
Preparation and property evaluation of electroless Ni-P coatings on AZ91D magnesium alloy
LI Xue-song(李雪松)1, 2, ZHANG Wen-xue(张文雪)1, JIANG Zhong-hao(江中浩)1
1. Key Laboratory of Automobile Materials, Department of Materials Science and Engineering,Jilin University, Changchun 130025, China;
2. College of Materials Science and Engineering, Changchun University of Technology, Changchun 130012, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Taking a bath with basic nickel carbonate as the source of nickel, sodium hypophosphite as the reducing agent and citric acid as the complexing agent, the Ni-P coatings on AZ91D magnesium alloy were prepared. The influence of pH value of the bath on the content of phosphorus, microhardness and corrosion resistance of the deposits was examined. The XRD analysis results show that the Ni-P coatings plated under all conditions have a mixed microstructure of amorphous and nanocrystalline and undergo a phase transformation to crystalline nickel and nickel phosphide upon heat-treatment. The microhardness of the Ni-P coatings increases with the increase of pH value of the bath and has a maximum at pH=6. The potentiodynamic polarization test in 3.5%(mass fraction) NaCl solution reveals that the Ni-P coatings exhibit a very good corrosion resistance to protecting AZ91D magnesium alloy.
Key words:
Ni-P coating; AZ91D magnesium alloy; preparation; property evaluation;
1 Introduction
Magnesium and its alloys play an important role in many fields, such as aerospace, electronics and automobile industries owing to their unique characteristics of higher specific strength and good damping capacity. However, the application of magnesium alloys has been limited due to their undesirable properties, mainly including poor corrosion and wear resistance. The corrosion of magnesium alloys depends on their metallurgy and environmental factors. To improve the practical usage of magnesium alloys, many researchers have attempted to develop anticorrosive and high wear-resistance strategies [1-8].
Among various surface techniques, electroless deposition is an important chemical deposition technology, which involves the deposition of metals from solution onto surfaces without using an external electric voltage[9] and is thought to be the simplest and most economic method. Another advantage of the electroless deposition technique is that high-quality deposits can be obtained without special requirements for substrate geometries and can be formed on either conductive or nonconductive parts. Among different plating metals, electroless-plated nickel exhibits more popularity due to its excellent properties such as high hardness, wear and corrosion resistance and has attracted extensive interests[10-14].
However, the electroless plating on magnesium alloys has many challenges in the processing of plating and there is limited reports on magnesium alloys [1, 15-18]. The magnesium alloy is extremely susceptible to galvanic corrosion that pits severely on metal surface and hence results in unattractive appearance as well as degraded mechanical properties. In this study, the electroless Ni-P deposits on the AZ91D magnesium alloy were prepared from a plating bath containing basic carbonate nickel after the alloy was pickled in an acid solution of chromium oxide and then was activated in a HF solution to form MgF2 film. The structure, morphology, corrosion characteristics and microhardness of the deposits were characterized by different methods.
2 Experimental
The substrate was AZ91D die cast magnesium alloy with a size of 30 mm×30 mm×3 mm, which was ground with 2000# SiC paper. The chemical composition of the alloy is listed in Table 1. The electroless plating bath was taken in a 1 L glass beaker and was kept at constant temperature in a thermostat. The bath composition and all operation parameters for the pretreatment and electroless Ni-P deposition are listed in Table 2.
The surface morphologies of the Ni-P coatings were observed by SEM (JSM-5310, Japan Electronics) and qualitative elemental analysis was performed using an attached EDS (INC250). The structures of the Ni-P coatings were studied by X-ray diffractometer (XRD, Rigaku Dymax, Japan) with a Cu Kα radiation (λ= 0.154 178 nm) and a monochromator at 50 kV and 300 mA with the scanning rate and step of 4?/min and 0.02?, respectively. The harnesses of the magnesium alloy and the Ni-P coatings were evaluated by a HXD-1000 microhardness tester with Vickers indenter employing a load of 2 N for 15 s. The corrosion resistance of the Ni-P coatings was measured by the electrochemical methods. The polarization curves of the Ni-P coatings were performed on an Electrochemical Analyzer (LANLIKE, Tianjin, China) by Linear Sweep Voltammetry technique at room temperature in a 3.5%(mass fraction) NaCl aqueous solution using a classic three-electrode cell. The working electrode was cleaned in acetone and agitated ultrasonically for 10 min before testing. The coated samples were masked with epoxy resin (EP 651) so that only 1 cm2 was exposed to the electrolyte. The samples were also degreased with acetone, and rinsed in deionized water before electrochemical test. Before the dynamic potential sweep experiment, the samples were immersed into electrolyte for about 10 min to stabilize the open-circuit potential (OCP) φ0. The scanning rate was 50 Mv/min for all measurements. The Tafel plots were transformed from the recorded data and the corrosion current density (Jcorr) was determined by extrapolating the straight-line section of the anodic and cathodic Tafel plots.
3 Results and discussion3.1 Functions of individual treatments
Since magnesium is one of the most electrochemically active metals, when it contacts with air or water, an oxide and hydroxide layer forms quickly on the surface [3], which has a detrimental effect on coating adhesion and uniformity. The quasi-passive film on magnesium is much less stable than the usual passive film formed on metals such as aluminum and stainless steels and provides only poor pitting resistance for magnesium. Therefore, a suitable pretreatment is necessary to insure that the deposition rate of metal ions is much higher than the corrosion rate of magnesium during the successive electroless deposition [18]. Alkaline cleaning was used to remove soils or greases on the surface of the magnesium alloy and the samples were then rinsed thoroughly in deionized water to remove all alkali. But such cleaning processes were not suitable for removing oxide [19]. So, when alkaline was cleaned the samples should be etched or pickled in a chromic
Table 1 Composition of AZ91D magnesium alloy(mass fraction, %)
![]()
Table 2 Composition and operating conditions of Ni-P plating bath(Samples are cleaned thoroughly with deionized water as quickly as possible between any two steps of treatments)
acid/nitric acid solution to remove any oxide layer or other chemical coatings that were not fully removed by alkaline cleaning. The AZ91D magnesium alloy consists of the primary α-Mg grains surrounded by a eutectic mixture of α and β-Mg17Al12 [1]. There is an internal galvanic corrosion caused by the second phases or impurities. The β-phase precipitates along grain boundaries, which exhibits higher cathodic reaction activity and lower corrosion current density than α-Mg [20]. So the next fluoride activation treatment is to create an equipotentialized film (MgF2) on the alloy surface. The pretreatment is an indispensable step in the process of electroless plating on the AZ91D magnesium alloys. According to Ref.[15], the time of fluoride activation should not be less than 10 min so as to form the completely MgF2 film on the alloy surface.
During the process of the electroless Ni-P plating, the role of basic carbonate nickel is to provide nickel ions. The sodium hypophosphite acts as a metal-reducing agent, and the citric acid as complexing agent of the nickel ions, and thiourea as solution stabilizer. The ammonia solution is used to adjust pH value of the plating bath. The fluoride in the plating bath is to inhibit the corrosion of the substrate during plating.
3.2 Composition and morphologies of coatings
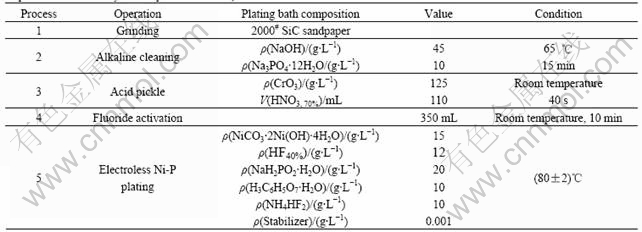
Fig.1(a) shows the XRD patterns of the AZ91D magnesium alloy. The structure of the substrate after the pretreatments is illustrated in Fig.1(b), which indicates that the substrate alloy consists of the primary α-Mg grains surrounded by a eutectic mixture of α and β-Mg17Al12 [18]. The emerging peak at about 36? signifies that the β-Mg17Al12 phase becomes obvious on the substrate surface after the pretreatments (Fig.1(b)). After the electroless Ni-P is plated, the substrate is fully covered by the electroless Ni-P alloy. The XRD patterns reveal that the electroless Ni-P coatings plated at different pH values are a mixed microstructure of
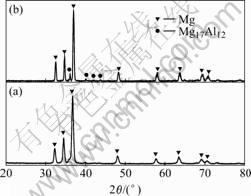
Fig.1 XRD patterns of electroless Ni-P deposition on AZ91D magnesium alloy (a) and AZ91D magnesium alloy substrate after pretreatment (b)
amorphous and nanocrystalline (omitted). The XRD patterns of the Ni-P alloy deposited in the bath of pH=6, on the as-plated and heat-treated conditions are shown in Fig.2, respectively. It can be seen that the Ni-P alloys undergo a phase transformation to crystalline nickel and nickel phosphide upon the heat-treatment.
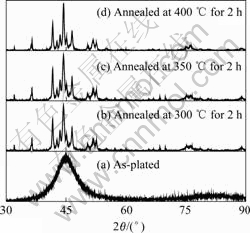
Fig.2 Electroless Ni-P alloys deposited in bath with pH=6 for 1 h under as-plated and heat-treated condition
The microstructures of the Ni-P coatings obtained from the bath with different pH values in Fig.3 are of the typical spherical nodular structure. It can be seen that when the samples were plated in the bath with pH=5, the substrate was much more corroded, which provides more chemically active spots on the substrate surface for the electroless platings. With the increase the pH value, the cluster of the Ni-P coatings becomes smaller and the Ni-P deposits are found to be more compact. The influence of the bath pH value on the chemical composition of the Ni-P coatings is presented in Table 3. As the pH value increases, the content of phosphorous decreases from 7.32% to 4.87%(mass fraction) according to the analysis by EDS. This result indicates that the structure of the as-deposited Ni-P coatings is a mixture of amorphous and nanocrystalline nickel [21].
Table 3 Composition of four coatings deposited in electroless baths with different pH values(mass fraction, %)

The microhardness HV of the Ni-P coating is shown in Fig.4, which is far higher than that of the AZ91D magnesium alloy substrate (about 100). When the bath pH value equals 6, the microhardness of the Ni-P coating reaches a maximum value of about 635.
Fig.3 Surface morphologies of electroless Ni-P coatings deposited in bath: (a) pH=5; pH=6; (c) pH=7; (d) pH=8
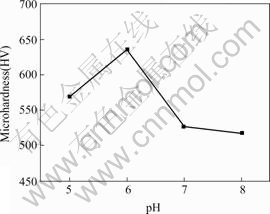
Fig.4 Dependence of microhardness of as-plated alloys on pH values
3.3 Corrosion characteristics of coatings
Magnesium alloy substrate is prone to galvanic corrosion because most other metals have a nobler electrochemical potential [3]. The nickel/Mg system is a classical example of cathodic coating on an anodic substrate. Therefore, the coating that only provides a physical barrier against the corrosion attack of magnesium alloy must be uniform, adherent and pore free. Otherwise, the corrosion rate will increase.
Fig.5 shows the polarization curves of the Ni-P coatings in 3.5% NaCl solution. It can be noted that both electroless Ni-P coatings have similar thickness (about 25 μm). The corrosion potential and corrosion current density obtained from the electrochemical polarization curves are summarized in Table 4. According to the polarization curves, no defects were detected in the samples tested [22]. As shown in Fig.5, the cathode reaction in the polarization curves corresponds to the evolution of the hydrogen, and the anodic polarization curve was the most important features related to the corrosion resistance [23]. All the Ni-P coatings show great positive shifts in the corrosion potential and evident decrease in the corrosion current density in comparison with their magnesium alloy substrate (curve a). It can be
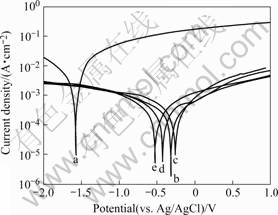
Fig.5 Polarization curves of AZ91D magnesium alloy substrate and substrate with different alloys in 3.5% NaCl aqueous solution: (a) Substrate; (b) pH=5; (c) pH=6; (d) pH=7; (e) pH=8
seen from Fig.5 and Table 4 that the Ni-P coatings of various phosphorus contents show a similar corrosion trend. The Ni-P coating deposited in the bath with pH=6 has the lowest Jcorr, as well as the highest φcorr, and thus exhibits potentially the best corrosion resistance compared with others. Moreover, the Ni-P coating deposited in the acidic baths with higher phosphorus contents exhibits better corrosion resistance than that in alkaline baths. Therefore, the properties of the Ni-P coating deposited in the bath with pH=6 is expected to improve the corrosion resistance of AZ91D magnesium alloy.
Table 4 Corrosion potential and corrosion current density values of as-plated alloys obtained from electrochemical polarization curves
The anodic potentiodynamic polarization curves of the Ni-P alloy deposited in the bath with pH=6 for the heat-treatments at 200, 300, 350 and 400 ℃ for 2 h are shown in Fig.6. The values of the corrosion potential φcorr and the anodic current density Jcorr were calculated from the intersection of the cathodic and anodic Tafel curves
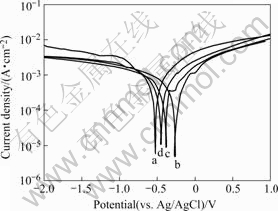
Fig.6 Polarization curves of substrate with alloys deposited in bath with pH=6 after heat-treatment in 3.5% NaCl aqueous solution: (a) 200 ℃, 2 h; (b) 300 ℃, 2 h; (c) 350 ℃, 2 h; (d) 400 ℃, 2 h
extrapolated from the cathodic and anodic potentiodynamic polarization curves and are shown in Table 5. It is obvious in Table 5 that the Ni-P alloy deposited after heat-treatment at 300 ℃ for 2 h has the lowest Jcorr, and the highest φcorr, and thus exhibits potentially the best corrosion resistance compared with others. But when being compared with the as-plated Ni-P alloy deposited in the bath with pH=6, φcorr is lower and Jcorr is higher than that subjected to the heat-treatment. This is due to the formation of the hard nickel phosphide phase (Ni3P) in the Ni-P coating and the Ni-P alloy crystallization. This result is in agreement with the classic corrosion theory that nanocrystalline would accelerate corrosion by forming much more micro electrochemical cells between the grain boundaries and the matrix, while the amorphous structure should exhibit inherently a high corrosion resistance because of its extremely homogenous structure that lacks preferential corrosion paths like grain boundaries or other structural defects.
Table 5 Corrosion potential and corrosion current density values of heat-treated alloys deposited in bath with pH=6 obtained from electrochemical polarization curves

1) The electroless Ni-P coatings are obtained directly on the AZ91D magnesium alloy by the electroless plating using basic nickel carbonate as the source of nickel.
2) The obtained electroless Ni-P coatings exhibit typical dense nodular structure consisting of a mixtured amorphous and nanocrystalline. Compositional analysis by EDS shows that the content of phosphorous decreases from 7.32% to 4.87%(mass fraction) with the increase of the pH value.
3) The Ni-P coating deposited in the bath with pH=6 has the highest microhardness of about HV 635.
4) The alloy deposited in the bath with pH=6 has the lowest Jcorr, as well as the highest φcorr, and thus provides potentially the best corrosion resistance. After being heat-treated for 2 h, the Ni-P coating crystallizes and produces the phosphide phase (Ni3P), so Jcorr increases, and thus leads to potentially the worse corrosion resistance compared with the as-plated alloy.
5) The Ni-P coatings on AZ91D magnesium alloy exhibit good performance and can further enhance the corrosion resistance of the substrate.
Reference
[1] AMBAT R, ZHOU W. Electroless nickel-plating on AZ91D magnesium alloy: Effect of substrate microstructure and plating parameters [J]. Surf Coat Tech, 2004, 179: 124-134.
[2] FUNATANIA K. Emerging technology in surface modification of light metals [J]. Surf Coat Tech, 2000, 133/134: 264-272.
[3] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys—a critical review [J]. J Alloys Comp, 2002, 336: 88-113.
[4] UMEHARA H, TAKAYA M, TERAUCHI S. Chrome-free surface treatments for magnesium alloy [J]. Surf Coat Tech, 2003, 169/170: 666-669.
[5] DABAL? M, BRUNELLI K, NAPOLITANI E, MAGRINI M. Cerium-based chemical conversion coating on AZ63 magnesium alloy [J]. Surf Coat Tech, 2003, 172: 227-232.
[6] ZHAO M, WU S, LUO J. R, FUKUDA Y, NAKAE H. A chromium-free conversion coating of magnesium alloy by a phosphate–permanganate solution [J]. Surf Coat Tech, 2006, 200: 5407-5412.
[7] KOUISNI L, AZZI M, ZERTOUBI M, DALARD F, MAXIMOVITCH S. Phosphate coatings on magnesium alloy AM60 part 1: Study of the formation and the growth of zinc phosphate films [J]. Surf Coat Tech, 2004, 185: 58-67.
[8] HSIAO H Y, TSAI W T. Characterization of anodic films formed on AZ91D magnesium alloy [J]. Surf Coat Tech, 2005, 190: 299-308.
[9] SHARMA A K, SURESH M R, BHOJRAJ H, et al. Electroless nickel plating on magnesium alloy [J]. Met Finish, 1998, 96: 10-14.
[10] MALLORY G O, HAJDU J B. Electroless plating: Fundamentals and applications[M]. Orlando, FL: AESF Publishing, 1990: 261.
[11] YAN H. New Techniques in electroless Ni and composite plating[M]. Beijing: National Defense Industry Press, 2001.
[12] ASTM Standards B733-97, Standard Specification for Autocatalytic (Electroless) Nickel- Phosphorus Coatings on Metal [S].
[13] RIEDEL W. Electroless Ni plating, ASM International[M]. England: Finishing Publications LTD, 1991.
[14] GU C D, LIAN J S, LI G Y, NIU L Y, JIANG Z H. High corrosion-resistant Ni-P/Ni/Ni-P multilayer coatings on steel [J]. Surf Coat Tech, 2005, 197: 61-67.
[15] ZHANG W X, HE J G, JIANG Z H, JIANG Q, LIAN J S. Electroless Ni-P layer with a chromium-free pretreatment on AZ91D magnesium[J]. Surf Cort Tecnol, 2007, 201: 4594.
[16] LIU X K, XIANG Y H, HU W B, DING W J. Adhesion mechanism of direct electroless nickel coating magnesium alloys [J]. J Chin Soc Corros Prot, 2002, 22(4): 235-239. (in Chinese)
[17] HUO H W, LI Y, WANG F H. Corrosion of AZ91D magnesium alloy with a chemical conversion coating and electroless nickel layer [J]. Corros Sci, 2004, 46(6): 1467-1477.
[18] GU C D, LIAN J S, LI G Y, NIU L Y, JIANG Z H. Electroless Ni-P plating on AZ91D magnesium alloy from a sulfate solution [J]. J Alloys Comp, 2005, 391: 104-109.
[19] ASTM Standard Designation B 480-88[EB/OL]. http://www.astm.org/cgi-bin/SoftCart.exe/DATABASE.CART/REDLINE_PAGES/B480.htm?E+mystore.
[20] SONG G, ATRENS A, DARGUSCH M. Influence of microstructure on the corrosion of diecast AZ91D [J]. Corros Sci, 1999, 41(2): 249-273.
[21] GUO Z, KEONG K G, SHA W. Crystallization and phase transformation behavior of electroless nickel phosphous platings during continuous heating[J]. J Alloys Comp, 2003, 358: 112-119.
[22] KERR C, BARKER D, WALSH F. Porosity and corrosion rate measurements for electroless nickel deposits on steel using electrochemical techniques [J]. Trans Inst Met Finish, 1997, 75: 81-89.
[23] DONG H, SUN Y, BELL T. Enhanced corrosion resistance of duplex coatings [J]. Surf Coat Tech, 1997, 90: 91-101.
Foundation item: Project(2004CB619301) supported by the National Key Basic Research Development Program of China; Project supported by 985 Project of Jilin University
Corresponding author: JIANG Zhong-hao; E-mail: jzh@jlu.edu.cn


