
J. Cent. South Univ. (2018) 25: 1317-1325
DOI: https://doi.org/10.1007/s11771-018-3828-2

Leaching of vanadium and chromium from converter vanadium slag intensified with surface wettability
YANG Qi-wen(杨其文)1, 2, XIE Zhao-ming(谢昭明)1, 2, PENG Hao(彭浩)1, 2,LIU Zuo-hua(刘作华)1, 2, TAO Chang-yuan(陶长元)1, 2
1. Chongqing Key Laboratory of Chemical Process for Clean Energy and Resource Utilization,Chongqing University, Chongqing 400044, China;
2. College of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400044, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
Technology intensified with surface wettability was introduced to leach vanadium and chromium from converter vanadium slag without roasting. Parameters affecting the leaching efficiency of vanadium and chromium were investigated: sulfuric acid concentration, MnO2-to-slag mass ratio, liquid-to-solid ratio, leaching time, leaching temperature, and sodium dodecyl sulfate (SDS)-to-slag mass ratio. The leaching efficiencies of vanadium and chromium were 33.46 % and 20.02 % higher in the presence of MnO2 and SDS, respectively, compared to the control. The leaching efficiencies of vanadium and chromium were 68.93 % and 30.74 %, respectively, under the optimum conditions: sulfuric acid concentration 40 wt%, MnO2-to-slag mass ratio 10.0 wt%, liquid-to-solid ratio 5:1 mL/g; 12 h; 90 °C; and SDS-to-slag mass ratio 0.25 wt%. The analysis of the reaction mechanism in the leaching process indicates that MnO2 combined with protons (H+) could oxidize low-valent vanadium and chromium; SDS could change the chemical behavior and decrease the surface tension of the aqueous solution to favor MnO2 oxidization.
Key words:
vanadium; chromium; leaching; surfactant; MnO2;
Cite this article as:
YANG Qi-wen, XIE Zhao-ming, PENG Hao, LIU Zuo-hua, TAO Chang-yuan. Leaching of vanadium and chromium from converter vanadium slag intensified with surface wettability [J]. Journal of Central South University, 2018, 25(6): 1317–1325.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3828-21 Introduction
Vanadium is an important strategic metal that is used in the manufacture of iron and steel and non-ferrous metals, and in the petrochemical industry because of its excellent physicochemical properties, such as tensile strength, hardness, and fatigue resistance [1–5]. In China, vanadium slag is typically produced by oxidation in the steelmaking process, representing a major vanadium resource [6–8]. Technologies have been developed to extract vanadium from converter vanadium slag, including direct leaching, roasting leaching, sub-molten salt leaching, and electro-oxidation leaching [9–12]. Roasting and leaching methods involve calcium salt roasting–acidic leaching and sodium salt roasting–water leaching processes. These processes require high temperatures and involve energy consumption. In particular, the sodium salt roasting–water leaching process causes serious environmental pollution in the form of poisonous gas and wastewater production [13–17]. Sub- molten salt technology has been applied to extracting valuable metals from treated amphoteric ores. However, this method requires high-concentration alkalinity (usually greater than 50 %), high reaction temperature, and concentrated solutions [18–20]. Acid oxidation leaching technology is a comparatively simple method to leach vanadium and chromium from converter vanadium slag [21, 22]. This technology results in lower poisonous and harmful gas emissions from the leaching process, and its operation is relatively simple. However, the process was only used to leach vanadium directly from converter vanadium slag, whereas other states of vanadium were hardly leached using this method. In general, this process requires high concentrations of acid and corrosion-resistant equipment. Therefore, it is necessary to develop a clean and high recovery technology to extract vanadium and chromium from converter vanadium slag.
Previous studies showed that the easiest and most effective method to improve leaching efficiency was to add fortifier to the leaching process [2]. The additive components react with the slag in different ways to destroy the spinel phase structure. MnO2 exhibits strong oxidation ability, such that low valence vanadium and chromium can combine with H+ to damage the structure of the converter vanadium slag. This reduces the amount of acid required for the leaching process, and improves the leaching efficiency of vanadium and chromium. In addition, according to the standard electrode potential (298 K) table, in acidic solution, φθMnO2/Mn2+=+1.23 V, φθVO2+/V3+=+0.359 V, φθVO2+/V3+=+0.680 V. This indicates that MnO2 could oxidize low-valent vanadium to high-valent vanadium oxide [21]. At the same time, surfactants can change the surface tension of the leaching solution and wetting properties of the variable leaching system [23]. This represents a clean and efficient way to leach vanadium and chromium with MnO2 and surfactants as enhancers of the leaching process.
The present work focused on strengthening the use of interface chemistry and controlling the chemical behavior of vanadium and chromium in aqueous solution. Through acid oxidation leaching with surfactants to extract vanadium and chromium from converter vanadium slag, the effects of sulfuric acid concentration, MnO2-to-slag mass ratio, liquid-to-solid ratio, leaching time, leaching temperature, and sodium dodecyl sulfate (SDS)-to- slag mass ratio on the leaching efficiency of vanadium and chromium were studied.
2 Materials and methods
2.1 Materials
The converter vanadium slag used in this study was obtained from Panzhihua, Sichuan Province, China. The vanadium and chromium present in the slag were low valence states, such as FeCr2O4 and Fe2VO4, as shown in Figure 1. Its chemical composition was analyzed by X-ray fluorescence, as shown in Table 1. X-ray diffractions (XRD) studies were performed using a Shimadzu XRD- 6000 diffractometer with a step size of 0.05° and an angular range of 5° to 90°. Scanning electron microscopy (SEM) images were recorded on a Shimadzu SSX-550 instrument and the applied X-ray fluorescence was obtained on a Shimadzu XRF-1800 instrument. The ultraviolet-visible (UV-vis) spectroscopy data were tested with Beijing Purkinje General Instrument TU-1901.
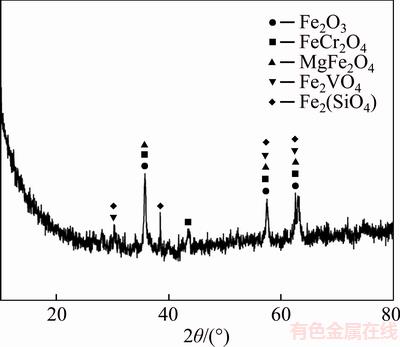
Figure 1 XRD pattern of converter vanadium slag
Table 1 Composition of converter vanadium slag (wt%)

All reagents used were of analytical grade, including sulfuric acid, MnO2 and the surfactants used for leaching, as well as the ammonium ferrous sulfate, urea, potassium permanganate, sodium nitrite, and N-phenylanthranilic acid used for chemical analysis.
2.2 Methods
2.2.1 Leaching process
The leaching experiments were conducted in a 300 mL beaker that was heated in a water bath, with the temperature maintained within ±0.1 °C. A predetermined amount of sulfuric acid and deionized water was added to the reactor to produce a homogeneous slurry under constant stirring (550 r/min to 560 r/min). The slurry was heated to a predetermined temperature and the converter vanadium slag and MnO2 were subsequently added to the beaker.
After the scheduled leaching time, the filtrate was separated from the slag by vacuum filtration. Titration with ammonium ferrous sulfate was used to determine the concentration of vanadium and chromium in the filtrate. Then, the leaching efficiencies of vanadium and chromium were calculated using the following formulae, respectively:
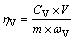 (1)
(1)
 (2)
(2)
where ηV is the leaching efficiency of vanadium; ηCr is the leaching efficiency of chromium; V is the volume of the leaching liquid, L; CV is the vanadium concentration in the leaching liquid, g/L; CCr is the chromium concentration in the leaching liquid, g/L; m is the mass of the converter vanadium slag, g; ωV is the vanadium content in the converter vanadium slag; and ωCr is the chromium content in the converter vanadium slag.
The error analysis was based on the relative standard deviation (RSD) and the error sources included instrumental and human errors.
2.2.2 Surface tension measurements
A surface tension meter was used to determine the surface tension of the leaching filtrate, with an accuracy of 0.01 (N/m). During the process of measurement, the temperature was maintained at 25 °C. The measurement precision was improved by averaging the test values of repeated measurements.
3 Results and discussion
3.1 Effect of sulfuric acid concentration
To determine the effect of sulfuric acid concentration on the leaching efficiency of vanadium and chromium, various concentrations of sulfuric acid were studied at 90 °C for 12 h with a liquid-to-solid ratio of 5:1 mL/g. The results are shown in Figure 2.
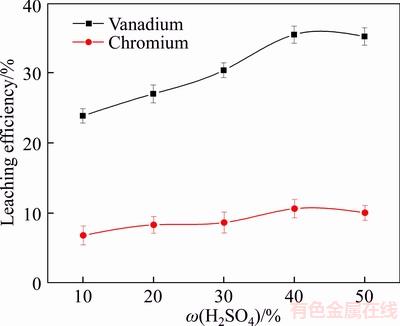
Figure 2 Effect of concentration of sulfuric acid on leaching efficiency of vanadium and chromium
Compared with other sulfuric acid concentrations, 40 wt% sulfuric acid was the most effective for leaching vanadium and chromium. At lower sulfuric acid concentrations, the rate at which protons (H+) destroyed the spinel lattice was slow, and as the sulfuric acid concentration increased, the rate of reaction also increased as sufficient protons (H+) were available to destroy the spinel lattice. However, the leaching efficiency of vanadium and chromium slightly reduced as the concentration of sulfuric acid increased from 40 wt% to 50 wt%. When the sulfuric acid concentration was higher than 40 wt%, impurities such as silicon and calcium sulfate covered the surface of the slag particles, reducing the rate of reaction [13]. Hence, based on the above results, 40 wt% sulfur acid was chosen as the optimal condition for further experiments.
3.2 Effect of mass ratio of MnO2-to-slag
To investigate its effect on vanadium and chromium leaching efficiency, the mass ratio of MnO2-to-slag was varied from 5.0 wt% to 15.0 wt% as the concentration of sulfuric acid was held constant at 40 wt%, and the leaching temperature and time were maintained at 90 °C and 12 h, respectively, with a liquid-to-solid ratio of 5:1 mL/g. The corresponding results are shown in Figure 3.
The leaching efficiencies of vanadium and chromium were 14.07% and 6.11% higher in the presence of MnO2, respectively, compared to the control. During the leaching process, protons (H+) entered the slag particles and destroyed the silicate phase in the converter vanadium slag, exposing low-valent vanadium to the solution. In addition, when V(III), which processed reducibility, came in contact with MnO2, the process of oxidation to high-valent vanadium was enhanced, thereby increasing the speed at which low-valent vanadium was oxidized to high-valent vanadium. Thus, MnO2 could combine with the protons (H+) to damage the slag structure and increase the leaching efficiency of vanadium and chromium [21].
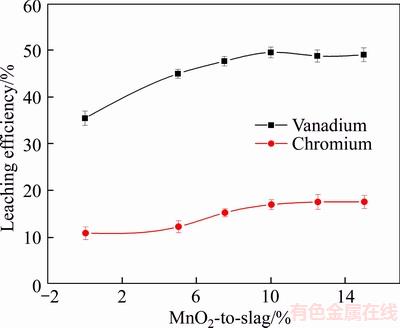
Figure 3 Effect of MnO2-to-slag mass ratio on leaching efficiency of vanadium and chromium
When the mass ratio of MnO2-to-slag was increased from 0 wt% to 15.0 wt%, the leaching efficiency of vanadium and chromium increased gradually. When the mass ratio of MnO2-to-slag was higher than 10.0 wt%, the leaching efficiency of vanadium and chromium was largely unchanged and considering the cost and quantity of the filtered slag, the mass ratio of MnO2-to-slag of 10.0 wt% was chosen as the optimum.
3.3 Effect of leaching time
To determine the effect of leaching time on the efficiencies of vanadium and chromium, different leaching times were studied. The concentration of sulfuric acid remained at 40 wt%, the MnO2-to-slag mass ratio was 10.0 wt%, the liquid-to-solid ratio was 5:1 mL/g, and the leaching temperature was 90 °C.
The results shown in Figure 4 indicate that the leaching efficiencies of vanadium and chromium increased significantly when the leaching time was less than 12 h, and longer durations did not significantly improve the leaching efficiency. When the leaching time was less than 12 h, increasing leaching time promoted contact between the slag and sulfuric acid solution. As the leaching time increased, silicon and calcium sulfate in the slag were leached, and these impurities covered the surface of slag particles and slowed the rate of subsequent reactions. Moreover, with increasing amounts of silicon and calcium sulfate, the viscosity of the pulp solution may increase and the mass transfer resistance at the acid-slag interface could decrease [13, 24]. Hence, the leaching time of 12 h was sufficient for leaching appreciable amounts of vanadium and chromium.
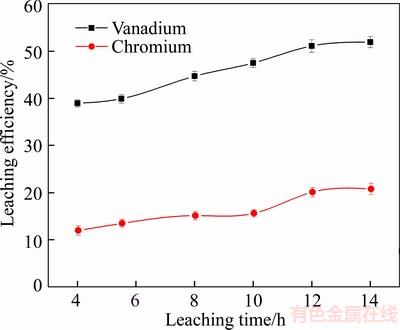
Figure 4 Effect of leaching time on leaching efficiency of vanadium and chromium
3.4 Effect of leaching temperature
To determine the effect of temperature on the leaching efficiencies of vanadium and chromium, the temperature was varied from 50 °C to 90 °C. The concentration of sulfuric acid remained constant at 40 wt%, the MnO2-to-slag mass ratio was 10.0 wt%, liquid-to-solid ratio was 5:1 mL/g, and leaching time was 12 h.
Figure 5 shows that the leaching efficiency of vanadium and chromium increased gradually with increasing leaching temperature. That was likely because the increased temperature increased the number of activated molecules, accelerated molecular motion and collision between vanadium and chromium slag particles, and ensured full contact among the reactants. At the same time, the elevated temperature accelerated oxidation, and decreased the viscosity of the medium, increasing the diffusion rate and favoring increased reactions [5, 21]. Thus, the optimum temperature was determined to be 90 °C in this study, considering the costs and energy requirements [2, 24].

Figure 5 Effect of leaching temperature on leaching efficiency of vanadium and chromium
3.5 Effect of liquid-to-solid ratio
To determine the effect of the liquid-to-solid ratio on the leaching efficiencies of vanadium and chromium, the liquid-to-solid ratio was varied from 3:1 to 7:1 mL/g, with sulfuric acid concentration remaining constant at 40 wt% and the temperature at 90 °C. The mass ratio of MnO2-to-slag was 10.0 wt%, and the leaching time was 12 h. The results are shown in Figure 6.
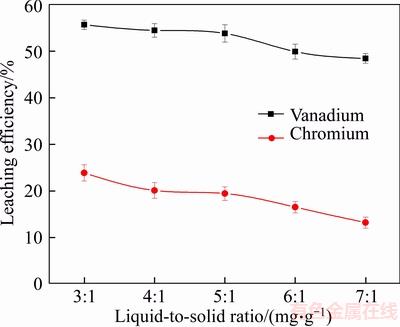
Figure 6 Effect of liquid-to-solid ratio on leaching efficiency of vanadium and chromium
From the above analysis, the effect of liquid- to-solid ratio on the leaching efficiencies of vanadium and chromium is clearly more significant than the other factors. Under the conditions of constant total leaching agent and increasing liquid-to-solid ratio, the volume of the liquid phase increased as the concentration of the leaching agent decreased. The chemical reaction equilibrium disfavored the leaching reaction, reducing the leaching efficiencies of vanadium and chromium [11]. When the liquid-to-solid ratio was 3:1 mL/g, the concentration of leaching agent was too high, which increased the difficulty of separating the leachate from the slag.
Meanwhile, a high liquid-to-solid ratio produced a large amount of industrial waste water, which resulted in significant environmental pollution. Moreover, the liquid-to-solid ratio of 5:1 mL/g was easy to filter, and the leaching efficiencies of vanadium and chromium were relatively high at this ratio. Therefore, the optimum ratio was set as 5:1 mL/g in this work.
3.6 Effect of surfactant types
According to the nature of the polar groups, surface-active agents can be divided into anionic, cationic, zwitterionic, and non-ionic surfactants. According to its properties and results from previous studies [23, 25], the preliminary experiment investigated these four types of surfactants.
To determine the effect of surfactant type on the leaching efficiencies of vanadium and chromium, four types of surfactant were investigated. The sulfuric acid concentration, MnO2-to-slag mass ratio, liquid-to-solid ratio, leaching temperature, and leaching time were held constant at 40 wt%, 10.0 wt%, 90 °C, 5:1 mL/g, and 12 h, respectively.
Figure 7 shows that different types of surfactants had different effects on the leaching process. SDS intensified the leaching efficiencies of vanadium and chromium while the others hindered the leaching process.
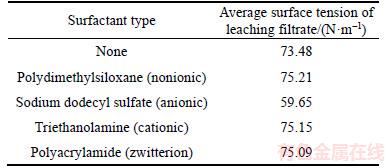
As shown in Table 2, the addition of different surfactant types resulted in different surface tensions. The addition of SDS reduced the average surface tension of the leachate, whereas the others increased the surface tension. The addition of SDS in the leaching process reduced the average surface tension of the leaching solution and decreased the adhesion strength between the liquid and solid phases. These factors likely reduced the moisture content of the slag to facilitate the filtration process. When the moisture content of the slag was reduced, the soluble vanadium present in the residual slag decreased. This phenomenon may increase the content of vanadium in the leaching solution and raise the leaching efficiency of vanadium [23]. Thus, SDS was selected for further study.
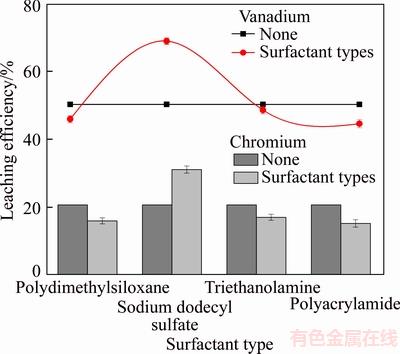
Figure 7 Effect of different surfactant types on leaching efficiencies of vanadium and chromium
Table 2 Lixivium of average surface tension by adding different surfactants (T=25.0 °C)
3.7 Effect of SDS-to-slag mass ratio
To determine its effect on the leaching efficiencies of vanadium and chromium, the mass ratio of SDS-to-slag was varied from 0 wt% to 5.0 wt% while the concentration of sulfuric acid remained at 40 wt% and the temperature at 90 °C. The leaching time was 12 h, the MnO2-to-slag ratio was 10.0 wt% and the liquid-to-solid ratio was 5:1 mL/g.
Figure 8 shows that increased SDS-to-slag mass ratio did not significantly change the leaching efficiency of vanadium and chromium. Table 3 shows that the average surface tension of the leaching filtrate was reduced by the addition of SDS.
During the leaching process of vanadium and chromium from the converter vanadium slag, the addition of SDS changed the properties of surface wettability to intensify leaching. SDS considerably reduced the surface tension of the leaching solution. This uniformly distributed the slag in the leaching solution, helping to maintain optimal contact with the leaching agent. This effect can be attributed to the surfactant, which can adsorb on the surface of slag grain, changing its wettability, and increasing the contact between the slag and leaching agent. Meanwhile, the surfactant exhibited the characteristics of parent molecular structure, which produced oriented adsorption at the interface of the solution, reducing the surface tension of the leaching solution, and enhancing the wettability and permeability of the solution. Thus, the leaching efficiency of vanadium was effectively improved. At the same time, the addition of the surfactant reduced the moisture content of the slag which aided filtration [23, 25].
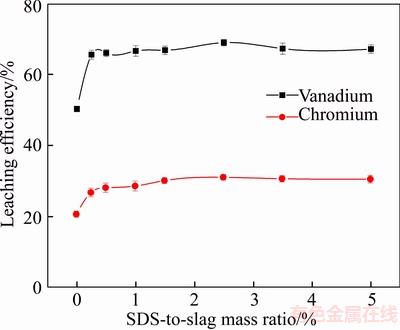
Figure 8 Effect of SDS-to-slag mass ratio on leaching efficiencies of vanadium and chromium
Table 3 Effect of SDS-to-slag mass ratio on surface tension (T=25.0 °C)
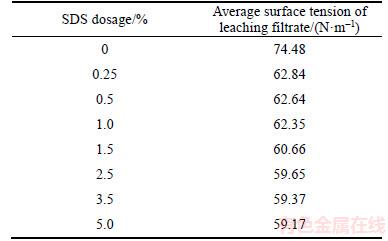
4 Reaction mechanism
Based on the acid oxidation reaction that occurred, the mechanism of acid oxidation leaching is depicted in Figure 9.
UV-vis spectroscopy is a powerful tool that can be used to elucidate the ion valence state of vanadium and chromium. According to previous studies, V(IV) and Cr(VI) absorb at about 760 nm and 373 nm, respectively [2], as shown in Figure 10. Vanadium and chromium were present in the forms of V(IV) and Cr(VI), and through the sulfuric acid leaching process, the pH of the leaching solution was less than 2. Vanadium was present in the leaching solution in the VO2+ ion.

Figure 9 Mechanism of vanadium leaching

Figure 10 UV-vis spectra of leaching solution
During the leaching process, protons (H+) damaged the silicate phase, exposing the spinel phase wrapped in the silicate phase in the process. Then, MnO2 oxidized the low-valent vanadium and chromium to improve the respective leaching efficiencies of vanadium and chromium. The main reaction occurring in the leaching process is as follows:
3MnO2+4H2SO4+2Fe2VO4=(VO2)2SO4+4H2O+2Fe2O3+3MnSO4 (3)
The addition of the surfactant SDS changed the chemical behavior of vanadium and chromium in the aqueous solution, which enhanced the efficiency of MnO2 oxidization and subsequent leaching [21, 26].
Surfactants reduced the surface tension of the leaching solution and changed the wetting properties in the variable leaching system. Surfactants had high surface activity and good diffusion and permeability, allowing them to quickly penetrate into the inner surface of the slag particle pores where they were adsorbed.
Adsorption of SDS on the surface of the slag in the leaching solution had a significant impact on the wetting effect. The surface of the converter vanadium slag generally exhibits significant electronegativity [23, 27]. For anionic surfactants, the nonpolar group was adsorbed on the surface of the converter vanadium slag. The polar group was oriented toward the leaching solution, which allowed the protons (H+) to enter the inner areas of the converter vanadium slag and promoted the leaching process. This mechanism is shown in Figure 11.
The results shown in Figure 12(a) indicate that the original converter vanadium slag particles were irregular in shape and unevenly distributed. From Figure 7(b), the irregular shapes were broken into molten particles, the sheet-like structures were broken into small irregular pieces, and the surface became rough after leaching. The silicate and spinel phases were partially broken after acid oxidation leaching.
5 Conclusions
1) Within the scope of the critical micelle concentration, the addition of SDS considerably affected the chemical properties of the pulp surface, increasing the hydrophilicity, and decreasing the surface tension of the leaching solution and moisture content of the slag. Hence, the addition of SDS could improve the leaching efficiencies of vanadium and chromium.
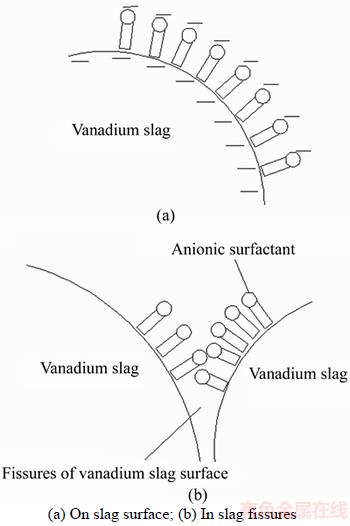
Figure 11 Adsorption of anionic surfactant on slag surface:
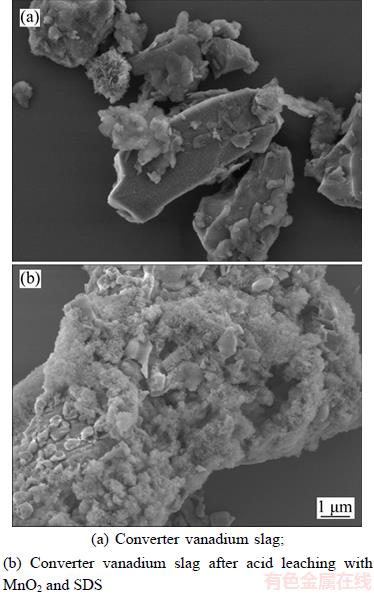
Figure 12 SEM images of converter vanadium slag before and after leaching:
2) Vanadium and chromium existed in low valence in the converter vanadium slag and were not directly leached to a great extent. The addition of MnO2 combined with protons (H+) damaged the slag structure and greatly improved the leaching efficiency of vanadium from the converter vanadium slag.
3) The leaching efficiencies of vanadium and chromium from the converter vanadium slag were 68.93 % and 30.74 %, respectively, under the optimal conditions: sulfuric acid concentration of 40 wt%; MnO2-to-slag mass ratio of 10.0 wt%; liquid-to-solid ratio of 5:1 mL/g; time of 12 h; temperature of 90 °C; and SDS-to-slag mass ratio of 0.25 wt%.
References
[1] CHEN Xiang-yang, LAN Xin-zhe, ZHANG Qiu-li, MA Hong-zhou, ZHOU Jun. Leaching vanadium by high concentration sulfuric acid from stone coal [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(s1): 123–126.
[2] LIU Zuo-hua, NUERAIHEMAITI A, CHEN Man-li, DU Jun, FAN Xing, TAO Chang-yuan. Hydrometallurgical leaching process intensified by an electric field for converter vanadium slag [J]. Hydrometallurgy, 2015, 155: 56–60.
[3] MOSKALY K R R, ALFANTAZI A M. Processing of vanadium: A review [J]. Minerals Engineering, 2003, 16(9): 793–805.
[4] MIRAZIMI S M J, RASHCHI F, SABA M. A new approach for direct leaching of vanadium from LD converter slag [J]. Chemical Engineering Research and Design, 2015, 94: 131–140.
[5] PENG Hao, LIU Zuo-hua, TAO Chang-yuan. Leaching kinetics of vanadium with electro-oxidation and H2O2 in alkaline medium[J]. Energy & Fuels, 2016, 30: 7802–7807.
[6] LI Xin-sheng, XIE Bing. Extraction of vanadium from high calcium vanadium slag using direct roasting and soda leaching[J]. International Journal of Minerals, Metallurgy, and Materials, 2012, 19(7): 595–601.
[7] YANG Zhao, LI Hong-yi, YIN Xu-chen, YAN Zhi-ming, YAN Xiao-man, XIE Bing. Leaching kinetics of calcification roasted vanadium slag with high CaO content by sulfuric acid [J]. International Journal of Mineral Processing, 2014, 133: 105–111.
[8] ZHANG Yi-min, BAO Shen-xu, LIU Tao, CHEN Tie-jun, HUANG Jing. The technology of extracting vanadium from stone coal in China: History, current status and future prospects [J]. Hydrometallurgy, 2011, 109(1, 2): 116–124.
[9] CHEN De-sheng, ZHAO Hong-xin, HU Guo-ping, QI Tao, YU Hong-dong, ZHANG Guo-zhi. An extraction process to recover vanadium from low-grade vanadium-bearing titanomagnetite [J]. Journal of Hazardous Materials, 2015, 294: 35–40.
[10] HE Dong-sheng, FENG Qi-ming, ZHANG Guo-fan, OU Le-ming, LU Yi-ping. An environmentally-friendly technology of vanadium extraction from stone coal [J]. Minerals Engineering, 2007, 20(12): 1184–1186.
[11] YANG Kang, ZHANG Xiao-yun, TIAN Xue-da, YANG Yong-long, CHEN Yan-bo. Leaching of vanadium from chromium residue [J]. Hydrometallurgy, 2010, 103(1–4): 7–11.
[12] ZHANG Guo-quan, ZHANG Ting-an, LV Guo-zhi, ZHANG Ying, LIU Yan, LIU Zhuo-lin. Extraction of vanadium from vanadium slag by high pressure oxidative acid leaching [J]. International Journal of Minerals, Metallurgy, and Materials, 2015, 22(1): 21–26.
[13] AARABI-KARASGANI M, RASHCHI F, MOSTOUFI N, VAHIDI E. Leaching of vanadium from LD converter slag using sulfuric acid [J]. Hydrometallurgy, 2010, 102(1–4): 14–21.
[14] YE Pu-pong, WANG Xue-wen, WANG Ming-yu, FAN Ye-ye, XIANG Xiao-yan. Recovery of vanadium from stone coal acid leaching solution by coprecipitation, alkaline roasting and water leaching [J]. Hydrometallurgy, 2012, 117–118: 108–115.
[15] SHAO Yan-hai, FENG Qi-ming, CHEN Yun, OU Le-ming, ZHANG Guo-fang, LU Yi-ping. Studies on recovery of vanadium from desilication residue obtained from processing of a spent catalyst [J]. Hydrometallurgy, 2009, 96(1, 2): 166–170.
[16] WANG Fei, ZHANG Yi-min, HUANG Jing, LIU Tao, WANG Yi, YANG Xiao, ZHAO Jie. Mechanisms of aid-leaching reagent calcium fluoride in the extracting vanadium processes from stone coal [J]. Rare Metals, 2013, 32(1): 57–62.
[17] WANG Tai-ying, XU Long-jun, LIU Chen-lun, ZHANG Zhao-di. Calcified roasting-acid leaching process of vanadium from low-grade vanadium-containing stone coal [J]. Chinese Journal of Geochemistry, 2014, 33(2): 163–167.
[18] WANG Zhong-hang, ZHENG Shi-li, WANG Shao-na, QIN Ya-ling, DU Hao, ZHANG Yi. Electrochemical decomposition of vanadium slag in concentrated NaOH solution [J]. Hydrometallurgy, 2015, 151: 51–55.
[19] WANG Zhong-hang, DU Hao, WANG Shao-na, ZHENG Shi-li, ZHANG Yi, HWANG S. Electrochemical enhanced oxidative decomposition of chromite ore in highly concentrated KOH solution [J]. Minerals Engineering, 2014, 57: 16–24.
[20] LIU Biao, DU Hao, WANG Yi, ZHENG Shi-li, LI Lan-jie. A novel method to extract vanadium and chromium from vanadium slag using molten NaOH-NaNO3 binary system [J]. AICHE Journal, 2013, 59(2): 541–552.
[21] YAN Wen-bin, HU Lan-shuang, GAO Feng, HAO Jun, HE Xin-bo. Effect of manganese dioxide on acid leaching of vanadium form stone coal [J]. Chinese Journal of Rare Metals, 2013, 37(1): 130–134. (in Chinese)
[22] WANG Ming-yu, XIAO Lian-sheng, LI Qing-gang, WANG Xue-wen, XIANG Xiao-yan. Leaching of vanadium from stone coal with sulfuric acid [J]. Rare Metals, 2009, 28(1): 1–4.
[23] WU Ai-xiang, AI Chun-ming, WANG Yi-ming, LI Xi-wen. Surfactant accelerating leaching of copper ores [J]. Journal of University of Science and Technology Beijing, 2013(6): 709–713. (in Chinese)
[24] PENG Hao, LIU Zuo-hua, TAO Chang-yuan. Selective leaching of vanadium from chromium residue intensified by electric field [J]. Journal of Environmental Chemical Engineering, 2015, 3(2): 1252–1257.
[25] QIN Jia, MAO Ming-fa, LIU Chun-lin, ZHANG Xiao, LI Zhao-dong. Study on effect of surfactant on copper ore leaching [J]. Mining and Metallurgical Engineering, 2013, 33(5): 115–118. (in Chinese)
[26] LIANG Tai-ran, DAI Zi-lin, HAO Wen-bin. Effect of acid- leaching reagent on extracting vanadium form stone coal [J]. Mining and Metallurgical Engineering, 2010, 30(6): 69–71. (in Chinese)
[27] PAN Zi-wei, WANG Da-wei, DU Hao, CHEN Gang, ZHENG Shi-li. Extraction technology of vanadium ang chromium from vanadium slags in presence of activated carbon [J]. Journal of Central South University, 2014, 24(8): 2171–2181.
(Edited by FANG Jing-hua)
中文导读
表面浸润强化转炉钒渣中钒和铬的浸出
摘要:介绍一种从转炉钒渣中提取钒和铬的表面浸润强化浸出技术,考察硫酸浓度、二氧化锰(MnO2)的添加量、液固比、浸出时间、浸出温度以及十二烷基硫酸钠(SDS)的添加量对钒和铬浸出率的影响。 结果表明:在浸出过程中添加MnO2和SDS,钒和铬的浸出率比未添加时分别高33.46% 和20.02%,在硫酸浓度40 wt%、反应时间 2.0 h、液固比 5:1 mL/g、反应温度90 °C、MnO2添加量为10.0 wt%以及SDS的添加量0.25 wt%时,钒和铬的浸出率分别为68.93%和30.74%。浸出过程反应机理表明,MnO2协同H+离子可以氧化低价钒和铬,SDS能改变溶液表面化学行为,降低溶液表面张力,有利于MnO2的氧化。
关键词:钒;铬;浸出;十二烷基硫酸钠;二氧化锰
Foundation item: Project(2015BAB17B00) supported by the National Key Technology R&D Program of China; Project(CYB15045) supported by the Program for Chongqing University Postgraduates’ Innovation Project, China
Received date: 2017-01-04; Accepted date: 2017-03-06
Corresponding author: XIE Zhao-ming, PhD; Tel: +86–13436059696; E-mail: xiezm@cqu.edu.cn; LIU Zuo-hua, PhD, Professor; Tel: +86–15922926287; E-mail: liuzuohua@cqu.edu.cn
Abstract: Technology intensified with surface wettability was introduced to leach vanadium and chromium from converter vanadium slag without roasting. Parameters affecting the leaching efficiency of vanadium and chromium were investigated: sulfuric acid concentration, MnO2-to-slag mass ratio, liquid-to-solid ratio, leaching time, leaching temperature, and sodium dodecyl sulfate (SDS)-to-slag mass ratio. The leaching efficiencies of vanadium and chromium were 33.46 % and 20.02 % higher in the presence of MnO2 and SDS, respectively, compared to the control. The leaching efficiencies of vanadium and chromium were 68.93 % and 30.74 %, respectively, under the optimum conditions: sulfuric acid concentration 40 wt%, MnO2-to-slag mass ratio 10.0 wt%, liquid-to-solid ratio 5:1 mL/g; 12 h; 90 °C; and SDS-to-slag mass ratio 0.25 wt%. The analysis of the reaction mechanism in the leaching process indicates that MnO2 combined with protons (H+) could oxidize low-valent vanadium and chromium; SDS could change the chemical behavior and decrease the surface tension of the aqueous solution to favor MnO2 oxidization.


