网络首发时间: 2018-08-24 16:26
纳米银材料的研究进展及应用前景
张敏 邱佳佳 殷涛 谭谆礼
北京交通大学机械与电子控制工程学院
北京师范大学中药资源保护与利用北京市重点实验室
摘 要:
介绍了国内外纳米银粒子及纳米多孔银的研究进展和应用现状,针对纳米银粒子和纳米多孔银在制备方法、微观结构、应用前景等方面进行了分析。纳米银粒子的粒径从几纳米至几百纳米,制备方法较多,已获得工业应用,如芯片产业链中的以纳米银填充的导电银浆、生物医药中的杀菌剂、化工生产中的催化剂等;纳米多孔银则是近年来发展起来的新材料,其独特的双连续结构和纳米量级的尺寸范围有利于开发新的应用。通过溶解合金中相对活泼元素而使银元素重组的去合金化法是制备纳米多孔银的有效方法之一,所需的前驱体包括晶态合金(Ag-Al,Ag-Zn,Ag-Mg合金等)和非晶态合金(AgCuSi,(Cu50 Zr50 )100-x Agx ,AgMgCa等),我国在前驱体制备和腐蚀工艺方面具有丰富的知识和技术储备。
关键词:
纳米银粒子 ;纳米多孔银 ;催化 ;去合金化 ;银浆 ;
中图分类号: O614.122;TB383.1
作者简介: 张敏(1981-),女,湖北荆州人,博士,副研究员,研究方向:金属材料的组织设计和结构表征;电话:010-51685462;E-mail:zhangm@bjtu.edu.cn;
收稿日期: 2018-07-18
基金: 基本科研业务费项目(2015RC079); 国家科技部重点基础研究发展计划项目(2017YFB0304504); 中国博士后科学基金项目(2015M570964)资助;
Research Progress and Application Prospect of Silver Nanoparticles and Nanoporous Silver Materials
Zhang Min Qiu Jiajia Yin Tao Tan Zhunli
School of Mechanical,Electronic and Control Engineering,Materials Science & Engineering Research Center,Beijing Jiaotong University
Beijing Area Major Laboratory of Protection and Utilization of Traditional Chinese Medicine,Beijing Normal University
Abstract:
The latest research progress of silver nanoparticles and nanoporous silver materials was introduced.The microstructures,preparation methodsand application prospects of the nano silver materials were studied and analyzed.There were several preparation methods of the silver nanoparticles and their grain sizes distributed from several nanometers to hundreds of nanometers.Silver nanoparticles were extensively used in the fields of silver paste,bactericides,catalysts,etc.Nanoporous silver materials were developed in recent years and they had characteristic double-continuous structure,which were beneficial for developing new applications.The typical sizes of the ligaments and pores varied from several nanometers to hundreds of nanometers.Dealloying was the most frequently-used preparation method of nanoporous silver.The precursors for dealloying process include both crystalline alloys(such as Ag-Al,Ag-Zn,Ag-Mg,etc.) and amorphous alloys(such as(AgCuSi,(Cu50 Zr50 )100-x Agx ,AgMgCa,etc.).Nowadays,there were rich reserves of knowledge and experience in precursor preparation and dealloying process at home.
Keyword:
silver nanoparticles; nanoporous silver; catalytic action; dealloying; nano silver paste;
Received: 2018-07-18
纳米材料因其特殊的微观结构,具备小尺寸效应、表面效应以及宏观量子隧道效应等特性。银元素作为贵金属的一种,块体材料的物理和化学性质都很稳定,具有良好的抗菌性和生物相容性。纳米银材料在具有这些特性的同时,还具有优异的催化性能等
[1 ,2 ,3 ]
,其应用涉及到生物学、医学、环境保护以及电子工业等诸多领域
[4 ,5 ,6 ]
。目前研究较多的纳米银材料按其微观结构主要分为纳米银粒子和纳米多孔银,本文将从材料的制备和应用两方面分析这两种纳米银材料的研究进展和应用前景,以及纳米银粉填充银浆的工业现状。
1 纳米银粒子
1.1 纳米银粒子的制备
纳米材料种类繁多,其制备方法多种多样。对于纳米银粒子材料的制备,目前广泛应用的主要有物理方法、化学方法和生物方法等
[7 ,8 ]
。
1.1.1 物理方法
物理法是通过机械球磨等物理手段将块体银破碎,经过反复研磨,制备成粒径为几纳米至几百纳米的单质银粒子。蒸发冷凝和激光烧蚀等也是制备纳米银粒子的物理方法。虽然其工作原理和生产工艺简单易懂,但是需要成本较高的大型设备,并且能耗大也是缺点之一。
机械球磨法是在封闭的研磨罐中放入研磨球,通过和大块单质银之间的碰撞打磨,改变金属银的形貌和尺寸,通过多次球磨,逐渐将晶粒细化。对于最终产物的形貌和粒径大小,研磨时间是重要的工艺参数之一,时间太短则没有细化到需求的粒径,时间太长则会发生团聚,这是因为纳米银粒子由于尺寸效应造成比表面积大,导致材料的粒径越小,表面能越高,团聚的倾向性越大
[9 ]
。Xu等
[10 ]
在-196℃的条件下,采用机械球磨法制得了平均粒径为20 nm的纳米银粒子。
蒸发冷凝法是指在惰性气体或者真空环境中,通过外加手段(如加热、激光、电弧高频感应等)产生高温,使块体原料发生气化或者形成等离子体,再在低温基板上凝结得到纳米银粒子。该方法的优点是产物纯度较高、粒径分布可通过调整功率和温度控制,但对技术设备要求极高,且制得的纳米银粒子易团聚。根据报道,通过蒸发冷凝法可制备出粒径范围为7~75 nm、并且形貌为球形的纳米银粒子
[11 ]
。
激光烧蚀法是利用激光的高能量密度,将块体银靶材瞬间加热到气化温度以上,使其表层原子迅速升温蒸发,然后在低温基板上冷凝,得到纳米银粒子产物。该方法与蒸发冷凝法有一定的类似之处,但是加热和气化在极短的时间内完成,得到的产物更加纯净。据报道,采用这种高能脉冲激光烧蚀技术,可成功制得粒径小于50 nm、分布均匀的球形纳米银粒子
[12 ]
。
1.1.2 化学方法
与物理方法需要大型机械设备相比,化学法制备条件简单、成本低、产量大,在工业生产中应用较为广泛,但生产过程中添加的分散剂、稳定剂以及有机溶剂等,会对环境造成污染。目前采用的化学法主要有化学还原法、微乳液法以及溶胶-凝胶法等。
化学还原法是指通过氧化还原方法,把银离子从它的盐溶液或有机体系中还原成纳米银粒子,随后洗涤、干燥获得纳米银粉末。该方法原理简单,对设备要求不高,产率高,可通过控制工艺参数制备粒度小、不易团聚的纳米材料,是目前实验室研究和工业生产上广泛采用的方法。但是反应是在液态环境中发生,所制得的纳米银粒子尺寸小,因而固液分离困难,产物粒度分布范围宽、容易发生团聚。
微乳液法是利用表面活性剂,使得溶有不同反应物的两种互不相溶的溶剂混合,形成乳液体系,反应产物在微泡中成核、聚结、团聚,经热处理后制得纳米银粒子
[13 ]
。微乳液法能够通过调控液滴的大小来控制所获得的纳米银粒子的粒径、形貌等特性,所需的实验装置简单,但是采用的有机化学试剂对环境和健康不友好。
溶胶-凝胶法是实验室中制备和合成常用的方法,指通过控制反应条件将金属化合物水解变成溶胶,将溶胶经过热反应过程制备纳米粒子粉末。溶胶-凝胶方法的反应条件容易控制,对设备要求不高,所获得产物粒子尺寸小、纯度高,但是制备过程时间较长
[14 ]
。通过溶胶-凝胶法制备得到的粒径20 nm左右的银粒子在太阳能电池中有潜在应用。
1.1.3 生物方法
目前应用于纳米银粒子制备的生物方法主要是微生物还原法和植物还原法等。自然界中一些细菌、真菌等和植物浸取液中的活性成分本身具有生物活性,可以在合成过程中起到还原剂和保护剂的作用,用来制备纳米银粒子
[15 ]
。这种制备方法条件简单、参数易控制,对环境无污染,成为近几年的研究热点。
微生物还原法利用微生物的生物机能,在胞内和胞外进行纳米银粒子的制备,有效的微生物原材料来源广泛、得到的纳米银粒子不易团聚。通过微生物还原法,Kowshik等
[16 ]
利用酵母菌,在胞外合成了粒径在2~10 nm间的纳米银粒子。
植物还原法利用植物中的活性有效成分(次生代谢物)的强还原电位,发生还原反应,可同时作为该反应的稳定剂和还原剂,在银粒子溶液中制备纳米银粒子,对环境无污染。该方法可以获得尺寸为18~39 nm的纳米银粒子,且不易发生团聚
[17 ,18 ]
。
各种方法制备纳米银粒子的比较如表1所示。
表1 制备纳米银粒子的方法比较 下载原图
Table 1 Comparison of features and product sizes of dif-ferent preparation methods
1.2 纳米银粒子的应用
1.2.1 医学领域的应用
银可以使细菌蛋白质变性,具有广谱杀菌的特点。而纳米银粒子由于粒径小、比表面积大,因此其杀菌性比块体银材料能显著增强。针对常见有害菌,如表皮葡萄球菌和大肠杆菌,将无纺布浸渍纳米银溶液可得到良好的抗菌性能
[19 ]
。
除可以有效杀灭和抑制细菌外,纳米银粒子对真菌、病毒、癌细胞等也有一定的抑制作用
[20 ]
,这些有效作用使得纳米银粒子在医疗和卫生领域有广阔的市场前景。
1.2.2 催化领域的应用
由于纳米银粒子具有粒径小、比表面积大并且有较多悬空键的特点,表现出良好的催化活性,可以被用作多种反应的催化剂。研究表明,纳米银粒子在合成螺氧化吲哚衍生物的缩聚反应中,与其他纳米材料催化剂相比,能够显著提高反应产量、缩短反应时间,且反应产物纯度更高
[21 ]
。在生物催化领域,纳米银粒子与蛋白质所含氨基酸中氨基之间发生作用,银的介入有效增强了酶的催化活性
[22 ]
。
1.2.3 电子工业的应用
在电子工业领域,银浆在使用过程中工艺简单,性能稳定,因此具有不可替代的作用。目前已发展成熟的导电银浆主要通过微米或亚微米尺度的银粉导电,随着电子工业中低温烧结和多层布线等新技术的快速发展,这些浆料的性能己不能满足要求,因此对材料本身的结构和性能提出了更高要求。当导电银浆中填充粒径为纳米量级的银粒子时,由于尺寸效应,其熔点降低,表面活性提高,比表面积增大,热导率升高,这些物理特性使得其可以满足大功率、高密度系统的散热要求,适合用于多级封装
[23 ,24 ]
。本文1.3节中将单独介绍纳米银粒子填充银浆的工业现状。
1.3 纳米银浆的工业现状
近年来,国内外正在开展纳米银粒子填充导电银浆的研究。德国HENKEL公司成功研发了无压烧结导电银浆,在原有的微米级银粉中掺杂一定比例的纳米级银粉,可实现高功率器件封装的批量生产。这种纳米银粒子填充导电银浆已经被用在一种高可靠性的芯片粘接材料Ablestik SSP2000上,适用于高功率LED产品等功率模块的集成
[25 ]
。
此外,日本京瓷集团(KYOCERA)和德国贺利氏集团(HERAEUS)也分别研发出了纳米银粒子填充的商业级纳米导电银浆并进行了推广。
在国内仅有少数企业掌握这项技术,其中北京中科纳通电子技术有限公司已成功研制出自主知识产权的纳米银填充导电浆料和纳米银喷墨导电墨水,并且实现产业化。由海外顶尖高校和研究所留学归国人员和国内高校教授联合创办的魔技纳米科技公司,在纳米材料制备、3D打印等领域均有较好的成绩,在纳米银材料的制备研发方面具有很好的人才和技术储备。弗朗施纳米科技公司也是国内一家人才和技术储备良好的公司,该公司自主研制了高纯度金属纳米粉制造设备,可生产尺寸1~100 nm的纳米银粉,为纳米银浆的制备提供原材料。
从国际和国内的研究情况来看,由于实验室小批量试制和工业批量生产对设备、工艺等参数的要求差异,一些已发表的研究成果在商业化过程中会遇到各种的问题,工业应用滞后于高校和研究院所的实验室研究。
2 纳米多孔银
纳米多孔银是继金、铂、钯等贵金属多孔材料之后发展起来一种具有三维连通的双连续结构的多孔材料,其三维结构中的系带由银晶粒组成,晶粒粒径为纳米量级,系带最小维度方向的尺寸和孔洞的最小维度方向尺寸也是纳米量级。目前制备的纳米多孔银平均粒径以20~40 nm为主。与纳米银粒子不同,纳米多孔银在宏观上连通,宏观可达厘米量级,这种连续结构既保持了纳米材料高比表面积的特点,又具有良好的导电性以及自持性。与金、铂、钯等贵金属的纳米多孔材料相比,由于银元素本身的杀菌作用,且成本更低,因此纳米多孔银具有非常大的应用潜力。
纳米多孔银的制备方法主要有模板法、去合金化法、电化学沉积法和化学沉积法等。下文将分别介绍模板法和去合金化法。
2.1 纳米多孔银的制备
2.1.1 模板法
模板法是将目标金属沉积在以已有的多孔材料模板上,然后去掉模板,获得类模板结构的多孔材料。按照模板材料类型的不同,可细分为乳液聚合物模板法,胶晶模板法,液晶模板法,生物模扳法和多孔氧化铝模板法等
[26 ,27 ]
。通过模板法制备获得的纳米多孔银的形貌及孔隙尺寸都与多孔模板本身的直接相关,可通过选择合适的模板,对形貌进行调控,获得所需的结构,但是制备工艺较复杂,对模板本身的微观结构要求较高。
2.1.2 去合金化法
去合金化法,又称选择性腐蚀法,是指先制备含有目标金属的二元或多元前驱体材料,然后将前驱体置于合适的腐蚀环境或者电化学环境中,利用前驱体合金中不同组元之间的电势差,通过控制反应时间、溶液温度等参数,选择性地优先腐蚀较活泼的金属,而较稳定的金属组元通过扩散、聚集等方式形成纳米多孔结构。
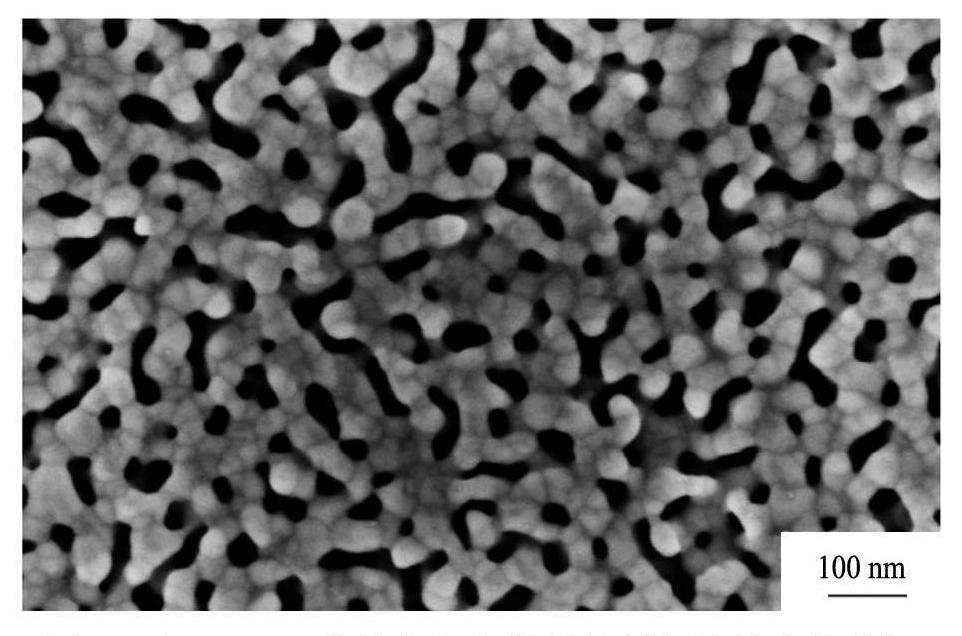
对于纳米多孔银材料,目前的研究表明,选择合适的前驱体(如AgCuSi非晶合金)和反应条件(HNO3 +HF),可实现去合金化后材料的宏观尺寸变化不大、而微观结构为银晶粒形成骨架的双连续(系带和孔洞均为连续结构)纳米多孔材料
[28 ,29 ,30 ,31 ,32 ]
。特征结构如图1所示。这种具有宏观尺度的纳米结构多孔材料可实现自持,同时具有小尺寸效应、表面孔效应、量子尺寸效应等性质
[33 ]
。
目前报道的纳米多孔银的制备方法中,前驱体主要有晶态合金和非晶态合金两种。晶态合金前驱体主要有Ag-Al,Ag-Zn和Ag-Mg合金等,这些合金的共同点是第二组元与银具有较大的电势差,易被腐蚀
[34 ,35 ,36 ]
。以这些二元合金为前驱体,腐蚀溶液可以选择盐酸、硝酸、氢氟酸或它们的混合溶液等,制备得到的纳米多孔银块体材料,系带尺寸为50~500 nm的纳米多孔材料。
由于晶态合金中有晶界、相界、位错等缺陷,以这些晶态合金作为前驱体,制备纳米多孔银的过程中,这些缺陷会被遗传,影响纳米多孔银微观结构的均匀性和完整性。
除晶态合金前驱体外,另一种为非晶态合金前驱体。非晶态合金又称作金属玻璃,主要组成元素为金属元素,其原子排列类似于液态金属,具有短程有序、长程无序的特征,原子之间以金属键连接,成分均匀,组织均匀,没有晶界、相界、位错等缺陷,各向同性。非晶态合金可以通过熔融态金属经过快速冷却获得。与晶态前驱体相比,采用非晶态合金作为前驱体制备纳米多孔银,其优势在于:非晶合金为单相非晶组织,成分均匀,且没有晶态材料中常见的缺陷,有利于在去合金化过程中形成均匀的纳米多孔结构;非晶合金的成分一般在相图中的深共晶点附近,范围比较宽,其组元可调性较大,便于通过合金成分设计实现对纳米多孔银微结构的调控
[37 ,38 ,39 ,40 ,41 ]
。
图1 以AgCuSi非晶合金为前驱体制备的纳米多孔银
Fig.1 Nanoporous Ag prepared by rapidly solidified AgCuSi after immersing in nitric acid for 6 min
[28]
目前用于制备纳米多孔银的非晶合金前驱体主要有AgCuSi
[28 ]
,(Cu50 Zr50 )100-x Agx
[42 ]
(x=10%,20%,30%和40%(原子分数)),AgMgCa
[43 ]
等,可获得系带约20 nm的双连续纳米多孔银。研究表明,韧带尺寸随着Ag含量和去合金化时间的增加而增加,但随着HF浓度的增加而减小。这种现象可归因于其他组元的溶解和Ag的扩散、聚集、成核和重结晶,导致相邻纳米晶体的取向附着。
2.2 纳米多孔银的应用
2.2.1 激光拉曼增强效应
表面增强活性拉曼光谱(surface-enhanced Raman spectroscopy,SERS)活性基底的制备是获得较好SERS信号的关键,目前关于SERS基底的研究已经很多,但是基底的增强能力、稳定性及重现性仍有待进一步改进和提高。由于纳米多孔银的制备工艺简单,形状可控,成本低,体系光损伤小又干净,具有出众的表面增强拉曼散射性能,已经成为使用较为广泛的一种SERS基底。具有三维纳米通孔结构的纳米多孔银基底可在表面等离子体共振和电荷转移的共同作用下,将探针分子的拉曼散射信号极大地增强,从而表现出优越的SERS效应
[44 ]
。
2.2.2 电催化氧化
由于纳米多孔银具有高比表面积、高通透率、系带和孔洞双连续通孔结构等特点,在能源电池、超级电容器等方面也有着巨大的应用潜力。
有研究表明,纳米多孔银电极在碱性环境中对甲醛的氧化电位达到-900 mV,比未经处理的光滑银电极低200 mV,甚至比金和铂的电化学催化氧化电位还要低150 mV,如果将该材料用作燃料电池的负极,将会使得燃料电池具有更高的输出电压
[45 ]
。
2.2.3 有机染料催化
有机染料是工业排放到水环境中的最主要的有害污染物,可能会对人们的健康造成严重的危害。而纳米多孔银由于其优异的催化性能,在废水处理(如有机染料的催化脱色)中发挥着重要作用。
有研究表明,纳米多孔银对还原有机染料(包括罗丹明和甲基橙)显示出极高催化的性能。而且由于其纳米多孔结构,可以实现有效染料的有效催化和连续脱色,具有优异的可重复使用性
[46 ]
。此外,它表现出优异的可回收性,即使在重复使用十次后,脱色效率仍可达99%,这为染料的连续催化脱色开辟了新的途径。
具有超细结构的纳米多孔金属材料能够表现出特殊的物理、化学和力学性能,目前已经发现的纳米多孔银的性能有高的激光拉曼增强效应、增大的杨氏模量、高的甲醛电催化氧化性能、偶氮染料催化降解等性能,还可用作灵敏传感器
[47 ,48 ,49 ,50 ,51 ]
,对于这些优良性能的工业化应用,前景广阔,但是目前尚没有实现纳米多孔银的工业化生产。
3 结论
纳米银粒子和纳米多孔银以其独特的结构和性能获得了广泛的关注。纳米银粒子可通过物理方法、化学方法、生物方法进行制备,所获得的纳米银粒子粒径范围从几个纳米到几百纳米,可被用于医学、催化、电子工业等领域;纳米多孔银可通过模板法和去合金化法进行制备,所获得的双连续纳米多孔结构中,银晶粒尺寸范围从几纳米至几十纳米,系带在最小维度方向上的尺寸范围从几纳米至几百纳米,可得到连通的块体材料,具有自持性,纳米多孔银的应用前景广阔。相较于国外,我国在纳米银粒子及其衍生产品的工业生产方面已涌现出一批有代表性的企业,有望打破国外垄断,实现自主知识产权;在纳米多孔银方面,去合金化法制备过程中所需的前驱体是关键因素,我国在前驱体的制备和腐蚀控制等方面具有丰富的知识和技术储备,在实际应用方面的探索也需更进一步。
参考文献
[1] Li X,Wang J,Zhang Y,Li M,Liu J.Surfactantless synthesis and the surface-enhanced Raman spectra andcatalytic activity of differently shaped silver nanomaterials[J].European Journal of Inorganic Chemistry,2010,(12):1806.
[2] Yang J,Zheng H,Han S,Jiang Z,Chen Z.The synthesis of nano-silver/sodium alginate composites and their antibacterial properties[J].RSC Advances,2014,5(4):2378.
[3] Liang S Y,Zhu X Y.Mechanistic study on preparation of silver nanoplates by photoinduced method[J].Chinese Journalof Rare Metals,2016,40(6):528.(梁诗宇,朱晓云.光诱导法制备片状纳米银的机制研究[J].稀有金属,2016,40(6):528.)
[4] Chou H H,Huang M T,Pender S L F,Ruslin M,Chou H H,Ou K L.The application of silver nano-particles on developing potential treatment for chronic rhinosinusitis:antibacterial action and cytotoxicity effect on human nasal epithelial cell model[J].Materials Science&Engineering C,2017,80:624.
[5] Mahadevan S,Vijayakumar S,Arulmozhi P.Green synthesis of silver nano particles from Atalantiamonophylla(L)Correa leaf extract,their antimicrobial activity and sensing capability of H_2O_2[J].Microbial Pathogenesis,2017,113:445.
[6] Hazer B,Ozlem A Kalayci.High fluorescence emission silver nano particles coated with poly(styrene-g-soybean oil)graft copolymers:antibacterial activity and polymerization kinetics[J].Materials Science&Engineering C,2016,74:259.
[7] Gao H,Yang H,Wang C.Controllable preparation and mechanism of nano-silver mediated by the microemulsion system of the clove oil[J].Results in Physics,2017,7:3130.
[8] Annadhasan M,Sankarbabu V R,Naresh R,Umamaheswari K,Rajendiran N.A sunlight-induced rapid synthesis of silver nanoparticles using sodium salt of Ncholyl amino acids and its antimicrobial applications[J].Colloids&Surfaces B Biointerfaces,2012,96(96):14.
[9] Liu F,Liu J,Cao X.Microwave-assisted synthesis silver nanoparticles and their surface enhancement Raman scattering[J].Rare Metal Materials&Engineering,2017,46(9):2395.
[10] Xu J,Yin J S,Ma E.Nanociystalline Ag formed by low-temperature high-energy mechanical attrition[J].Nanostructured Materials,1997,8(1):91.
[11] Gromov D G,Pavlova L M,Savitsky A I,Trifonov A Y.Nucleation and growth of Ag nanoparticles on amorphous carbon surface from vapor phase formed by vacuum evaporation[J].Applied Physics A,2015,118(4):1297.
[12] Boutinguiza M,Comesana R,Lusquinos F,Riveiro A,Val J D,Pou J.Production of silver nanoparticles by laser ablation in open air[J].Applied Surface Science,2015,336:108.
[13] Liu J,Li X,Zeng X.Silver nanoparticles prepared by chemical reduction-protection method,and their application in electrically conductive silver nanopaste[J].Journal of Alloys&Compounds,2010,494(1):84.
[14] Parvulescu V I,Cojocaru B,Parvulescu V,Richards R,Li Z,Cadigan C,Granger P,Miquel P,Hardacre C.Sol-gel-entrapped nano silver catalysts-correlation between active silver species and catalytic behavior[J].Journal of Catalysis,2010,272(1):92.
[15] Soni N,Prakash S.Microbial synthesis of spherical nanosilver and nanogold for mosquito control[J].Annals of Microbiology,2014,64(3):1099.
[16] Kowshik M,Ashtaputre S,Kharrazi S,Vogel W,Urban J,Kulkarni S K,Paknikar K M.Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3[J].Nanotechnology,2003,14(1):95.
[17] Borase H P,Salunke B K,Salunkhe R B,Patil C D,Hallsworth J E,Kim B S,Patil S V.Plant extract:a promising biomatrix for ecofriendly,controlled synthesis of silver nanoparticles[J].Applied Biochemistiy&Biotechnology,2014,173(1):1.
[18] Khader S Z A,Ahmed S S Z,Sathyan J,Mahboob M R,Venkatesh K P,Ramesh K.A comparative study on larvicidal potential of selected medicinal plants over green synthesized silver nanoparticles[J].Egyptian Journal of Basic&Applied Sciences,2018,5(1):54.
[19] Deng X,Nikiforov A,Vujosevic D,Vuksanovic V,Mugosa B,Cvelbar U,Geyter N D,Morent R,Leys C.Antibacterial activity of nano-silver non-woven fabric prepared by atmospheric pressure plasma deposition[J].Materials Letters,2015,149:95.
[20] Sportelli M C,Picca R A,Cioffi N.Recent advances in the synthesis and characterization of nanoantimicrobials[J].Trac.Trends in Analytical Chemistry,2016,84:131.
[21] Rashid Z,Moadi T,Ghahremanzadeh R.Green synthesis and characterization of silver nanoparticles using Ferulalatisecta leaf extract and their application as a cat-alyst for the safe and simple one-pot preparation of spirooxindoles in water[J].New Journal of Chemistry,2016,40(4):3343.
[22] Wang A M,Wang H,Zhou C,Du Z Q,Zhu S M,Shen S B.Ag-induced efficient immobilization of papain on silica spheres[J].Chinese Journal of Chemical Engineering,2008,16(4):612.
[23] Jiang H,Moon K,Li Y,Wong C.Surface functionalized silver nanoparticles for ultrahigh conductive polymer composites[J].Chemistry of Materials,2006,18(13):2969.
[24] Teng Y,Yan F C,Li W L,Du J H,Zhang J M,Yan J K.Properties of lead-free conductive silver paste with different morphology and particle size of silver[J].Chinese Journalof Rare Metals,2017,41(11):1273.(滕媛,闫方存,李文琳,杜景红,张家敏,严继康.银粉形貌及粒径对无铅导电银浆性能的影响[J].稀有金属,2017,41(11):1273.)
[25] Li M,Li H,Zhou Z H.Semi-supervised document retrieval[J].Information Processing&Management,2009,45(3):341.
[26] Shen W,Zhang L,Du Y,Zhao B,Zhou X.Synthesis,characterization,and properties of porous silver spheres using rape pollen as novel bio-templates[J].Materials Letters,2018,213:7.
[27] Fang J H,Spizzirri P,Lin L,Roberts A,Praweret S.Template controlled fabrication of silver nano-structures using porous anodic aluminium oxide[J].Journal of the Australian Ceramic Society,2010,46(1):46.
[28] Zhang M,Junior A M J,Pang S J,Zhang T,Yavar A R.Fabrication of nanoporous silver with open pores[J].ScriptaMaterialia,2015,100:21.
[29] Viswanath R P.A patent for generation of electrolytic hydrogen by a cost effective and cheaper route[J].Hydrogen Energy,2004,29(11):1191.
[30] Meng N,Leung M K H,Leung D Y C.Energy and exergy analysis of hydrogen production by a proton exchange membrane(PEM)electrolyzer plant[J].Energy Conversion and Management,2008,49(10):2748.
[31] Fierro S,Kapalka A,Comninellis C.Electrochemical comparison between Ir02 prepared by thermal treatment of iridium metal and Ir02 prepared by thermal decomposition of H_2IrCl_6 solution[J].Electrochemistry Communications,2010,12(1):172.
[32] Wang J T.Biological hydrogen production and hydrogen generation[J].Energy Conservation Tecnology,2010,28(159):57.
[33] Ding Y.Nanoporous metals:a new class of nanostructured energy materials[J].Journal of Shandong University(Natural Science),2011,46(10):121.(丁轶.纳米多孔金属:一种新型能源纳米材料[J].山东大学学报(理学版),2011,46(10):121.)
[34] Wang X,Qi Z,Zhao C,Wang W,Zhang Z.Influence of alloy composition and dealloying solution on the formation and microstructure of monolithic nanoporous silver through chemical dealloying of Al-Ag alloys[J].Journal of Physical Chemistry C,2009,113(30):13139.
[35] Zhang C,Sun J Z,Xu J L,Wang X G,Ji H,Zhao C C,Zhang Z H,et al.Fonnation and microstructure of nanoporous silver by dealloying rapidly solidified Zn-Ag alloys[J].Electrochimica Acta,2012,63:302.
[36] Ji H,Zhang C,Xu J,Zhao C,Wang X,Zhang Z.On the vacancy-controlled dealloying of rapidly solidified Mg-Ag alloys[J].Ciystengcomm,2011,13(15):4846.
[37] Berenguer R,Quijada C,Morallon E.Electrochemical characterization of SnO_2 electrodes doped with Ru and Pt[J].Electrochimica Acta,2009,54(14):5230.
[38] Marshall A T,Haverkamp R G.Electrocatalytic activity of IrO_2-RuO_2 supported on Sb-doped SnO_2 nanoparticles[J].Electrochimica Acta,2010,55(6):1978.
[39] Mathias M,Roth J,Fleming J,Lehnert W.Diffusion media materials and characterisation.Handbook of Fuel Cells-Fundamentals,Technology and Applications[M].New York:John Wiley and Sons,Ltd;2003.Capter46,4.
[40] Escribano S,Blachot J F,Etheve J,Morin A,Mosdale R.Characterization of PEMFCs gas diffusion layers properties[J].Power Sources,2006,156(1):8.
[41] Ismail M S,Damjanovic T,Ingham D B,Pourkashanian M,Westwood A.Effect of polytetrafluoroethylene-treatnent and microporous layer-coating on the electrical conductivity of gas diffusion layers used in proton exchange membrane fuel cells[J].Power Sources 2010,195(9):2700.
[42] Wang H,Xiao S G,Zhang T.Fabrication of nanoporous silver by de-alloying Cu-Zr-Ag amorphous alloys[J].International Journal of Minerals Metallurgyand Materials,2016,23(7):835.
[43] Li R,Liu X J,Wang H,Wu Y,Chu X M,Lu Z P.Nanoporous silver with tunable pore characteristics and superior surface enhanced Raman scattering[J].Corrosion Science,2014,84(8):159.
[44] Fang C,Ellis A V,Voelcker N H.Electrochemically prepared porous silver and its application in surface-enhanced Raman scattering[J].Journal of Electroanalytical Chemistry,2011,659(2):151.
[45] Singh B,Seddon B,Dempsey E,Redington W,Dickinson C.Porous core-shell platinum-silver nanocatalyst for the electrooxidation of methanol[J].Electroanalysis,2015,27(1):135.
[46] Yang Y,Chen Z,Wu X,Zhang X,Yuan G.Nanoporous cellulose membrane doped with silver for continuous catalytic decolorization of organic dyes[J].Cellulose,2018,25(4):1.
[47] Bian W W,Liu Z,Lian G,Wang L,Wang Q L,Zhan J H.High reliable and robust ultrathin-layer gold coating porous silver substrate via galvanic-free deposition for solid phase microextraction coupled with surfaceenhanced Raman spectroscopy[J].Analytica Chimica Acta,2017,94:56.
[48] Zabihzadeh S,Petegem S V,Holler M,Diaz A,Duarte L I,Swygenhoven H V.Defonnation behavior of nanoporous polycrystalline silver.Part I:microstructure and mechanical properties[J].Acta Materialia,2017,131:467.
[49] Li Z,Lu X,Li B,Bai L,Wang Q.Research on electrochemical oxidation of formaldehyde on the nanoporous silver electrode in alkaline solution[J].ECS Electrochemistry Letters,2015,4(6):24.
[50] Xu M,Sui Y,Wang C,Zhou B,Wei Y J,Z B.Design of porous Ag platelet structures with tuneable porosity and highly catalytic activity[J].Journal of Materials Chemistiy A,2015,3(44):22339.
[51] Zhou Y,Tang L,Zeng G,Zhu J,Dong H R,Zhang Y,Xie X,Wang J,Deng Y C.A novel biosensor for silver(Ⅰ)ion detection based on nanoporous gold and duplexlike DNA scaffolds with anionic intercalator[J].RSC Advances,2015,5(85):69738.