of Zr65Cu27.5Al7.5 alloy, TTT- diagrams of two alloys were constructed by isothermal DSC measurements. The isothermal DSC curves of the two alloys at 698 K and 678 K are shown in Fig.3. Similar to the continuously-heating DSC curves, the isothermal DSC curves of Alloy L kept its single crystallization mode and the isothermal DSC curves of Alloy UH maintained its double-stage crystallization characteristic. From the isothermal DSC curves, the onset time, tonset, for pre-crystallization or main- crystallization event corresponding to each isothermal annealing temperature could be determined according to the method used by NAGASE and UMAKOSHI[13]. The tonset can be taken as a parameter for characterizing the isothermal stability of glassy alloy at a given temperature. It can be seen from Fig.3 that the relative isothermal stability between Alloy L and Alloy UH depends on the isothermal annealing temperature used.
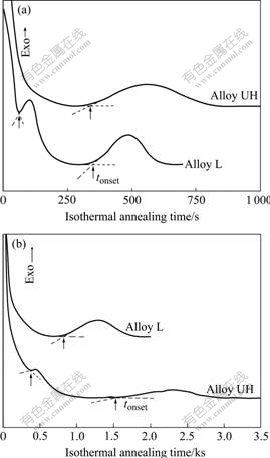
Fig.3 Isothermal DSC curves of alloys with different oxygen contents at 698 K (a) and 678 K (b)
The tonset was too long at the temperature below 658 K and too short at the temperature above 698 K. In order to ensure the measurement accuracy, isothermal DSC measurements were carried out within the temperature range of 658-698 K. The relationship between the onset time, tonset, and isothermal annealing temperature, T, in the temperature range below the nose, Tn, of TTT-diagrams can be expressed by the following equation[13]:
![]() (1)
(1)
where t0 and E are the time constant and the activation energy for isothermal crystallization respectively; R is the gas constant. In Eqn.(1), t0 and E can be estimated by fitting the experimentally observed onset time, tonset, at the isothermal annealing temperature, T, to the following relation:
![]() (2)
(2)
Fig.4(a) shows the plots of ln(tonset) and 1/T for the two alloys. The values of t0 and E estimated from the slopes and intercepts of fitted straight lines are listed in Table 1. It is interesting to note that although the time constants for isothermal pre- and main-crystallization of Alloy UH were much smaller than those of Alloy L, its corresponding activation energies for isothermal pre- and main-crystallization were much higher. The enhanced tendencies for the activation energy of Zr-based BMGs by oxygen were reported elsewhere[3,12,14]. By applying t0 and E into Eqn.(1), the partial TTT-diagrams of two alloys for pre- and main-crystallization onsets are shown in Fig.4(b). If the pre-crystallization event was taken as thermal stability criterion, it can be clearly seen from Fig.4(b) that there was a critical temperature TC(≈ 655 K, near to the Tg of Alloy UH). Although Alloy L had longer onset time in the temperature range above TC, Alloy UH had longer onset time at the temperature range below TC. Moreover, if the main-crystallization event was taken as thermal stability criterion, Alloy UH had longer onset time within a rather large supercooled temperature range. Phase precipitation corresponding to the main-crystallization peak of Zr65Cu27.5Al7.5 alloy was retarded by oxygen within the large temperature range.
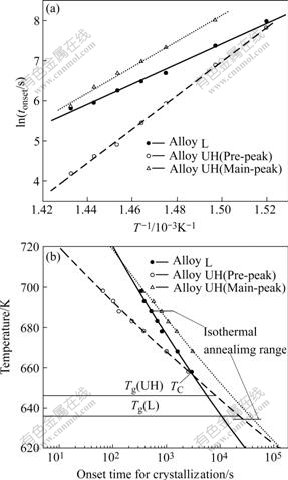
Fig.4 Plots of ln(tonset) and 1/T (a) and partial TTT-diagrams (b) for crystallization onset of alloys containing different oxygen contents constructed by isothermal DSC measurements
Table 1 Time constants and activation energies for isothermal crystallization of two alloys
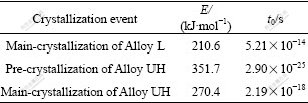
To examine the reason for the changed thermal stability of Zr65Cu27.5Al7.5 alloy within different temperature ranges, the primary phases for the Alloy L and Alloy UH were checked at 683 K (above TC), and 633 K (below TC), respectively. The isothermal annealing time ranges were chosen according to the constructed partial TTT- diagrams in Fig.4(b) (indicated by thick bar). XRD patterns corresponding to each annealing condition are shown in Fig.5. It can be seen from Fig.5 that only two phases were identified for all the annealing conditions: I-phase and stable bct-Zr2Cu phase. For the annealing at 683 K (Fig.5(a)), I-phase precipitated as primary phase in Alloy UH after 0.3 ks annealing, and the phase could persist up to 1.1 ks. However, bct-Zr2Cu formed as primary phase in Alloy L after 0.7 ks annealing, and the phase largely formed (high XRD intensity) only after much longer time annealing. It is clear that the formation of I-phase at higher temperature in Alloy UH decreased the thermal stability, nevertheless, the precipitation of bct-Zr2Cu phase in the alloy was not accelerated by oxygen at higher temperature. For the annealing at 633 K (Fig.5(b)), I-phase also precipitated as primary phase in Alloy UH after 56 ks annealing; bct-Zr2Cu formed as primary phase in Alloy L after only 24 ks annealing; and the phase largely formed (high XRD intensity) after much shorter time annealing. It was also clear that the formation of I-phase in Alloy UH at lower temperature did not decrease the thermal stability of the alloy. On the contrary, Alloy UH had much higher thermal stability at lower temperature. All the results mentioned above validated the changing tendencies of the thermal stability of Zr65Cu27.5Al2.5 alloy reflected by the partial TTT- diagrams in Fig.4(b).
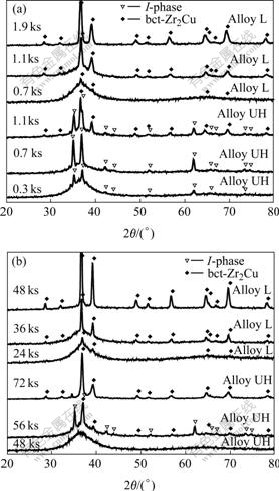
Fig. 5 Change in XRD patterns for alloys with different oxygen contents after isothermal annealing at 683 K (a) and 633 K (b) for corresponding time ranges indicated in Fig.3(a)
4 Discussion
4.1 Controlling factor for oxygen-induced I-phase precipitation in Zr65Cu27.5Al7.5 glassy alloy
It was proposed that oxygen was a necessary constituent for the precipitation of I-phase during crystallization in the Zr-Cu-Al and Zr-Cu-Ni-Al alloys without noble elements[5,9]. Oxygen-induced I-phase precipitated as the oxygen concentration in both quaternary and ternary Zr-based alloys approached a critical amount of about 0.4%(molar fraction)[15]. The formation of oxygen-induced I-phase was connected with three conditions: the quenched-in icosahedral short-range order existing in supercooled glassy alloys[16], the smaller interfacial energy between icosahedral structure and glassy matrixes[5], and the decrease of Gibbs free energy of I-phase after oxygen addition[12,15]. The former two conditions are the intrinsic properties of glassy alloys and icosahedral structure; however, the third condition depends on the oxygen level in I-phase. It was found that the oxygen level in the precipitated I-phase was much higher than the critical value. MURTY et al[12] investigated the oxygen distribution in Zr65Cu27.5Al7.5 alloy with 0.82% (molar fraction) oxygen after primary crystallization by 3DAP analysis. It was reported that the oxygen concentration in the I-phase approached 5%, six times higher than the nominal oxygen content of the alloy. Similar phenomenon was also reported even in a Zr-Cu-Al-Pd system. CHEN et al[1] researched the oxygen distribution in Zr65Cu15Al10Pd10 alloy with 0.1% (molar fraction) oxygen after the precipitation of palladium-induced I-phase by 3DAP analysis. It was also found that although the oxygen distribution in the as-quenched glassy alloy was uniform, oxygen concentration in the precipitated I-phase approached 4%, forty times higher than the nominal oxygen content of the alloy. The large increase of oxygen in I-phase demonstrates that much higher oxygen level is needed to make the crystallization driving force for a glass to I-phase transformation larger than that for a glass to a stable phase transformation.
Based on the fact mentioned above, it was proposed that oxygen redistribution in glassy alloys should occur before I-phase nucleation[1]. The first step for I-phase precipitation was the formation of oxygen-enriched regions by an oxygen redistribution process, and oxygen diffusion kinetics was the controlling factor for oxygen-induced I-phase precipitation in Zr65Cu27.5Al7.5 glassy alloy. In the higher temperature range above TC, oxygen diffused fast because of the low viscosity of supercooled liquid. Oxygen-enriched regions suitable for I-phase precipitation could easily form in the alloy with higher oxygen, thus I-phase precipitated after a shorter time isothermal annealing. However, oxygen diffusion could be very slow at the lower temperature range near Tg because of the increased viscosity of supercooled liquid, and the delay for forming oxygen-enriched regions retarded the nucleation and growth of I-phase. The easy precipitation of oxygen-induced I-phase at the higher temperature range above TC caused the thermal stability of Zr65Cu27.5Al7.5 glassy alloy at the temperature range to decrease, which has been well accepted. On the other hand, the alloy was also endowed with enhanced thermal stability at the lower temperature range near Tg by oxygen. The very high activation energy for I-phase precipitation onset possessed by the Alloy UH should be the reflection of above micro-mechanism.
From the I-phase density shown in Fig.6, it can be seen that the nucleation rate of I-phase was larger at the higher temperature above TC than that at the lower temperature near Tg, which is supposed to be the reflection of oxygen diffusion controlled kinetics for I-phase precipitation.
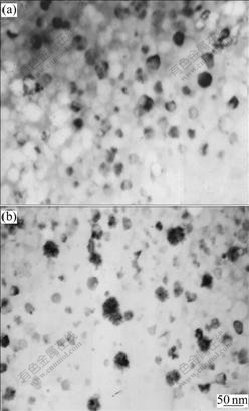
Fig.6 Bright-field TEM images of Alloy UH after isothermal annealing at 683 K for 0.7 ks (a) and at 633 K for 56 ks (b)
4.2 Retarding effect of oxygen on bct-Zr2Cu phase precipitation in Zr65Cu27.5Al7.5 glassy alloy
Another result from the partial TTT-diagrams for the crystallization onset of Alloy L and Alloy UH shown in Fig.4(b) was that bct-Zr2Cu phase precipitation corresponding to the main-crystallization peak was retarded by the addition of oxygen within a rather large supercooled temperature range. That is to say, when taking the bct-Zr2Cu precipitation event as thermal stability criterion, oxygen enhanced the thermal stability of the alloy. This reflects the different crystallization kinetic characteristics of oxygen-induced I-phase and Zr2Cu phase.
In a raw material of α-Zr (A3 structure) with certain oxygen content, oxygen atoms are distributed at the centers of the octahedral configurations consisting of six Zr atoms. ALTOUNIAN et al[17] proposed that oxygen and zirconium could keep a semi-permanent bond even in a liquid state because of the very strong binding between Zr and O, that is to say, they could keep the Zr—O octahedral configurations with an O atom surrounded by six Zr atoms even in a liquid Zr-based alloy. When the liquid was quenched, these atomic clusters could be quenched into the glass. It has been proposed that the Zr—O octahedral configurations in a Zr-based alloy could promote the precipitation of such phase with the structural comparability of Zr—O octahedral configuration in its unit cell. For example, ‘big-cube’ fcc-Zr2Ni crystalline phase, which was first reported by ALTOUNIAN et al[17] in the Zr-Ni metallic glass containing oxygen impurity, has a E93 structure, space group fd3m (227) with a=1.227 nm and 96 atoms (64 Zr and 32 Ni) per unit cell. Because there are four octahedral configurations formed by six Zr atoms in each fcc-Zr2Ni unit cell, the easy precipitation of fcc-Zr2Ni phase has been related with its heterogenous nucleation triggered by the Zr—O atomic clusters in the Ni-contained Zr-based alloy. However, bct-Zr2Cu phase has a C11b structure, space group i4/mmm (139) with a=0.322 nm, c=1.118 nm and 6 atoms (4 Zr and 2 Cu) per unit cell [18]. There is at least one Cu atom at the corner angles of atomic octahedral configurations in each bct-Zr2Cu unit cell. If the Zr—O octahedral configurations in the present Ni-free glassy alloy acted as the seeds for the precipitation of Zr2Cu, at least one of the strong Zr—O bonds should be destroyed and substituted by weak Cu—O bond, which needs more energy. Thus, Zr—O atomic clusters in Zr65Cu27.5Al7.5 glassy alloy could not preferentially promote the nucleation of Zr2Cu. In addition, LIU et al[19] found that oxygen was rejected out from Zr2Cu phase when crystallization took place in the glassy matrix of Zr50Cu50 alloy, so it can be proposed that Zr2Cu is a phase with very limited oxygen solubility. Consequently, oxygen could act as a nucleation and growth barrier for the precipitation of Zr2Cu in Zr65Cu27.5Al7.5 glassy alloy. In this case, oxygen-stabilized glass could be obtained and its thermal stability was enhanced. Higher activation energy for Zr2Cu phase precipitation onset in Alloy UH should also be the reflection of above micro-mechanism. The retarding effect of oxygen on Zr2Cu precipitation provided the opportunity for oxygen-induced I-phase to exist for longer time.
By comparing the density of the Zr2Cu phase precipitated in Alloy L shown in Fig.7(a) with that of the oxygen-induced I-phase formed in Alloy UH (in Fig.6), it is clear that coarse-grained microstructure was the intrinsic characteristic of Zr2Cu phase. It could be also seen from Fig.7(b) that the Zr2Cu phase precipitated after I-phase formation in Alloy UH had smaller nucleation rate. This should be the evidence that the precipitation of Zr2Cu phase corresponding to the main-crystallization peak of Zr65Cu27.5Al7.5 alloy was retarded by oxygen. In order to achieve ultrafine-grained microstructure upon crystallization and improve the mechanical properties of the glassy alloy, the precipitation of Zr2Cu phase should be avoided.
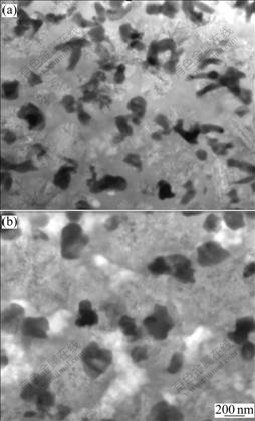
Fig.7 Bright-field TEM images of Alloy L after isothermal annealing at 633 K for 36 ks (a) and Alloy UH after isothermal annealing at 633 K for 68 ks (b)
4.3 Possible utilization of oxygen-induced I-phase in Zr65Cu27.5Al7.5 glassy alloy
According to the crystallization characteristic of the Zr65Cu27.5Al7.5 glassy alloy containing 0.68%[O], there is a possibility to prepare in-situ Zr-based BMG composite containing oxygen-induced I-phase. First, glassy alloy powders need to be prepared by appropriate atomization method. In this situation, high GFA of the alloy is not necessary because of the small size of particles, and relatively cheap raw materials with higher oxygen such as sponge Zr can be used, which will increase the commercial attractiveness of the alloy for industrial applications. Secondly, the alloy powders with higher oxygen content need to be consolidated by HP processing. Because oxygen-induced I-phase is a desired phase and only Zr2Cu phase needs to be avoided in microstructure, the adoptable alloy powders containing higher oxygen will have longer onset time for crystallization, which may provide longer time and higher temperature for the powders to be consolidated. Moreover, the oxygen-induced I-phase will precipitate during the HP processing. Bulk shape and suitable microstructure may be simultaneously obtained if sintering parameters based on the TTT-diagram are properly controlled. The bulk samples prepared can also provide an opportunity for investigating the effect of oxygen-induced I-phase on the mechanical properties of the glass. Of course, the HP processing technology for the high oxygen-contained Zr65Cu27.5Al7.5 glassy alloy needs to be further studied.
5 Conclusions
1) The ‘panoramic effects’ of oxygen impurity on the thermal stability of Zr65Cu27.5Al7.5 glassy alloy are the reflection of different crystallization kinetic characteristics of oxygen-induced I-phase and Zr2Cu phase in the alloy.
2) When taking the pre-crystallization event for oxygen-induced I-phase as the thermal stability criterion of Zr65Cu27.5Al7.5 glassy alloy, the alloy containing lower oxygen has higher thermal stability above 655 K. However, the alloy containing higher oxygen has longer onset time near Tg. Oxygen can enhance the thermal stability of the alloy near Tg.
3) When taking the main-crystallization event for bct-Zr2Cu phase as thermal stability criterion of Zr65Cu27.5Al7.5 glassy alloy, the alloy with higher oxygen content has longer onset time within a rather large supercooled temperature range. The retarding effect of oxygen on bct-Zr2Cu phase may provide a possibility to prepare in-situ Zr65Cu27.5Al7.5 glassy alloy-based composite containing oxygen-induced I-phase via powder consolidation by HP sintering process.
References
[1] CHEN M W, INOUE A, SAKURAI T, PING D H, HONO K. Impurity oxygen redistribution in a nanocrystallized Zr65Cr15Al10Pd10 metallic glass [J]. Appl Phys Lett, 1999, 74(6): 812-814.
[2] SIEGRIST M E, SIEGFRIED M, L?FFLER J F. High-purity amorphous Zr52.5Cu17.9Ni14.6Al10Ti5 powders via mechanical amorphization of crystalline pre-alloys [J]. Mater Sci Eng A, 2006, 418(1/2): 236-240.
[3] SUN J. Effect of microalloying on glass-forming ability and crystallization kinetics of Zr52.5Cu17.9Ni14.6Al10Ti5 alloy [J]. Scripta Mater, 2006, 54(6): 1081-1085.
[4] HE Lin, SUN Jun. Effect of oxygen on the thermal stability of Zr-Cu-Ni-Al-Ti bulk amorphous alloy [J]. Acta Metallurgica Sinica, 2006, 42(2): 134-138 (in Chinese).
[5] ECKERT J, MATTERN N, ZINKEVITCH M, SEIDEL M. Crystallization behavior and phase formation in Zr-Al-Cu-Ni metallic glass containing oxygen [J]. Mater Trans JIM, 1998, 39(6): 623-632.
[6] INOUE A, ZHANG T, SAIDA J, MATSUHITA M, CHEN M W, SAKURAL T. High strength and good ductility of bulk quasicrystalline base alloys in Zr65Al7.5Ni10Cu17.5-xPdx system [J]. Mater Trans JIM, 1999, 40(10): 1137-1143.
[7] HAJLAOUI K, YAVARI A R, DAS J, VAUGHAN G. Ductilization of BMGs by optimization of nanopartical dispersion [J]. J Alloys Compd, 2007, 434/435: 6-9.
[8] XIE G Q, ZHANG W, LOUZGUINE-LUZGIN D V, KIMURA H, INOUE A. Fabrication of porous Zr-Cu-Al-Ni bulk metallic glass by spark plasma sintering process [J]. Scripta Mater, 2006, 55(8): 687-690.
[9] MURTY B S, PING D H, HONO K, INOUE A. Direct evidence for oxygen stabilization of icosahedral phase during crystallization of Zr65Cu27.5Al7.5 metallic glass [J]. Appl Phys Lett, 2000, 76(1): 55-57.
[10] SCUDINO S, ECKERT J, YANG X Y, SORDELET D J, SCHULTZ L. Conditions for quasicrystal formation from mechanically alloyed Zr-based glassy powders [J]. Intermetallics, 2007, 15(4): 571-582.
[11] HAJLAOUI K, YAVARI A R, DOISNEAU B, LEMOULEC A, BOTTA W J, VAUGHAN F G, GREER A L, INOUE A, ZHANG W, KVICH ?. Shear delocalization and crack blunting of a metallic glass containing nanoparticles: In situ deformation in TEM analysis [J]. Scripta Mater, 2006, 54(11): 1829-1834.
[12] MURTY B S, PING D H, HONO K, INOUE A. Influence of oxygen on the crystallization behavior of Zr65Cu27.5Al7.5 and Zr66.7Cu33.3 metallic glasses [J]. Acta Mater, 2000, 48(15): 3985-3996.
[13] NAGASE T, UMAKOSHI Y. Electron irradiation induced crystallization of the amorphous phase in Zr65.5Al7.5Ni10.0Cu17.5 metallic glass [J]. Sci Tech Advanced Mater, 2004, 5(1/2): 57-67.
[14] HE Lin, ZHANG Shuai, SUN Jun, ZHANG Chang-jun. Effect of oxygen impurity on the long-term thermal stability of Zr-based metallic glasses below glass transition temperature [J]. Trans Nonferrous Met Soc China, 2006, 16(5): 992-997.
[15] MURTY B S, HONO K. Nanoquasicrystallization of Zr-based metallic glasses [J]. Mater Sci Eng, 2001, A312(1/2): 253-261.
[16] QIANG J B, ZHANG W, INOUE A. Formation of glassy and icosahedral phases in as-cast (Zr9Ni4)75(Al1-xTix)25 alloys [J]. Scripta Mater, 2006, 55(7): 617-620.
[17] ALTOUNIAN Z, BATALLA E, STROM-OLSEN J O, WALTER J L. The influence of oxygen and other impurities on the crystallization of NiZr2 and related metallic glasses [J]. J Appl Phys, 1987, 61(1): 149-155.
[18] HE L, WU Z G, JIANG F, SUN J. Enhanced thermal stability of Zr65Cu17.5Ni10Al7.5 metallic glass at temperature range near glass transition by oxygen impurity [J]. J Alloys Compd. 2008, 456(1): 181-186.
[19] LIU Z Y, HRILJAC J A, CHANG I T H, JONES I P, HARRIS I R. Crystallisation of oxygen-stabilised amorphous phase in a Zr50Cu50 alloy [J]. Intermetallics, 2001, 9(12): 1029-1036.
(Edited by YANG Bing)


