文章编号:1004-0609(2016)-05-1027-07
碳辅助氢还原制备纳米钨粉的工艺及机理
吴 桐,唐建成,叶 楠,卓海鸥,薛滢妤,周旭升
(南昌大学 材料科学与工程学院,南昌 330031)
摘 要:
摘 要:以偏钨酸铵和葡萄糖为原料,采用碳辅助氢气还原的方法制备出纳米W粉,并研究W、C摩尔比和还原工艺对W粉的平均粒度和残碳量的影响。结果表明:还原产物、W粉的平均粒度和残C量与还原温度和W、C摩尔比密切相关,而当还原时间超过1 h后,还原时间对W粉的平均粒度和残C量没有明显的影响。还原产物决定于还原温度,W、C摩尔比为1:2.6的前驱体在700、750、800 ℃还原以后得到的产物分别为W+WO2、W+W2C+WC和W。W粉的粒度随着W、C摩尔比由1:2.0增加到1:3.0而由98 nm减小到42 nm,但W粉的残C量在W、C摩尔比小于1:2.6时都在0.05%(质量分数)以下,W、C摩尔比继续增加时,W粉的残C量显著增加。W、C摩尔比为1:2.6的原料经800 ℃还原1 h后得到无明显团聚的W粉,其平均粒度为46 nm,残C量为0.05% (质量分数)。
关键词:
中图分类号:TF123.7 文献标志码:A
W具有高密度、高熔点、高硬度、高耐磨性、低热膨胀系数、优异的导电导热性能以及良好的耐腐蚀性而在高密度合金和硬质合金等领域得到广泛的应 用[1-2],制备出均匀细小无团聚的W粉是改善钨基高密度合金和WC-Co硬质合金的关键[3-4]。
目前,W粉的制备方法主要有氧化钨氢还原法[5-7]和氧化W、C还原法[8-19]。氧化钨氢还原具有反应温度低和W粉纯度高等优点,但氢还原时会产生大量的水蒸汽,而水蒸汽会加速氧化钨的挥发,W粉会通过“挥发-沉积”的过程快速长大,从而难以制备出纳米W粉[10-13]。傅小明[14]以仲钨酸铵为原料还原出的球形亚微米W粉粒度约为100 nm,WU[15]即使利用纳米针紫钨为原料,利用氧化钨氢还原法也难以得到100 nm以下的W粉。氧化W、C还原尽管可以消除水蒸汽对氧化钨挥发的影响,但C还原所需要的高温会加速W粉的长大,SWIFT等[16]通过还原包覆了碳的WO3前驱体制得纳米W粉也无法解决此难题。因此,克服氢还原和C还原制备W粉的不足,开发出适用于制备性能优良的纳米W粉的新工艺是硬质合金合金领域目前的一个研究难点。
为了克服氧化钨氢还原和C还原制备W粉时存在的问题,本文作者将氢还原与碳还原两种制备方法相结合,利用C与水蒸汽在一定温度下会生成一氧化碳和氢的特性,消除水蒸气对氧化钨挥发的影响,采用C辅助氢还原的方法制备出均匀细小无团聚的纳米W粉,并研究还原温度、W、C摩尔比和还原时间对还原产物、纳米W粉粒度和纯度的影响[17]。
1 实验
本实验按W、C摩尔比1:2.0~1:3.0配置偏钨酸铵与葡萄糖的水溶液,溶液蒸发干燥后在氮气气氛下经400 ℃煅烧2 h后得到前驱体粉末,前驱体粉末在管式气氛炉中分别在700、750、800 ℃保温30~150 min进行碳辅助氢还原,待降至400 ℃时通入氩气进行钝化处理。
利用X射线衍射仪分析样品的物相,利用扫描电子显微镜(SEM)观察样品的显微组织,对SEM像进行2D-晶粒尺寸分析,在每个样品不同视场内取300个W晶粒,采用直线截距法测量晶粒尺寸[18],利用化学分析方法测量样品的残C量。
2 结果与讨论
2.1 还原温度对还原产物的影响
偏钨酸铵粉末与葡萄糖以W、C摩尔比1:2.6的比例混合煅烧后制备的前驱体以及前驱体分别在700、750、800 ℃下还原1 h的产物的XRD谱如图1所示。由图1可以看出,前驱体的衍射峰为漫散峰,说明前驱体由非晶态的钨氧化物组成。前驱体经700 ℃还原1 h后非晶态物质完全消失,此时主要由WO2和W组成,这说明前驱体的还原反应在700 ℃已经开始,但还原反应不充分,产物中还有氧化钨存在。当还原温度升高到750 ℃时,还原产物的物相发生了显著的变化,样品中除了有W存在外,还有W2C和WC存在,这说明前驱体经750 ℃还原1 h后除了发生还原反应外,还发生碳化反应。当前驱体经800 ℃还原1 h后,样品中的W2C和WC完全消失,得到纯净的W粉,而且W的衍射峰有明显的宽化,说明此时W粉的粒度有所细化。
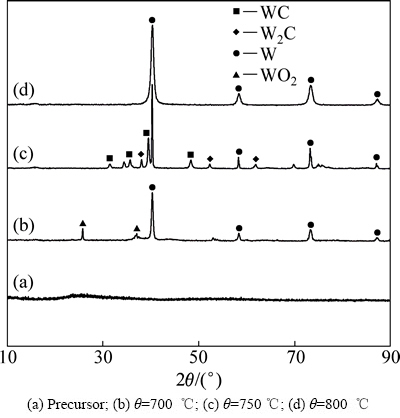
图1 W、C摩尔比为1:2.6的前驱体及前驱体在不同温度下还原1 h后产物的XRD谱
Fig. 1 XRD patterns of precursor with tungsten carbon molar ratio of 1:2.6 and products prepared by carbon assisting hydrogen reduction of precursor at different temperatures for 1 h
偏钨酸铵粉末与葡萄糖以W、C摩尔比1:2.6的比例混合煅烧后制备的前驱体以及前驱体分别在700、750、800 ℃下还原1 h后产物的SEM像如图2所示。由图2(a)中可以看出,前驱体粉末呈现松散的不规则形状。不同还原温度条件下得到的产物颗粒形貌有明显区别。经700 ℃还原1h制备出的粉末的SEM像如图2(b)所示,颗粒大部分呈絮状,有部分颗粒表面部形核成近球形的颗粒,仍保留了前驱体松散的状态。经750 ℃还原1 h制备出的粉末的SEM像如图2(c)所示,此时,仍存在大量的絮状颗粒,在絮状颗粒的表面部已经大量开始形核生成近球形的W2C/WC颗粒,但生成的W2C/WC颗粒有明显的团聚现象,且W2C/WC颗粒的尺寸不均匀。经800 ℃还原1 h制备出的粉末的SEM像如图2(d)所示,得到的W粉呈近球形,表面光滑,颗粒细小而均匀,而且没有明显的团聚现象,经测量,平均粒径约为46 nm。
当没有碳存在时,氧化钨氢还原法制备W粉是一个渐变的过程,由WO3一步步还原成WO2.9、WO2.7、WO2.0,最后得到纯净的W粉,每一步的反应方程式分别为
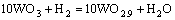 (1)
(1)
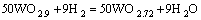 (2)
(2)
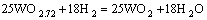 (3)
(3)
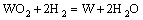 (4)
(4)
由以上4个反应方程式可以看出,氧化钨氢还原制备W粉时,有大量的水蒸汽产生。W的氧化物具有挥发性,W粉在氢还原过程中的长大是通过氧化钨的“挥发-沉积”来实现的。而且氧化钨氢还原过程中产生的水蒸汽会促进氧化钨的挥发,并与氧化钨生成水合氧化钨WO2(OH)2,继而与H2发生均相还原反应,还原产物会沉积在已经形核的W粉上,从而致使W粉长大[19]。水蒸汽的存在会促进W粉的长大,温度越高,水蒸气浓度越高,W粉长大越明显,这是氢还原难以制备纳米W粉的难点。
当有C存在时,碳辅助氢还原制备W粉时,除了有氢还原制备W粉的4个反应外,C会与H2O发生反应,其化学反应方程为
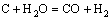 (5)
(5)
代入热力学数据[20],其ΔG=131307-133.65T,由此可以计算出此反应的ΔG在温度高于709 ℃时为负,即此反应在709 ℃以上会发生。因此,当还原温度低于709 ℃时,C对W粉的还原没有作用,当还原温度大于709 ℃时,C能与H2O反应生成CO和H2,从而可以消除水蒸汽对氧化钨挥发的影响,这与实验结果相符。

图2 W、C摩尔比为1:2.6的前驱体在不同温度下还原1 h产物的SEM像
Fig. 2 SEM images of products prepared by carbon assisting hydrogen reduction of precursor with tungsten carbon molar ratio of 1:2.6 at different temperatures for 1 h
而C与H2O反应生成的CO会与W发生反应生成W2C,进而会生成WC,其反应的方程式为
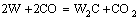 (6)
(6)
代入热力学数据,其ΔG=-198827+190.606T,由此可以计算出此反应的ΔG在温度低于770 ℃时为负,即此反应在770 ℃以下会发生。当还原温度在770 ℃以下时,生成的CO会与钨反应生成W2C/WC,还原温度只有在770 ℃以上时碳辅助氢还原才能制备出纯W粉,这与实验结果相符。
2.2 还原时间对纳米W粉粒度的影响
偏钨酸铵粉末与葡萄糖以W、C摩尔比1:2.6的比例混合煅烧后制备的前驱体在800 ℃下分别还原30、60、90、120、150 min的产物的XRD谱如图3所示。从图3可以看出,还原时间为30 min时,氧化钨的还原并不充分,产物中存在部分WO2;当还原时间超过60 min时,还原产物全部转化为W。
图4所示为偏钨酸铵粉末与葡萄糖以W、C摩尔比1:2.6的比例混合煅烧后制备的前驱体在800 ℃下分别还原30、60、90、120、150 min产物的SEM像。从图4(a)可以看出,当还原时间为30 min时,还原产物团聚严重,大颗粒氧化钨上沉积了还原的小颗粒W粉,W粉颗粒形貌及大小不均匀,这与XRD的检测结果相符。从图4(b)~(e)可以看出,当还原时间超过60 min时,氧化钨已经完全还原成W粉,W粉的形貌、粒度以及分散性均无明显变化,均呈近球形,表面光滑,颗粒细小而均匀,分散性良好。
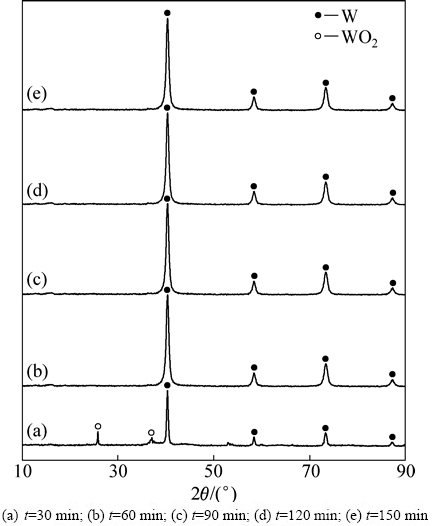
图3 W、C摩尔比为1:2.6的前驱体在800 ℃下还原不同时间的产物的XRD谱
Fig. 3 XRD patterns of products prepared by carbon assisting hydrogen reduction of precursor with tungsten carbon molar ratio of 1:2.6 at 800 ℃ for different reduction time
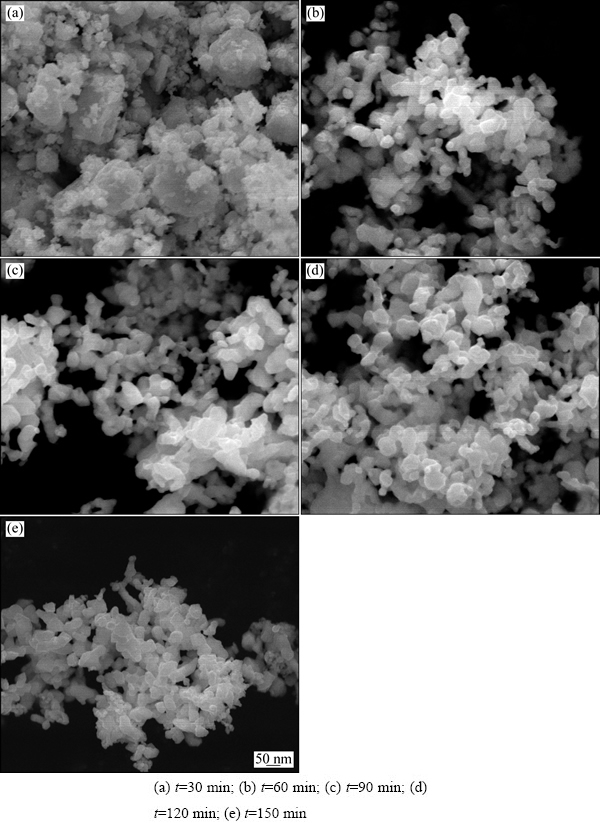
图4 W、C摩尔比为1:2.6的前驱体在800 ℃下还原不同时间后产物的SEM像
Fig. 4 SEM images of products prepared by carbon assisting hydrogen reduction of precursor with tungsten carbon molar ratio of 1:2.6 at 800 ℃ for different reduction time
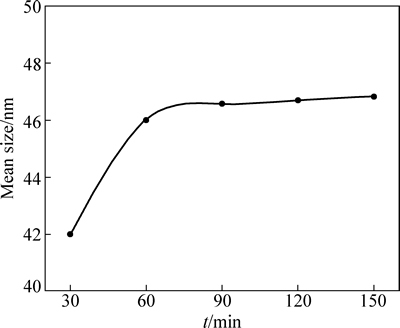
图5 还原时间对W粉平均粒度的影响
Fig. 5 Effects of reduction time on mean size of tungsten powders
对SEM像进行2D-晶粒尺寸分析,以粒度d为纵坐标,还原时间t为横坐标得到W粉平均粒度与还原时间的关系图如图5所示。由图5可以看出,当还原时间低于60 min,W粉粒度呈增大的趋势;当还原时间超过60 min,W粉粒度保持在46~47 nm的范围内,还原时间的延长对W粉粒度的影响不明显。出现这种现象的原因是当还原时间低于60 min时,W粉晶粒未发育完全,所以W粉粒度变化较为明显;还原时间超过60 min时,氧化钨被完全还原,延长还原时间只会增加水蒸汽的浓度,促进还原过程中的气相迁移,但C会消耗生成的水蒸汽,还原过程中水蒸汽的浓度始终保持在较低的范围内,从而抑制细小的W粉沉积在较大的W粉颗粒上,因此,即使还原时间延长,W粉的粒度没有显著变化。
2.3 W、C摩尔比对纳米W粉粒度与残C量的影响
前驱体经合适的还原工艺还原后可以得到W粉。当还原时间超过1 h,还原时间对W粉的平均粒度和含C量的影响不明显,W粉的平均粒度和含C量与W、C摩尔比密切相关。
当还原温度为800 ℃,还原时间为1 h时,W、C摩尔比对W粉粒度和残C量的影响的如图6所示。由图6可以看出,W、C摩尔比会显著影响纳米W粉的粒度和残C量。当W、C摩尔比小于1:2.6时,残C量都小于0.05%(质量分数);但W、C摩尔比大于1:2.6时,W粉中的残C量显著增加。当W、C摩尔比较低时,C会全部与水蒸汽发生反应,在W粉中不会残留,还原出纯度较高的W粉。而当W、C摩尔大于1:2.6时,由于C并不会参与氧化钨的还原反应,也不会发生碳化发应,只能以游离的形式存在于W粉中,从而影响W粉的纯度,发生残C量显著升高的现象。
W粉粒度随着配碳量的增加逐渐减小,并且粒度下降的幅度逐渐减慢,当W、C摩尔比由1:2.0升高到1:3.0,W粉的粒度由98 nm降到42 nm。还原过程中,C会消耗还原反应中生成的水蒸汽,W、C摩尔比越高,消耗的水蒸汽越多,对氧化钨挥发的抑制越显著,W粉的粒度越细;当W、C摩尔比较低时,由于反应中产生的水蒸汽的浓度较高,少量的C难以显著降低水蒸汽的浓度,抑制氧化钨的效果会减弱,W粉粒度下降的幅度因而减慢。
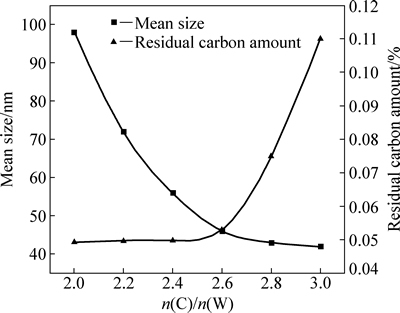
图6 W、C摩尔比对W粉粒度和残C量的影响
Fig. 6 Effects of tungsten carbon molar ratio on mean size and residual carbon amount of tungsten powders
以偏钨酸铵和葡萄糖为原料,采用碳辅助氢气还原的方法可以制备出纳米W粉,W粉的平均粒度和残C量与W、C摩尔比和还原温度密切相关,W、C摩尔比为1:2.6的原料经800 ℃还原1 h后,可以得到无明显团聚的W粉,其平均粒度为46 nm,残留C含量为0.05%。
3 结论
1) 以偏钨酸铵和葡萄糖为原料,采用碳辅助氢气还原的方法制备出纳米W粉,还原产物、W粉的平均粒度和残C量与还原温度和W、C摩尔比密切相关,还原时间超过1 h对W粉的平均粒度和残C量的影响不明显。
2) 当还原温度为700、750和800 ℃时,还原产物分别为W+WO2、W+W2C+WC和W。热力学分析表明:当还原温度低于709 ℃时,C对W粉的还原没有作用;当还原温度大于709 ℃时,C能与H2O反应生成CO和H2,从而可以消除水蒸汽对氧化钨挥发的影响;当还原在770 ℃以下时,生成的CO会与W反应生成W2C/WC,还原温度只有在770 ℃以上时,C辅助氢还原才能制备出纯W粉。
3) W粉的粒度随着W、C摩尔比由1:2.0增加到1:3.0由98 nm减小到42 nm,而W粉的残C量在W、C摩尔比小于1:2.6时都在0.05%(质量分数)以下,但W、C摩尔比继续增加时,W粉的含残C量显著增加。
4) W、C摩尔比为1:2.6的原料经800 ℃还原1 h后,得到分散性能良好的W粉,其平均粒度为46 nm,残C量为0.05%(质量分数)。
REFERENCES
[1] 吴冲浒, 聂洪波, 肖满斗. 中国超细硬质合金及原料制备技术进展[J]. 中国材料进展, 2012, 31(4): 39-46.
WU Chong-hu, NIE Hong-bo, XIAO Man-dou. Development on preparation technology of ultrafine-grained cemented carbides and their raw materials in China[J]. Materials China, 2012, 31(4): 39-46.
[2] FANG Z Z, WU W, RYU T, HWANG K S, SOHN H Y. Synthesis, sintering, and mechanical properties of nanocrystalline cemented tungsten carbide-a review[J]. International Journal of Refractory Metals and Hard Materials, 2009, 27(2): 288-299.
[3] ZHANG H B, BAI L Y, HU P, YUAN F L, Li J L. Single-step pathway for the synthesis of tungsten nanosized powders by RF induction thermal plasma[J]. International Journal of Refractory Metals and Hard Materials, 2012, 31: 33-38.
[4] BONACHE V, SALVADOR M D. Microstructural control of ultrafine and nanocrystalline WC-12Co-VC/Cr3C2 mixture by spark plasma sintering[J]. Ceramics International, 2011, 37(3): 1139.
[5] MOIYSOHI Y. The preparation and characterization of ultrafine tungsten powder[J]. Journal of Materials Science Letters, 1997, 16: 347-349.
[6] SCHUBERT W D, LASSNER E. Production and characterization of hydrogen-reduced submicron tungsten powders. PartⅡ: Controlled decomposition of APT and hydrogen reduction of the oxides[J]. International Journal of Refractory Metals and Hard Materials, 1991, 10(4): 171-183.
[7] 宋志华, 郭亨群, 吴冲浒, 吴其山. 以紫钨为原料制备纳米钨粉体的研究[J]. 稀有金属材料与工程, 2011, 40(12): 2216-2220.
SONG Zhi-hua, GUO Heng-qun, WU Chong-hu, WU Qi-shan. Preparation of nano-tungsten powder from WO2.72[J]. Rare Metal Materials and Engineering, 2011, 40(12): 2216-2220.
[8] FARID A, ISLAM S H, ASKARI S J, TIAN J J, GUO Shi-ju. Effect of WC particle size on the microstructure, mechanical properties and fracture behavior of WC-(W, Ti, Ta) C-6wt% Co cemented carbides[J]. International Journal of Refractory Metals and Hard Materials, 2007, 25(5/6): 405-410.
[9] ZHANG J X. Effect of reduction and carburization temperature of tungsten powder on WC-phase substructure and mechanical properties of WC-Co cemented carbide[J]. International Journal of Refractory Metals and Hard Materials, 1988, 7(4): 224-228.
[10] 王 岗, 李海华, 黄忠伟, 陈美华, 王庆康, 钟伟良. 蓝钨与紫钨氢还原法生产超细钨粉的比较[J]. 稀有金属材料与工程, 2009, 38(S1): 548-552.
WANG Gang, LI Hai-hua, HUANG Zhong-wei, CHEN Mei-hua, WANG Qing-kang, ZHONG Wei-liang. Comparison of ultrafine tungsten powder produced by hydrogen reduction of blue tungsten oxide and violet tungsten oxide[J]. Rare Metal Materials and Engineering, 2009, 38(S1): 548-552.
[11] 郭 峰. 氧化钨氢气还原制备超细纳米钨粉的研究现状[J]. 粉末冶金材料科学与工程, 2007, 12(4): 205-210.
GUO Feng. Research status of preparing ultrafine tungsten powders by H2-reducing tungsten oxide[J]. Materials Science and Engineering of Powder Metallurgy, 2007, 12(4): 205-210.
[12] 雷纯鹏, 唐建成, 刘 刚, 刘 原. 氧化钨还原过程中的形貌结构遗传特性研究[J]. 稀有金属与硬质合金, 2012, 40(5): 1-6.
LEI Chun-peng, TANG Jian-cheng, LIU Gang, LIU Yuan. Reasearch on the genetic characteristics of morphology structure of tungsten oxide during the reduction process[J]. Rare Metals and Cemented Carbides, 2012, 40(5): 1-6.
[13] RICCERI R, MATTEAZZI P. A study of formation of nanometric W by room temperature mechanosynthesis[J]. Journal of Alloys and Compounds, 2003, 358(1/2): 71-75.
[14] 傅小明. 仲钨酸铵循环氧化还原法制备亚微米球形钨粉[J]. 稀有金属材料与工程, 2010, 39(S1): 468-471.
FU Xiao-ming. Submicron spherical tungsten powder prepared with ammonium paratungstate through the circulatory oxidization-reduction method[J]. Rare Metal Materials and Engineering, 2010, 39(S1): 468-471.
[15] WU C H. Preparation of ultrafine tungsten powders by in-suit reduction of nano-needle violet tungsten oxide[J]. International Journal of Refractory Metals and Hard Materials, 2011, 29(6): 686-691.
[16] SWIFT G A, KOC R. Tungsten powder from carbon coated WO3 precursors[J]. Journal of materials science, 2001, 36(4): 803-806.
[17] ZHANG L, CHEN S, CHENG X, WU H P, MA Y, XIONG X J. Effects of cubic carbides and La addition on WC grain morphology, hardness and toughness of WC-Co alloys[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(7): 1680-1685.
[18] 廖寄乔, 黄伯云, 王 华. 超细钨粉的粒度表征[J]. 中国有色金属学报, 2002, 12(3): 448-453.
LIAO Ji-qiao, HUANG Bai-yun, WANG Hua. Characterization of particle size of ultrafine tungsten powder[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(3): 448-453.
[19] WU X W, LUO J S, LU B Z, XIE C H, PI Z M, HU M Z, XU Tao, WU G G, YU Z M, YI D Q. Crystal growth of tungsten during hydrogen reduction of tungsten oxide at high temperature[J]. Transactions of Nonferrous Metals Society of China, 2009, 19(S3): s785-s789.
[20] 叶大伦. 实用无机物热力学数据手册[M]. 北京: 冶金工业出版社, 2002: 26-1151.
YE Da-lun. Thermodynamic data directory of inorganic matter[M]. Beijing: Metallurgical Industry Press, 2002: 26-1151.
Preparation technology and mechanism of tungsten nano-powders by carbon assisting hydrogen reduction
WU Tong, TANG Jian-cheng, YE Nan, ZHUO Hai-ou, XUE Ying-yu, ZHOU Xu-sheng
(School of Materials Science and Engineering, Nanchang University, Nanchang 330031, China)
Abstract: Tungsten nano-powders were prepared by carbon assisting hydrogen reduction using ammonium metatungstate (AMT) and glucose as raw materials. The effects of tungsten carbon molar ratio and reduction technology on the mean particle size and residual carbon amount of tungsten powders were investigated. The results show that the reduction products, the mean particle size and residual carbon amount of tungsten powders depend on the reduction temperature and tungsten carbon molar ratio, there is not apparent effect of reduction duration on the mean particle size and residual carbon amount of tungsten powders when reduction duration is more than 1 h. The reduction products depend on reduction temperature. When the reduction temperature is 700, 750 and 800 ℃, the reduction products are W+WO2, W+W2C+WC and W, respectively. When the tungsten carbon molar ratio increases from 1:2.0 to 1:3.0, the mean particle size of tungsten powders decreases from 98 nm to 42 nm. The residual carbon amount of tungsten powders is less than 0.05% (mass fraction) when the tungsten carbon molar ratio is less than 1:2.6, but the continuous increase of the tungsten carbon molar ratio results in the significant increase of the residual carbon amount of tungsten powders. Tungsten nano-powders without obvious agglomeration are prepared by the carbon assisting hydrogen reduction of the precursor with tungsten carbon molar ratio of 1:2.6 at 800 ℃ for 1 h. The mean size and residual carbon amount of tungsten nano-powders are 46 nm and 0.05% (mass fraction), respectively.
Key words: tungsten nano-powder; carbon assisting hydrogen reduction; reduction temperature; reduction time
Foundation item: Project (51271090, 51364036, 51471083) supported by the Natural Science Foundation of China
Received date: 2014-07-21; Accepted date: 2016-01-10
Corresponding author: TANG Jian-cheng; Tel: +86-791-83969559; E-mail: tangjiancheng@ncu.edu.cn
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(51271090,51364036,51471083)
收稿日期:2014-07-21;修订日期:2016-01-10
通信作者:唐建成,教授,博士;电话:0791-83969559;E-mail:tangjiancheng@ncu.edu.cn
[1] 吴冲浒, 聂洪波, 肖满斗. 中国超细硬质合金及原料制备技术进展[J]. 中国材料进展, 2012, 31(4): 39-46.
[7] 宋志华, 郭亨群, 吴冲浒, 吴其山. 以紫钨为原料制备纳米钨粉体的研究[J]. 稀有金属材料与工程, 2011, 40(12): 2216-2220.
[10] 王 岗, 李海华, 黄忠伟, 陈美华, 王庆康, 钟伟良. 蓝钨与紫钨氢还原法生产超细钨粉的比较[J]. 稀有金属材料与工程, 2009, 38(S1): 548-552.
[11] 郭 峰. 氧化钨氢气还原制备超细纳米钨粉的研究现状[J]. 粉末冶金材料科学与工程, 2007, 12(4): 205-210.
[12] 雷纯鹏, 唐建成, 刘 刚, 刘 原. 氧化钨还原过程中的形貌结构遗传特性研究[J]. 稀有金属与硬质合金, 2012, 40(5): 1-6.
[14] 傅小明. 仲钨酸铵循环氧化还原法制备亚微米球形钨粉[J]. 稀有金属材料与工程, 2010, 39(S1): 468-471.
[18] 廖寄乔, 黄伯云, 王 华. 超细钨粉的粒度表征[J]. 中国有色金属学报, 2002, 12(3): 448-453.


