
Recovery of oxalic acid from mother-liquor containing hydrochloric acid and cobalt by solvent extraction with P350
TIAN Qing-hua(田庆华), GUO Xue-yi(郭学益), LI Zhi-hai(李治海)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract:
The solvent of P350 was applied to extract and separate the oxalic acid from the mother-liquor originated from the precipitation of cobalt by oxalic acid, and its extraction mechanism was deduced. Some factors, including the concentration of P350, the concentration of hydrochloric acid and the concentration of oxalic acid were investigated to determine the best distribution coefficient of the oxalic acid. In the case of phase ratio (O/A ) at 2.0, the extraction of the oxalic acid was more than 95% and its concentration in the extraction raffinate was lower than 0.004 0 mol/L after six-stage counter-current extraction. While the phase ratio (O/A) of the stripping was at 1.0, the recovery of oxalic acid attained more than 95% after ten-stage counter-current stripping.
Key words:
P350; counter-current; solvent extraction; oxalic acid; stripping;
____________________________________________________________________________________________
1 Introduction
At present, precipitation process with ammonium oxalate as the precipitant is the main supporting technique for producing the cobalt oxalate in industry, which consumes a lot of ammonia. A great deal of ammonia-containing wastewater will be produced in this process[1]. The non-treatment wastewater is directly discharged, thereby not only bringing about material loss but also polluting the environment[2]. A new method to produce cobalt oxalate by oxalic acid without ammonia involved was presented[3]. In this process, huge mount of hydrochloric acid was produced, which can be used to dissolve the raw materials. However, to increase the precipitation efficiency, excess oxalic acid is added and results in excess oxalic acid left in the mother-liquor. If the mother-liquor was used to dissolve the raw material directly, it will result in the precipitation of metal oxalate and decrease the leaching efficiency of the valuable metals. So, the oxalic acid needs to be separated from mother-liquor containing hydrochloric acid and cobalt.
The main methods to deal with the oxalic acid include oxidation method[4-6], precipitation method [7-9] and solvent extraction[10-12]. The mother-liquor originated from the precipitation of cobalt by oxalic acid contains excess oxalic acid, hydrochloric acid and some cobalt chloride. The precipitation method and oxidation method have some problems, such as bringing impurity to the solution and consuming hydrochloric acid[13]. So, the optimum method to separate the oxalic acid from the mother liquor is solvent extraction.
A novel method of selective extraction of oxalic acid from the mother-liquor by the complexing agent P350 was proposed, which was used in recycling the mother-liquor after precipitating cobalt effectively. The oxalic acid separated from the mother-liquor can be reused in precipitation process. The phase ratio and the stages of counter-current extraction were determined, and the extracting ratio of the oxalic acid by P350 and the extracting mechanism were discussed, respectively, by the method of slope and the infra-red absorption spectroscopy.
2 Experimental
The mother-liquor containing oxalic acid, hydrochloric acid and cobalt chloride was prepared by precipitating cobalt chloride with oxalic acid. All chemicals were of analytical purity from Shanghai Chemical Reagents Co., China.
P350 and the oxalic acid with phase proportion of 1:1 were added in a 150 mL separation funnel and oscillated completely. The optimum concentration of P350 was determined by measuring the distributing proportion and the liquidity of the mixture solution. P350 with optimum concentration and oxalic acid was added in the funnel again and oscillated completely in the different conditions. The concentration of hydrochloric acid was determined by NaOH, and the concentration of oxalic acid was determined by (NH4)2Ce(NO3)6.
3 Results and discussion
3.1 Composition of extracted complex
The slope method was used to study the composition of the extracted complex. When the extraction ratio of the oxalic acid by P350 is fixed to be 1/n, the equation when the extraction reaction is balanced as:
HOOC—COOH+nP350=nP350? HOOC—COOH (1)
The equilibrium extraction constant is
![]() (2)
(2)
The equilibrium distribution coefficient is
![]() (3)
(3)
In the aqueous solution, the ionization equations of the oxalic acid are
![]() (4)
(4)
![]() (5)
(5)
![]() (6)
(6)
![]() (7)
(7)
According to Eqs.(1)-(7), the equilibrium distribution coefficient (D) is
![]() (8)
(8)
Because of diluted concentration of the oxalic acid in the solution, the concentration of P350 could be supposed to keep constant. Then, the formula obtained is
lgD=nlg[P350]+A (9)
where A is a constant.
Fig.1 shows the curve of lgD-lg[P350] by changing the concentration of P350. The concentrations of the hydrochloric acid were 0.5 and 1.0 mol/L, respectively.
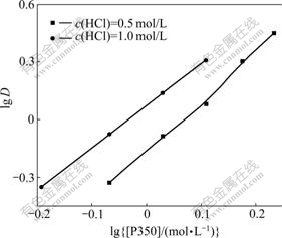
Fig.1 Relation between lg[P350] and lgD at different concentrations of HCl
From the slope of the two lines shown in Fig.1, the extraction ratio is about 2, so the composition of the extracted complex of the oxalic acid by P350 is
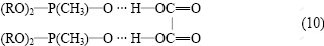
3.2 Extraction mechanism
P350 is a neutral phosphide solvent and its function group is P=O. Many researchers studied the mechanism of extracting the carboxylic acid by the neutral phosphide extraction solvent[14]. The associating mechanism of the oxalic acid and P350 could be judged by the change of infra-red absorption spectroscopy before and after extracting the oxalic acid by P350, which is shown in Fig.2.
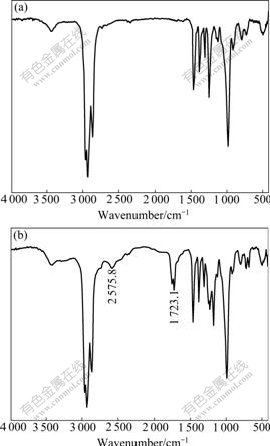
Fig.2 IR spectra before and after extraction: (a) Free organic phase; (b) Organic phase with oxalic acid
From Fig.2, there were two more wave crest after extracting the oxalic acid by P350. In the point of wave- number of 2 575.8 cm-1, the broad diagnostic crest P—OH could be seen. The other appears are the point of wavenumber of ![]() and it is the C=O diagnostic crest. Simultaneously, the P=O diagnostic crest moved apparently from 1 248.1 cm-1 to 1 226.2 cm-1, which proved the mechanism of the complex of the oxalic acid and P350 was hydrogen bonding[15].
and it is the C=O diagnostic crest. Simultaneously, the P=O diagnostic crest moved apparently from 1 248.1 cm-1 to 1 226.2 cm-1, which proved the mechanism of the complex of the oxalic acid and P350 was hydrogen bonding[15].
3.3 Composition of P350 and sulfonated kerosene
The composition of P350 and the sulfonated kerosene was decided by distribution coefficient and viscosity. On the condition of the phase ratio of 1.0, the concentration of oxalic acid in the aqueous solution of 0.23 mol/L, the temperature of 30 ℃, the distribution coefficient of extracting the oxalic acid by P350 in different volume fractions is shown in Fig.3.
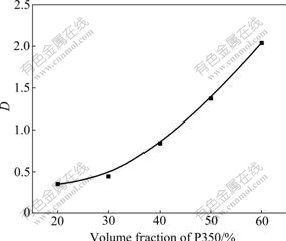
Fig.3 Relationship between distribution coefficient and volume fraction of P350
Fig.3 indicted that the distribution coefficient increases with the increase of the volume fraction of P350. The increasing trend was obvious in the case of high content of P350. At the same time, the viscosity increased and it was more difficult to separate the different phases. When the volume fraction of P350 is more than 70%, the separation speed of different phases slows down, and the third phase (emulsification) appears in which the aqueous phase is separated from the organic phase 24 h later. When P350 volume fraction was 50%- 60%, besides insuring the high distribution coefficient, the speed of separating the different phases was ensured. Thus, the volume fraction of P350 was determined to be 50% in the latter experiments.
3.4 Effect of concentration of H+ on distribution coefficient
When the oxalic acid was dissolved in water, three kinds of ions appeared in the aqueous solution: C2O42-, HC2O4- and H2C2O4. Increasing the concentration of H+ will promote the reaction as follows:
H++C2O42- HC2O4- (11)
HC2O4- (11)
H++HC2O4- H2C2O4 (12)
H2C2O4 (12)
Based on the extraction mechanism, oxalic acid was associated with P350 by hydrogen bond. As a result, in the aqueous phase, increasing the concentration of the oxalic acid helped it to associate with P350. On the condition of O/A=1.0, the concentration of the oxalic acid of 0.24 mol/L and the temperature of 30 ℃, with increasing the concentration of HCl, the molecule of the oxalic acid was easily extracted by P350, so the distribution coefficient(D) increased. In the case of increasing the concentration of HCl to 1.7 mol/L, D value reached the maximum (about 1.8), which could explain that in high acidity, the reaction keeps constant and the concentration of the oxalic acid in the aqueous solution increased to the maximum.
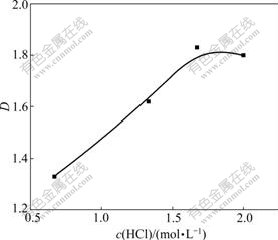
Fig.4 Effect of concentration of H+ on distribution coefficient
3.5 Effect of oxalic acid concentration on distribution coefficient
The concentration of the oxalic acid is an important factor affecting the distribution coefficient. Fig.5 showed that the distribution coefficient decreased with the increase of the concentration of oxalic acid. When the concentration of the oxalic acid was low, most of the oxalic acid in the aqueous phase was extracted into the organic phase and associated with P350 by hydrogen bond, so the distribution coefficient was high. Otherwise, the proportion of the oxalic acid associated with P350 decreased, and more oxalic acid dissolved in the aqueous solution than in the organic phase. When the concentration of the oxalic acid increased to a certain value, most P350 in the organic phase extracted the oxalic acid, which meant the extraction by P350 was saturated and the distribution coefficient was small.
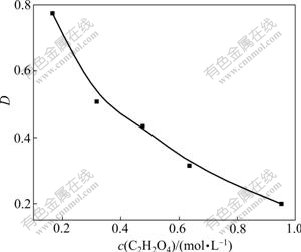
Fig.5 Effect of oxalic acid concentration on distribution coefficient
The counter-current extraction was easily destroyed even in the case of low or high concentration of the oxalic acid. In the case of high concentration of the oxalic acid, in order to meet the requirement, more extracting stages were needed, which resulted in long flow sheet and hard control. On the other hand, larger phase ratio was needed, which brought in emulsification and destroyed the normal extraction. In the case of low concentration of the oxalic acid, though less extracting stages were needed, poorer aqueous phase brought in the emulsification and breakage of the normal extraction.
3.6 Counter-current extraction
The optimum conditions were obtained from above experiments and shown below: the volume fraction of P350 was 50%; the concentration of the hydrochloric acid was 1.7 mol/L and the concentration of the oxalic acid was 0.2-0.4 mol/L. The main components in the mother liquor from the precipitation of the cobalt by the oxalic acid were: the concentration of Co2+ of about 1 g/L, the concentration of HCl and H2C2O4 of about 1.0 and 0.25 mol/L, respectively.
In the experiments of counter-current extraction, the content of P350 was determined to be 50% in the condition of the concentration of oxalic acid of 0.24 mol/L, the concentration of hydrochloric acid of 1.0 mol/L and temperature of 30 ℃. The extracting stages were determined by the equilibrium isothermal method, proved by the method of the simulation again, to meet the requirement, in which the concentration of the oxalic acid should be decreased to 0.004 0 mol/L.
1) Equilibrium isothermal method
The equilibrium isothermal and operating lines with the phase ratio O/A of 1.0, 1.5 and 2.0 are shown in Figs.6-8. The formula of the operating line are
![]() (13)
(13)
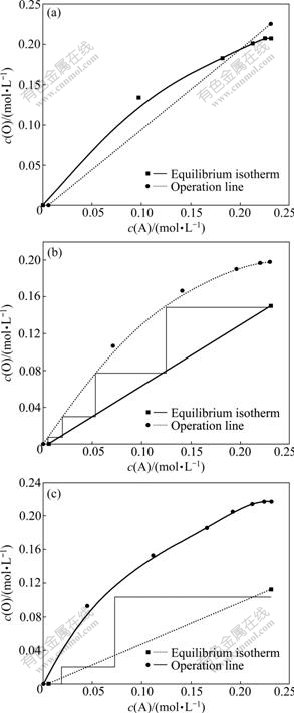
Fig.6 Equilibrium isotherms at different O/A ratio: (a) 1.0; (b) 1.5; (c) 2.0
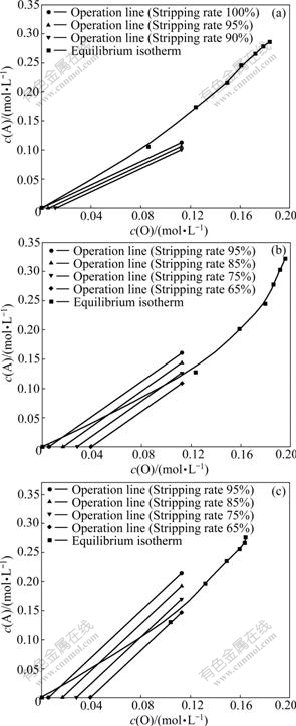
Fig.7 Stripping equilibrium isotherms at different O/A ratio: (a) 1.0; (b) 1.5; (c) 2.0
where y1 is the equilibrium concentration in the exit of the oxalic acid inorganic phase; yn+1 is the equilibrium concentration in the entrance of the oxalic acid in organic phase; x0 is the equilibrium concentration in the entrance of the oxalic acid in aqueous phase; xn is the equilibrium concentration in the exit of the oxalic acid in aqueous phase.
From Fig.6(a), in the case of O/A=1.0, there was a point of intersection between the equilibrium isothermal and the operation lines, which proved that on the condition of O/A=1.0, there is no way to meet the requirement other than diluting the concentration of the oxalic acid in the mother liquor. In the case of the phase ratio of O/A>1.5, the requirement could be met and more stages were needed if the phase ratio was decreased. The numbers of the stage were 4 and 3 when the phase ratio were 1.5 and 2.0, as shown in Figs.6(b) and (c).
2) Simulation testing method
According to the phase ratio and the number of stage from the method of the equilibrium isothermal, the 3-stage counter-current extraction was simulated when O/A=2.0. The results are shown in Table 1.
Table 1 Results of counter-current multi-stage simulation testing
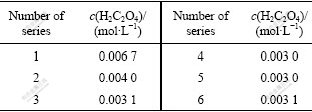
Commonly, the number of stages was 2-3 in industry, which is more than the formulation experiment, so the final number of the stages was determined to be 6.
3.7 Reactivation of P350
The oxalic acid was stripped by the de-ionized
water and the extraction solvent was reactivated. The results are shown in Fig.7 by the equilibrium isothermal method. From these figures, the stripping ratio was less than 100%. In the case of phase ratio O/A of 1.5 and 2, the stripping ratios were only 80% and 75%, but in the case of phase ratio O/A of 1.0, more than 95% of the stripping ratio was obtained and most oxalic acid could be recovered, so O/A=1.0 was chosen. In this case, the number of stages was obtained to be 7 according to Fig.7(a) on the condition of stage-efficient of 0.75; and the final numbers of stage was 10.
4 Conclusions
1) The oxalic acid in the mother liquor originated from the precipitation of cobalt was separated efficiently by the extraction of P350 with the sulphnoated kerosene as a thinner.
2) P350 is a neutral extractive solvent and can combine the oxalic acid with hydrogen pond. The combining proportion n(H2C2O4):n(P350) is 1:2 and the structure is 2P350?HOOC—COOH.
3) The optimum volume fraction of P350 is 50%. The concentration of the hydrochloric acid is an important factor to affect the distribution coefficient. With the increase of the concentration of hydrochloric acid, the distribution coefficient of the oxalic acid extracted by P350 is increased. In the case of the concentration of HCl of 1.7 mol/L, it reaches the maximum. The concentration of the oxalic acid also affects the distribution coefficient, and its optimum concentration is 0.2-0.4 mol/L.
4) The oxalic acid is separated by several-stage counter-extraction. On the conditions of known components in the mother liquor, the phase ratio is determined to be 2.0 by the method of the equilibrium isothermal and the simulation, and the number of stages is 6. The concentration of oxalic acid in the extraction residual is lower than 0.004 0 mol/L. The oxalic acid from the organic phase is stripped by de-ionized water, and the optimum phase ratio O/A is 1.0 determined by the equilibrium isothermal. The stripping number of stages is 10 and the recovery of the oxalic acid is more than 95%.
References
[1] HE Huan-hua, CAI Qiao-fang. Metallurgy of nickel and cobalt in China [M]. Beijing: Metallurgy Industry Press, 2000: 541-557. (in Chinese)
[2] ZHENG H, HANAKI K, MATSUO T. Production of nitrous oxide gas during nitrification of wastewater [J]. Water Science and Technology, 1994, 30(6): 133-141.
[3] TIAN Qing-hua. Study on preparation of cobalt oxide by precipitation-thermolysis process of oxalic acid without ammonia and recovery utilization of the mother liquor [D]. Changsha: Central South University, 2009: 21-22. (in Chinese)
[4] ANDREOZZI R, ISOLA A, CAPRO V, MAROTTA R, TUFANO V. The use of manganese dioxide as a heterogeneous catalyst for oxalic acid ozonation in aqueous solution [J] . Applied Catalysis A: General, 1996, 138(1): 75-81.
[5] ZHAO Lei, SUN Zhi-zhong, MA Jun. Catalytic ozonation by ceramic honeycomb for the degradation of oxalic acid in aqueous solution [J]. Enviroment Science, 2007, 28(11): 2533-2538. (in Chinese)
[6] PCC Faria, JJM Orfao, MFR Pereira. Activated carbon catalytic ozonation of oxamic and oxalic acids [J]. Applied Catalysis B: Environmental, 2008, 79(3): 237-243.
[7] PENG Cun-yao, LI An-ping, DANG Peng-xian. Recovery of oxalic acid from waste mother liquor of terraycin [J]. Henan Chemical Industry, 2001(5): 31-32. (in Chinese)
[8] Haselhuhn Frank, Kind Matthias. Pseudo-polymorphic behavior of precipitated calcium oxalate [J]. Chemical Engineering and Technology, 2003, 26(3): 347-353.
[9] WANG Shu-min, YANG Geng-liang, LIU Hai-yan. Reclaimation of oxalic acid in waste fluid from oxytertracycline factory using lead sulfate [J]. Journal of Hebei University (Sci & Tech), 2003, 23(1): 45-47. (in Chinese)
[10] LI Yu-xin, WANG Yun-dong, DAI You-yuan. Effect of diluents on the extraction of oxalic acid by trialkylphosphine oxide [J]. Chinese Journal of Chemical Engineering, 2004, 12(1): 143-148.
[11] KIRSCH T, MAURER G. Distribution of oxalic acid between water and organic solutions of tri-n-octylamine [J]. Ind Eng Chem Res, 1996, 35(5): 1722-1735.
[12] QIN Wei, LIU Guo-jun, YU Ll-xin, DAI You-yuan. Oxalic acid recovery in a TOA-based system [J]. Journal of Tsinghua University (Sci & Tech), 2000, 40(10): 43-46. (in Chinese)
[13] BENITEZ ISA O, TALHAM DANIEL R. Calcium oxalate monohydrate precipitation at membrane lipid rafts [J]. Journal of the American Chemical Society, 2005, 127(9): 2814-2815.
[14] DAI You-yuan, QIN Wei, ZHANG Jin, et al. The extracting technology of organic compound [M]. Beijing: Beijing Industry Press, 2007: 248-252.
[15] YIN Yong-jia. Handbook of physics and chemisitry [M]. Beijing: Higher Education Press, 1988: 652-668.
________________________
Corresponding author: TIAN Qing-hua; Tel: +86-731-88877863; E-mail: qinghua@mail.csu.edu.cn


