
Microstructure evolution in nanocrystalline Cu-Nb alloy during
mechanical alloying
LEI Ruo-shan(雷若姗),WANG Ming-pu(汪明朴),GUO Ming-xing(郭明星),
LI Zhou(李 周), DONG Qi-yi(董琦祎)
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 15 July 2007; accepted 10 September 2007
![]()
Abstract:
The microstructural evolution of nanocrystalline Cu-10%Nb(mass fraction) alloy during mechanical alloying (MA) was investigated by using X-ray diffraction, optical microscopy(OM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) observation. Upon milling of Cu-Nb powders with coarse grains, the grain size is found to decrease gradually with lengthening milling time, and reach the minimum value (about 9 nm) after 100 h milling. The microstrain and the microhardness of the powders increase during the grain refinement. And Cu lattice parameter increases steadily over 100 h milling. The mechanisms of solid solution extension during milling were discussed. The results show that up to 10%Nb can be brought into solid solution by MA. The extension of solid solution is found to relate closely with the formation of nanocrystalline.
Key words:
mechanical alloying; nanocrystalline material; Cu-Nb alloy; microstructure;
![]()
1 Introduction
Nanostructured materials with a grain size below 100-200 nm have been much studied in the last decade due to their unusual mechanical, electrical and magnetic properties that are normally attributed to the ultrafine grains and the large amounts of grain boundaries (GB’s)[1]. The mechanical strength of nanocrystalline metallic materials is enhanced as the dislocation motion is also hindered by grain boundaries, compared with metals with coarse grains[2]. For example, pure nanocrystalline Cu has a yield strength in excess of 400 MPa, which is six times higher than that of coarse-grained counterpart Cu[3-5]. Furthermore the strength of copper can be improved by alloying with insoluble elements, such as Cr, Nb, Ta, etc[6]. BOTCHAROVA et al[6-8] have reported that up to 10%(mole fraction) Nb can be dissolved within a Cu matrix by MA though the solubility of Nb in Cu is only negligible under thermodynamic equilibrium conditions. The compacted samples of Cu-5%Nb(mole fraction) alloys show a high mechanical strength of about 1 GPa and a relatively good conductivity of about 50%IACS after annealing at 900 ℃ for 1 h[9]. And since the elastic properties of Nb and Cu are quite similar, a large ductility of the alloys can be expected. Therefore the mechanically alloyed Cu-Nb alloys shall be ready for the application as a new class of conductor.
In this study, the microstructure evolution of nanocrystalline Cu-10%Nb(mass fraction) alloy during MA was investigated in order to understand the alloy behavior. Microstructural parameters such as the lattice strain, grain size and lattice parameter were measured. The relationship between the formation of nanocrystalline and the extension of solid solution during milling was discussed.
2 Experimental
The Cu-Nb alloy with niobium content of 10%(mass fraction) was prepared. MA was performed using a QM-1F Mini-planetary ball mill with stainless steel containers and balls at a rotational speed of 300 r/min. Ball milling was carried out for 100 h under an argon atmosphere with an ratio of ball to powder of 14. After different milling times, samples were taken for microstructure investigations, such as OM, SEM, TEM observation and X-ray diffraction. The hardness measure- ments of the powders were carried out. Hardness tests were performed using a HVA-10A Vickers microhardness tester with a load of 19.6 N at a load time of 30 s. The microstructure of mechanically alloyed powders was investigated by X-ray diffraction analysis using CuKα radiation and scanning rate of 2(?)/min. The lattice parameters were determined from the measured diffraction data using the DBWS Rietveld program[10]. The grain size and the internal strain were obtained from the measured data using Williamson–Hall plots[11]. The powders were examined by SEM (KYKY-2800) and optical microscope (NEOPHOT-21). Furthermore, TEM investigation of the powders was carried out on ion-beam-thinned large powders using a FEI TECNAIG2 operating at 200 kV.
3 Results
3.1 X-ray diffraction analysis
XRD patterns of mechanically alloyed Cu-10%Nb powders for different milling times are shown in Fig.1. It confirms the change of the microstructure of the powers during the milling process. With increasing milling time the diffraction patterns of the powders show a remarkable line broadening and an intensity reduction in the first 40 h of milling. In addition, after long milling time (≥40 h), the niobium peaks are no longer detectable and the copper peaks move towards the lower angles, which indicates that up to 10% niobium may be dissolved within the copper lattice by MA. In order to pursue the dissolution of niobium in the copper lattice, the copper lattice parameter was determined (Fig.2). The lattice parameter determined on unmilled powder (0.361 3 nm) is lower than the true value (0.361 5 nm [6]), probably because of slight instrumental errors. From Fig.2, it can be seen that the lattice parameter of the copper phase increases steadily over 100 h milling and reaches the maximum of about 0.364 1 nm. The dependence of the copper lattice parameter on milling time can be explained by the observed dissolution of niobium, which can be assumed to take place by a substitutional mechanism. This leads to an increase of the copper lattice parameter due to the larger atomic radius of niobium compared with copper.
To extract the peak broadening contribution of the sample (β) from the total line broadening βg a Wagner relation was assumed: β=βg-βi2/βg[12], where βi is the instrumental line broadening. In order to separate the effects of small grain size and internal strain on line broadening, a quantitative analysis can be carried out by using a plot according to the Williamson-Hall method [11]: ![]() , where λ is the X-ray wavelength; θ is the Bragg angle; d is the grain size; ε is the internal strain; and K is the Scherrer constant, K≈1. For each state, a linear dependence of β cos θ on sin θ is expected, with the intercept corresponding to the particle size(d) as d=Kλ/(βcos θ). The slope of the straight line corresponds to the contribution of the internal strain 2ε. Fig.3 shows the evolution of the copper grain size and internal strain during milling. In the first 40 h of milling, the average grain size of copper decreases rapidly, then with further milling the speed of decrease becomes slow till it reached the minimum value of about 9 nm (Fig.3(a)). Therefore MA can form a nanocrystalline grain structure in the Cu-10%Nb powder. Fig.3(b) shows that the internal strain in copper increases fast during the first 40 h which is opposite to the grain size, then it increases slowly. At the beginning of milling concurrent deformation, fracture, and welding of powder particles result in the fast increase of dislocation, the solution of niobium in copper and distortion of copper matrix, so the internal strain increases fast. With further milling, as more and more powders fracture and grain size decreases, part of the internal strain is released. Therefore, the internal strain is relatively stable after 40 h milling.
, where λ is the X-ray wavelength; θ is the Bragg angle; d is the grain size; ε is the internal strain; and K is the Scherrer constant, K≈1. For each state, a linear dependence of β cos θ on sin θ is expected, with the intercept corresponding to the particle size(d) as d=Kλ/(βcos θ). The slope of the straight line corresponds to the contribution of the internal strain 2ε. Fig.3 shows the evolution of the copper grain size and internal strain during milling. In the first 40 h of milling, the average grain size of copper decreases rapidly, then with further milling the speed of decrease becomes slow till it reached the minimum value of about 9 nm (Fig.3(a)). Therefore MA can form a nanocrystalline grain structure in the Cu-10%Nb powder. Fig.3(b) shows that the internal strain in copper increases fast during the first 40 h which is opposite to the grain size, then it increases slowly. At the beginning of milling concurrent deformation, fracture, and welding of powder particles result in the fast increase of dislocation, the solution of niobium in copper and distortion of copper matrix, so the internal strain increases fast. With further milling, as more and more powders fracture and grain size decreases, part of the internal strain is released. Therefore, the internal strain is relatively stable after 40 h milling.
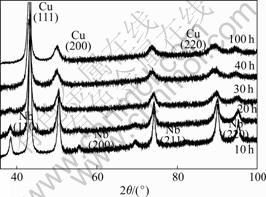
Fig.1 XRD patterns of Cu-10%Nb for different milling times
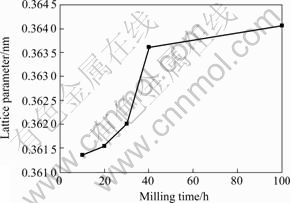
Fig.2 Lattice parameter of Cu matrix with respect to milling time
3.2 Microhardness measurements
Fig.4 shows the microhardness of Cu-10%Nb increases strongly during the milling process, and reaches 4.75 GPa after 100 h milling which is very high for copper alloys![]() . The change of the microhardness is corresponding to the change of the internal strain. And the high microhardness may be resulted from the formation of nanocrystalline according to the Hall-Petch relation and also the solution of niobium in copper according to solution strengthening.
. The change of the microhardness is corresponding to the change of the internal strain. And the high microhardness may be resulted from the formation of nanocrystalline according to the Hall-Petch relation and also the solution of niobium in copper according to solution strengthening.
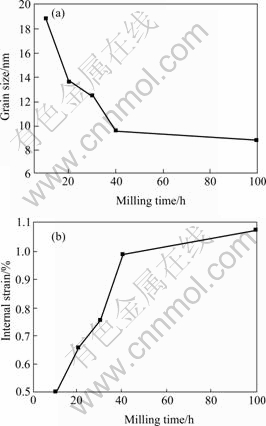
Fig.3 Copper grain size(a) and internal strain of copper matrix(b) with respect to milling time
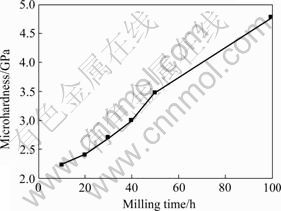
Fig.4 Microhardness of powder with respect to milling time
3.3 Microstructural analysis
Fig.5 shows the microstructural evolution of Cu-10%Nb powder during milling. The layered structure forms (Fig.5(a)) and becomes obvious (Fig.5(b)) and fine with the milling time because of repeated deformation, fracture and welding. The fine layered structure may speed the solution of niobium in copper to form high concentration Cu-Nb alloys, because the diffusion distance decreases and large amount of the interfacial energy can provide driving force for diffusion. And during the milling process, the distribution of niobium becomes more and more homogeneous. After 100 h milling, a very homogeneous microstructure forms, just very few niobium particles with small sizes remain (Fig.5(c)).
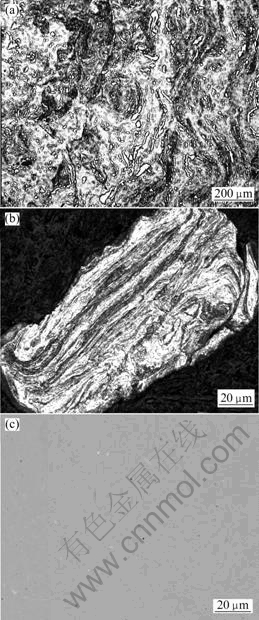
Fig.5 Change of microstructure of Cu-10%Nb powder with respect to milling time: (a)10 h (OM); (b) 50 h (OM); (c) 100 h (SEM)
A TEM investigation of the microstructure of the powder after 100 h milling shows a very fine nanocrystalline grain structure. Fig.6(a) shows a strong strain contrast because of milling and the grain size of the powder is 5-20 nm corresponding to the results of XRD. As proved by EDX analysis in the TEM, the composition of the powder is very homogeneous. Furthermore electron diffraction of the powder does not show niobium reflections (Fig.6(b) and Fig.6(c)). In conclusion, these investigations reveal the successful formation of a supersaturated and metastable Cu-Nb solid solution with nanocrystalline grain size by MA.
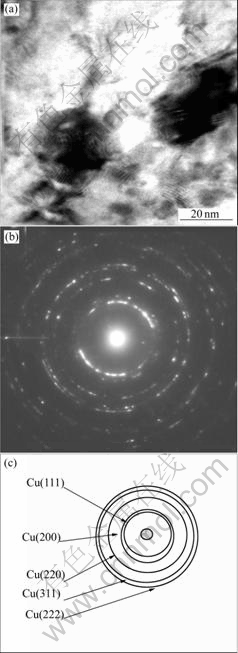
Fig.6 TEM examination of Cu-10%Nb powder: (a) TEM image after milling for 100 h; (b) SAD pattern of (a); (c) Calibration of (b)
4 Discussion
Nanocrystalline and supersaturated Cu-Nb solid solution can be formed by MA as confirmed by XRD, SEM, TEM. In fact, the mechanisms of solid solubility extension induced by MA are not completely known yet. So far, there are 6 mechanisms which may be the reasons of the extension of solid solution: 1) the effect of grain boundaries or phase boundaries[13]; 2) the influence of impurities [14]; 3) the effect of dislocation [13, 15]; 4) the effect of sharp-angle [16]; 5) the effect of the energy from milling [17]; 6) the effect of highly increased temperature. BOTCHAROVA et al[6] have explained the achievement of an extended solid solution of niobium in the copper matrix by the dislocation solute-pumping mechanism. In fact, the formation of nanocrystalline is very important to the formation of supersaturated solid solution. Fine nanocrystalline can be formed because of repeated heavy deformation and fracture during milling. There is an increase in the grain boundary volume in a nanocrystalline structure. As shown by theoretical calculation, the concentration of solute at the grain boundaries may be 10-100 times than inside the grain. Therefore the diffusivity of the component atoms is increased due to the large amount of structural defects and the local stresses in such a material. And as nanocrystalline stores very large amount of specific interfacial free energy and elastic energy as the driving force of alloying[18], the alloy is in metastable state. From the principle of solubility of metastable phase[19], it can be deduced that the solid solution can be extended. And also as niobium can be cut into a few namometer during milling, if the sizes of niobium particles can be decreased to the critical size, then they may dissolve into copper to decrease energy. Therefore under these conditions, niobium atoms substitute copper atoms in the grain boundaries and vice versa. The rapid diffusion of atoms from one grain into the other leads to quick homogenization and results in the formation of solid solutions. Therefore the formation of nanocrystalline and supersaturated solid solution are closely related.
5 Conclusions
1) MA can result in a supersaturated solid solution of niobium in copper and the formation of nanocrystalline. A dissolution of up to 10% niobium in the copper lattice can be achieved. And the extension of solid solution can be achieved only when nanocrystalline is formed.
2) Due to the formation of nanocrystalline, the dissolution of niobium in the copper matrix and also the strong plastic deformation exerted during milling, the value of the microhardness of the powders after 100 h milling is as high as 4.75 GPa.
References
[1] ZHAO Y H, SHENG H W, LU K. Microstructure evolution and thermal properties in nanocrystalline Fe during mechanical attrition[J]. Acta Materialia, 2001, 49 (2): 365-375.
[2] BENSON D J, FU H H, MEYERS M A. On the effect of grain size on yield stress: Extension into nanocrystalline domain[J]. Materials Science and Engineering A, 2001, 319/321: 854-861.
[3] WANG Yin-ming, CHEN Ming-wei, ZHOU Feng-hua. High tensile ductility in a nanostructured metal[J]. Nature, 2001, 419(6910): 912-914.
[4] LERGOS M, ELLIOTT B R, RITTNER M N, WEERTMAN J R, HEMKER K J. Microsample tensile testing of nanocrystalline metals[J]. Philosophical Magazine A, 2000, 80(4): 1017-1026.
[5] VALIEV R Z, ALEXANDROV I V, ZHU Y T. Paradox of strength and ductility in metals processed by severe plastic deformation[J]. Journal of Materials Research, 2002, 17 (1): 5-8.
[6] BOTCHAROVA E, HEILMAIER M, FREUDENBERGER J, DREW G, KUDASHOW D, MARTIN U, SCHULTZ L. Supersaturated solid solution of niobium in copper by mechanical alloying[J]. Journal of Alloys and Compounds, 2003, 351(1/2): 119–125.
[7] BOTCHAROVA E, FREUDENBERGER J, SCHULTZ L. Cu–Nb alloys prepared by mechanical alloying and subsequent heat treatment[J]. Journal of Alloys and Compounds, 2004, 365 (1/2):157–163.
[8] FREUDENBERGER J, BOTCHAROVA E, SCHULTZ L. Formation of the microstructure in Cu-Nb alloys[J]. Journal of Materials Science, 2004, 39(16/17): 5343–5345.
[9] BOTCHAROVA E, FREUDENBERGER J, SCHULTZ L. Mechanical and electrical properties of mechanically alloyed nanocrystalline Cu–Nb alloys[J]. Acta Materialia, 2006, 54(12): 3333-3341.
[10] RIETVELD H M. ![]() A profile refinement method for nuclear and magnetic structures[J]. J Appl Crystallogr, 1969, 2(2): 65-71.
A profile refinement method for nuclear and magnetic structures[J]. J Appl Crystallogr, 1969, 2(2): 65-71.
[11] WILLIAMSON G K, HALL W H. X-ray line broadening from filed aluminium and wolfram[J]. Acta Metall, 1953, 1: 22–31.
[12] KUSCHKE W M, KELLER R M, GRAHLE P, MASON R, ARZT E. Mechanisms of powder milling investigated by X-ray diffraction and quantitative metallography[J]. Z Metallkde, 1995, 86(12): 804–813.
[13] SURYANARAYANA C.Mechanical alloying and milling[J]. Progress in Materials Science, 2001, 46 (1/2): 1-184.
[14] YAVARI A R, DESRE P J M. Mechanically driven alloying of immiscible elements[J]. Materials Science Forum, 1994, 155/155: 463-474.
[15] ESTRIN Y, RABKIN E. Pipe diffusion along curved dislocations: an application to mechanical alloying[J]. Scripta Materialia, 1998, 39 (12): 1731-1736.
[16] MA E, SHENG H W, HE J H, SCHILLING P J. Solid-state alloying in nanostructured binary systems with positive heat of mixing[J]. Materials science and engineering A, 2000, 286: 48-57.
[17] SCHAFFER G B, FORESTER J S. Influence of collision energy and strain accumulation on the kinetics of mechanical alloying[J]. J Mater Sci, 1997, 32 (12): 3157-3162.
[18] VELTL G, SCHOLZ B, KUNZE H-D. Amorphization of Cu-Ta alloys by mechanical alloying[J]. Mater Sci and Engng A, 1991, 134: 1410-1413.
[19] YAVARI A R. Phase transformations in nanocrystalline alloys[J]. Mater Sci Eng A, 1994, 179-180: 20-26.
Foundation item: Project(2006AA03Z517) supported by the National High-Tech Research and Development Program of China
Corresponding author: LEI Ruo-shan; Tel: +86-731-8830264; E-mail: leiruo@163.com


