
Effect of surfactants on preparation of nanometer TiO2 by pyrohydrolysis
CHAI Li-yuan(柴立元)1, YU Yan-fen(于延芬)1, 2,
ZHANG Gang(张 刚)1, PENG Bing(彭 兵)1, WEI Shun-wen(韦顺文)1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Changsha Environmental Protection College, Changsha 410004, China
Received 23 June 2006; accepted 26 October 2006
Abstract:
The nanometer TiO2 was prepared by pyrohydrolysis with titanic solution. The effects of species and content of the surfactants on the particle size, morphology and phase of TiO2 were studied by using LPA, XRD and TEM respectively. The results show that it is beneficial to reducing the aggregation of TiO2 particles with adding surfactants to the solution. Nanometer TiO2 powders with the size of 40-55 nm are obtained by adding the anion surfactants in the optimal content of 1.5% (mass fraction). The effect of cationic surfactant for reducing particle aggregation is not as good as that of anion. The crystal phase constitutent of TiO2 is dependent on the temperature of thermal treatment and complete anatase can be achieved after calcining in the temperature range of 350-750 ℃.
Key words:
surfactant; nanometer TiO2; pyrohydrolysis;
1 Introduction
Nano-powder of titanium dioxide has extinguished physicochemical properties, such as large surface area, high activity, nontoxicity, long-term stability, excellent absorbency of light especially for ultraviolet ray, which makes it widely applied in pigments, catalysts, cosmetics, sensor, and environmental protection[1-4]. A lot of methods have been developed for preparing nanometer titanium dioxide powder such as micromusion[5], sonochemical[6], gas pyrohydrolysis[7], and sol-gel[8]. Titanic solution, which was of low cost and easily available raw materials, was adopted in this study to prepare nano titanium dioxide by pyrohydrolysis. However, severe aggregation of hydrolyzed products appeared in the pilot-scale preparation.
ROOSEN and HAUSNER[9] pointed out that the aggregation of powders was mostly caused by the combination of free hydroxyls though hydrogen bonds on the surface of particles in the process of sol reaction. Many methods were developed to disperse the aggregated powders such as organical washing[10], freezing drying[11], and adding surfactants[12]. However, the disadvantage of organical washing method is its high cost and that of freezing drying is great energy consumption. It is the most economical and efficient way to add surfactants for preventing the rigid aggregation of powders up to the present. ZOU et al[13] prepared nano-TiO2 with small size using TiCl4 as raw material in complex reaction system consisted of HCl, DBS, xylene and H2O. ZHOU et al[14] adopted DBS as surfactant to prepare the nano-TiO2 using hydrolysis method, with the result of obtaining TiO2 particles of the homogeneous dispersion, narrow particle distribution and homogeneous size. HU et al[15] used Ti(SO4)2 and CO(NH2)2 to make nano-TiO2 by harmonious precipitation and it was found to improve the particle size and distribution of TiO2 by adding complex surfactants.
Therefore, the effects of anionic surfactants DI, cationic surfactants EA and non-ionic surfactants PT on aggregated particle size and phases of TiO2 in the pyrohydrolysis are investigated in detail in this paper, which is aimed at determining the optimal parameters for preparing dispersed nanometer TiO2.
2 Experimental
2.1 Preparation of titania powders
The hydrous titania (H2TiO3) from Zhuzhou Chemical Plant, China, was dissolved by hot sulfuric acid to form TiOSO4 solution. And the purified solution was adjusted to a certain concentration of TiOSO4 in the range of 50-56 g/L and H+ concentration of 2.6-3.0 mol/L and then pyrohydrolyzed in RXQ-SG46-280 of autoclave at 0.14 MPa and the temperature range of 100-130 ℃ with adding some surfactants. The ultra-fine hydrous titania was rinsed using the distilled water and alcohol respectively and filtrated in a centrifugal machine until no existence of sulfuric acid in the filtrated water. Finally, the specimen was dried and calcined.
2.2 Characteristics of powder
The average particle size and size distribution of TiO2 were measured by using the laser diffraction particle size analyzer(LPA). Phases of sample were detected by using X-ray diffiraction(XRD) with the apparatus of Japanese Rigaku Dmax rA X-ray diffractometer employing Cu Kα (Ni filtered) radiation of wavelength 15.4 nm. The morphology of the samples was checked by using Hitachi H-800 transmission electron microscopy(TEM) under the accelerating voltage of 200 kV.
3 Results and discussion
3.1 Effect of surfactant type on particle size and distribution of TiO2
In order to prevent the growth of nano-TiO2, the influence of the surfactant type on the particle size and distribution of TiO2, including anionic surfactant DI, cationic surfactant EA and non-ionic surfactant PT were studied respectively. The results are listed in Table 1.
Table 1 Effects of surfactant type on TiO2 particle size
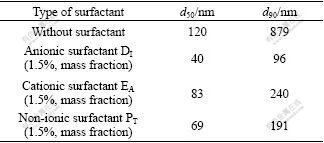
It can be seen from Table 1 that adding suitable surfactant is helpful to reducing the particle size of TiO2 due to the sorption of surfactants on the surface of nanometer particles. The reaction of the interfacial energy among particles prevents the aggregation of powders from the static repulsion and special hindrance. The particle with the size of 83 nm is obtained with adding the cationic surfactants EA, which is larger than that with adding the anion DI or non-ionic PT obviously. In addition, from the results of particle size distribution, the effect of adding the anionic surfactant DI apparently is smaller than others. This means that anionic DI is the best choice to reduce the aggregation of TiO2 powders in the hydrolysis process of TiOSO4 solution.
The TEM morphology of TiO2 powders with various kinds of surfactants is given in Fig.1. It can be seen that particles are in the form of sphere and the samples without adding surfactants are seriously aggregated to the diameter range of 120-130 nm. On the other hand, the aggregation of the samples is alleviated and the particle size is reduced to 40-50 nm with adding anionic surfactant. In general, the preparation of H2TiO3 is carried out in the strongly acidic solutions and the particle takes the positive charges from the ionization of the hydroxy groups on the surface of powders. These positive charges are easy to attract the opposite ones to form the electric double layer[16], which is absorbed by the polar groups of surfactants and produces the electrostatic repulsion barrier and steric hindrance. The cationic surfactant shows poor dispersion for the bad absorption of the positive charge. The effect of non-ionic surfactants on the dispersion of nano-TiO2 is in the range of the above additives.
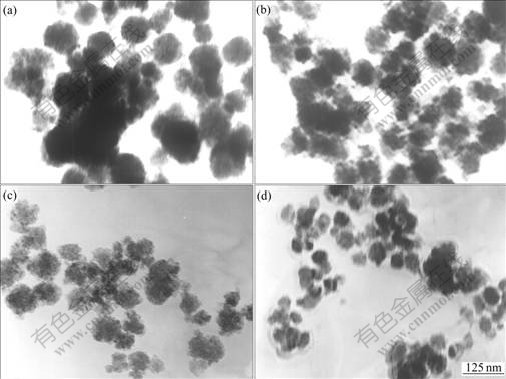
Fig.1 TEM photographs of TiO2 powder with different surfactants: (a) Without surfactants; (b) 1.5% cationic surfactants EA; (c) 1.5% non-ionic surfactants PT; (d) 1.5% anionic surfactants DI
3.2 Effect of surfactant content on preparation of nanometer TiO2
Table 2 illustrates the relationship between the content of the anionic surfactant DI and particle size of TiO2. It can be found from Table 2 that the hydrolyzed TiO2 grows up fast without additive. Under this condition there are no covers of surfactant on the surface of hydrolyzed products and no nanometer TiO2 can be acquired. In addition, the particle size decreases with the increase of the content of anionic additive, especially at 1.5%. The particle size of TiO2 would rise contrarily when the additive content is over 1.5%. On one hand, no steric hindrance takes place when the content of additive is too low to cover TiO2 particles. It is easy to cause the bridging across the particles[17] and the aggregation of powders. On the other hand, the content of surfactant is too high to increase the solubility of active reagent in the strongly acidic system. The viscosity of solution increases, the particle movement becomes difficult and the bridging of the surfactants leads to the increase of the surface tension and large particle size. The TEM photographs of TiO2 produced with adding different contents of the anionic surfactants are displayed in Fig.2. From these images, it is clear that the lower the content of additive, the bigger the particle size of TiO2 (Figs.2(a) and (b)). The dispersion of powders would be improved under suitable content and the ultrafine particles would be achieved (Fig.1(d) and Fig.2(c)). The dispersion of samples is reduced under contents of the additive (Fig.2(d)).
Table 2 Effect of anionic surfactant DI content on TiO2 particle size
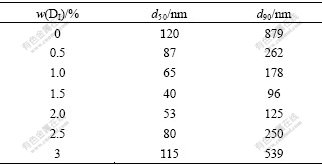
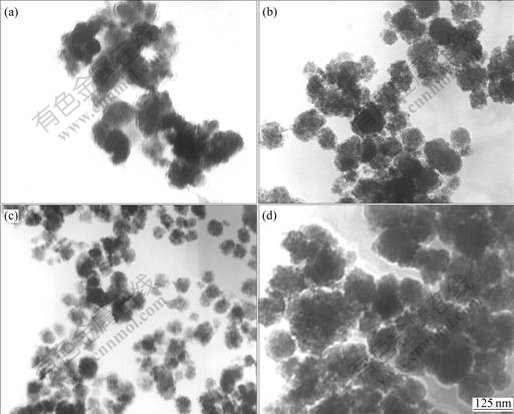
Fig.2 TEM photographs of TiO2 with adding various contents of surfactant DI: (a) Without surfactants; (b) 0.5% anion surfactants DI; (c) 1% anion surfactants DI; (d) 2% anion surfactants DI
3.3 Effect of acidity of solution on particle size and distribution of TiO2
When the anionic surfactant DI is introduced into TiOSO4 solution, the concentration of H+ has an apparent effect on the hydrolysis and particle size of TiO2[18-19]. The profile of the relationship between TiO2 crystal dimension and initial concentration of H+ is shown in Fig.3. The data for the profile are acquired by hydrolyzing with 1.5% of additive DI and thermal treatment at 550 ℃ for 1 h.
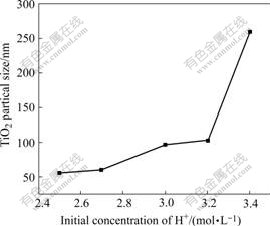
Fig.3 Relationship between TiO2 particle size and initial concentration of H+
It is found from Fig.3 that the particle size of TiO2 increases with the increase of H+ concentration. When the concentration of H+ is below 3.2 mol/L, the crystal size increases slowly. But when the concentration of H+ is over 3.2 mol/L, the particles grow up rapidly to large grain. In addition, when the concentration of H+ is below 3.2 mol/L, the process of hydrolysis can produce the unlayered colloid. This is due to low-level acidity and narrow nucleating region and the time for hydrolysis is so short that a mass of particles are created. The crystal is difficult to grow up and the size is controlled. It is also found that when the concentration of H+ is over 3.2 mol/L, the ionic concentration of solution increases and the crystalline rate is accelerated, which leads to the increase of particle size and the decrease of nucleation rate. Moreover, the high-level H+ interferes the reaction and descends the hydrolysis rate. Then the new nuclear has enough time to grow up and aggregates into large-size TiO2 grains. Fig.4 presents the TEM photograph of TiO2 sample prepared under the H+ concentration of 2.6 mol/L. It can be seen that when the concentration of H+ is kept at low level, the small particle size and favorable dispersion are achieved.
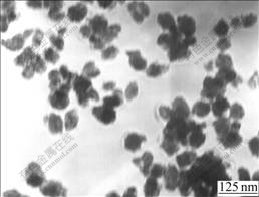
Fig.4 TEM photograph of TiO2 powders at [H+] of 2.6 mol/L
3.4 XRD pattern of TiO2 powder
Fig.5 shows X-ray diffraction patterns of TiO2 prepared with adding 1.5% anionic surfactants DI. It can be concluded that the TiO2 phase begins to appear in the anatase structure at 250 ℃. With increasing calcining temperature, it is easy to find that the width of diffraction peaks becomes narrower. According to Scherrer equation, it is implied that the crystallite size of particles becomes finer. It can be assumed that increasing thermal treating temperature can strengthen the process of re- crystallization and accelerate the crystal growth[20].
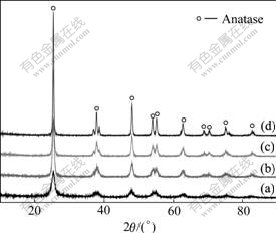
Fig.5 XRD patterns of TiO2 calcined at various temperatures: (a) 250 ℃; (b) 350 ℃; (c) 550 ℃; (d) 750 ℃
This behavior of TiO2 powders is different from other titanium dioxides. The transformation temperature of the crystal prepared by sol-gel is in the range of 500-650 ℃[21]. This indicates that the type of the crystal is related to not only the thermal treating temperature but also the preparing methods and raw materials. The samples obtained in this research include only one phase of the anatase treated in a wide range of temperature.
4 Conclusions
1) The aggregation and particle size of TiO2 can be improved with adding surfactants. The anionic additive shows the best result among the additives.
2) The best dispersion is obtained when the content of anionic surfactant is controlled at 1.5%(mass fraction) and the particle size ranges from 40 nm to 55 nm.
3) The crystal phase constituent of TiO2 is related to thermal treating temperature, fabricating methods and raw materials. The TiO2 samples in complete anatase can be achieved after thermal treatment at 350-750 ℃.
References
[1] ZHANG Li-de, MOU Ji-mei. Nano-meterials and Nano-structure [M]. Beijing: Science Press, 2002: 88-91. (in Chinese)
[2] ULRIKE D. The surface science of titanium dioxide [J]. Surface Science Reports, 2003, 48: 59-64.
[3] MARDARE D, TASCA M, DELIBAS M, RUSU G L. On the structural properties and optical transmittance of TiO2 sputtered thin films [J]. Applied surface Science, 2000, 156: 200-206.
[4] WEI C, LIN W Y, ZAINAL Z, MILLIAMS N E. Bactericidal activity of TiO2 photocatalyst in aqueous media: Toward a solar-assisted water disinfection system [J]. Environ Science Thchnology, 1994, 28(5): 934-938.
[5] MANJARI L. Preparation of nanosized TiO2 by microemulsion method [J]. Mater Res, 1998, 13(5): 1249-1254.
[6] GUO Wei-lin, WANG Xi-kui, WANG Gang-zhi. Preparation of the nanocrystalline titanium dioxide by sonochemical method [J]. China Powder Science and Technology, 2004, 32(8): 1008-1015. (in Chinese)
[7] SPICER P T, CHAOUL O, TSANTILIS S, PRATSINIS S E. Titania formation by TiCl4 gas phase oxidation, surface growth and coagulation [J]. Journal of Aerosol Science, 2002, 33(1): 17-34.
[8] DING X Z, LIU X H. Synthesis and microstructure control of nanocrystalline titania powders via a sol-gel process [J]. Materials Science and Engineering A, 1997, A224(1/2): 210-215.
[9] ROOSEN A, HAUSNER H. Techniques for agglomeration control during wet-chemical powder synthesis [J]. Adv Ceram Mater, 1988, 3(2): 131-136.
[10] The newsroom of The Cyclopedia of Chemical Industry. The Cyclopedia of Chemical Industry (Vol.12) [M]. Beijing: Chemical Industry Press, 1998: 428. (in Chinese)
[11] RIGHERINK M D. Advances in technology of the cryochemical process [J]. Am Ceram Soc Ball, 1972, 51(2): 158-161.
[12] XING Guang-jian, YAO Wang, CHEN Guang-hua. Preparation of TiO2 nano-powder by hydrolyzing titanate controlled by surfactant [J]. Journal of Beijng Universtry of Technology, 2004, 30(3): 354-358. (in Chinese)
[13] ZOU Bing-suo, LIN Jin-gu, WANG Li, XU Ji-ren, ZHAO Jia-long. Structural, electronic state characterization and properties of coated TiO2 nanoparticles [J]. Acta Physica Sinica, 1996, 45(7): 1239-1243. (in Chinese)
[14] ZHOU Wu-yi, TANG Shao-qiu, ZHANG Shi-ying, ZHOU Xi-rong. Study on preparation of nano TiO2 by hydrolyzation of titanium-salt coated with DBS [J]. Journal of the Chinese Ceramic Society, 2003, 31(9): 858-861. (in Chinese)
[15] HU Xiao-li, CHEN Dong-dan, HU Xiao-hong, YING Hong. The effect of surfactants on the particle size and shape of TiO2 [J]. China Ceramic Industry, 2003, 10(4): 25-28. (in Chinese)
[16] XU Jia-qiang, PAN Qing-yi, SUN Yu-an, LI Zhan-cai. Emulsion synthesis, microstructure and gas sensing properties of nanometer ZnO ceramics [J]. Journal of Inorganic Chemistry. 1998(3): 355-359.
[17] DENG Tong, ZHAO Xue-fan. Interfacial Electrical Image [M]. Beijing: Peking University Press, 1992. (in Chinese)
[18] JIU Ling, LUO Ju-rong. Influence factors on preparing process of TiO2 nanoscale particles [J]. Journal of Inorganic Materials, 1999, 14(2): 264-270. (in Chinese)
[19] ZHANG Qing-jin, HU Xiao-hong, YANG Ming. Analysis of the influences on the preparation of TiO2 UFP by liquid phase precipitation [J]. Journal of South China University of Technology (Natural Science), 1996, 24(7): 52-56. (in Chinese)
[20] KANG M. Preparation of titania photocatalyst film and its catalytic performance for 1, 1’-dimethyl-4, 4’-bipyidium dichloride decomposition [J]. Appl Catal B—Environ, 2002, 37(3): 187-196. (in Chinese)
[21] KAMAL A M, SRINNIVAS V, SOTIRIS E P. Competition between TiCl4 hydrolysis and oxidation and its effection product TiO2 powder [J]. AICh E J, 1994, 40(7): 1183-1188. (in Chinese)
Foundation item: Project(04GK2007) supported by the Key Project of Science and Technology Plan of Hunan Province, China
Corresponding author: CHAI Li-yuan; Tel: +86-731-8836921; E-mail: lychai@mail.csu.edu.cn


