
Novel aerosol method for aligned carbon nanotubes synthesis
CHEN Xiao-hua(陈小华)1, 2, LI Wen-hua(李文华)2, YANG Zhi(杨 植)2, XU Long-shan(许龙山)2,
CHEN Chuan-sheng(陈传盛)2, WANG Yan-guo(王岩国)2
1. College of Materials Science and Engineering, Changsha University of Science and Technology,Changsha 410076, China;
2. College of Materials Science and Engineering, Hunan University, Changsha 410082, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
A novel aerosol method for the production of alighed carbon nanotubes was presented. Multi-walled carbon nanotubes (MWNTs) with fairly uniform diameters and aligned MWNT bundles were obtained by using solutions of organometallics such as ferrocene in hydrocarbon solvents. A hollow and multi-walled structure was observed with diameter ranging from 15 to 50 nm, leading aspect ratio to be over 200. The quality of the product is dependent on the pyrolysis temperature, carrier gas flow rate and the catalyst precursor concentration. For the sample synthesized at 900 ℃, G/D is 1.81. G/D of samples obtained at 850 ℃ and 800 ℃ are 1.18 and 0.77, respectively. The inclusion of acetylene to an atomized spray of ferrocene in hexane yield aligned MWNT bundles with a narrow diameter distribution.
Key words:
aerosol; carbon nanotubes; aligned morphology;
1 Introduction
Since carbon nanotubes were discovered by Iijima in 1991[1], considerable efforts have been made to study their preparation, growth mechanism and potential applications[2-4]. Aligned and well separated CNT morphology is important for many potential applications, where a high electric-field concentration is needed in field emission applications, and a large surface area is desirable in catalytic reactions, such as fuel cell or battery applications. It is very desirable to directly synthesize aligned carbon nanotubes (ACNTs). Up to now, a lot of studies have been done on the synthesis of ACNTs by the CVD method[5-9]. They can be broadly divided into chemical and physical methods according to the way that carbon atoms are released from carbon-containing precursor molecules. In the physical methods, high-energy input is used to release the carbon atoms. The chemical methods rely on carbon atomization via catalytic decomposition of carbon precursors on the surface of transition metal particles. In CVD methods,
the carbon precursor decomposition and CNT formation take place on the surface of catalyst particles that are supported on a substrate. Conversely, in nebulized spray synthesis, the catalyst particles are suspended in the gas-phase throughout the entire CNT formation process. Nebulized spray is generated by an ultrasonic atomizer. When a high frequency (100 kHz-10 MHz) ultrasonic beam is directed to a gas/liquid interface, geyser forms at the surface, and the height of the geyser is proportional to the acoustic intensity. The formation of the geyser is accompanied by the generation of a spray, resulting from the vibrations at the liquid surface and cavitation at the gas/liquid interface[10]. Nebulized spray pyrolysis has been employed for the preparation of submicron sized particles and epitaxial thin films[11-12] of complex metal oxides. The advantage of nebulized spray is the ease of scaling into an industrial scale process, as the reactants are fed into the furnace continuously. In this study, the nebulized spray pyrolysis process involving liquid aerosol containing both the carbon and the catalyst sources was used and prepare clean to well-aligned carbon nanotubes.
2 Experimental
Silicon substrates were placed in the region of the reactor to collect the products. In a typical synthesis, 2 g metallocene was dissolved in 100 mL of a hydrocarbon and the solution nebulized using a 1.54 MHz ultrasonic beam was carried into a 40 mm quartz tube, placed in a SiC furnace maintained at the required temperature (800-1 000 ℃). Ultra pure argon was used as the carrier gas and the gas flow rate was controlled using mass flow controllers. In a typical procedure, the carrier gas flow rate was kept at 1 000 mL/min and the pyrolysis was carried for 30 min. After the reaction, the flow rate was reduced to 80 mL/min and the furnace was allowed to cool. The products were collected after the tube was cooled to room temperature. Ferrocene was used as both catalyst and carbon sources. Hexane was used as solvents for the catalysts and acted as additional carbon sources.
Morphology of the samples was characterized using a JSM-6700F SEM and JEM-3010 HRTEM. Raman spectrum was measured using a Jobin Yvon-Horiba Labran-010 Raman spectrometer with an excitation wavelength of 632.8 nm.
3 Results and discussionGenerally, samples are composed of carpets of clean MWNTs perpendicularly aligned to the substrate surface. SEM observation (Fig.1) shows the section of a carpet composed of well-aligned MWNTs obtained when the aerosol flow passes through the reactor during 30 min. The length of the aligned CNTs are approximately 200 μm. Fig.1(d) shows a magnified SEM image of Fig.1(c). The aligned CNTs are shown with a high number density. The low magnification TEM image (Fig.2) shows that the carbon nanotubes are the product obtained and no trace of carbon nanoparticles, which indicates the high selectivity of the synthesis method in good agreement with the SEM results. A hollow and multi-walled structure is observed with diameter ranging from 15 to about 50 nm, leading to an aspect ratio over 200.
The optimum temperature for the growth of MWNTs is 900 ℃. As the temperature decreases the large quantities of carbon spheres are obtained, as shown in Fig.3. Furthermore, the quantity of the reactants carried inside the furnace is directly proportional to the Ar flow rate. The flow rate of the carrier gas afects the yield and quality of the MWNTs. At low argon flow rate of 200 mL/min, yield of MWNTs is negligible. Short MWNTs and large particles are obtained at a high flow rate of 2 000 mL/min of argon. A flow rate of 1 000 mL/min of argon is found to be ideal for the growth of MWNTs. In addition, the concentration of ferrocene in hexane is crucial in determining the yield of the MWNTs. As the concentration of ferrocene increases, the yield of MWNTs also increases. A concentration of 20 g/L is found to be ideal while at higher concentration catalyst particles are obtained. GLERUP et al[13] observed the diameter of the MWNTs to be proportional to the concentration of the catalyst. The effect of the concentration in this experiment, however, is different. The higher concentration indicates that there are multiple nucleation centers within the droplet. Even though there is multiple nucleation, the diameter distribution of MWNTs obtained is found to be narrow. Any significant changes are not observed in diameter when the concentration changes.
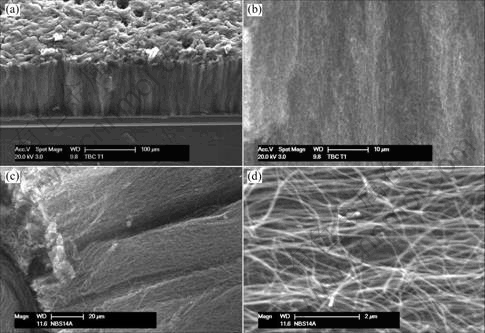
Fig.1 SEM images of sample obtained from pyrolysis of 5%(mass fraction) ferrocene solution in hexane at 900 ℃ during 30 min
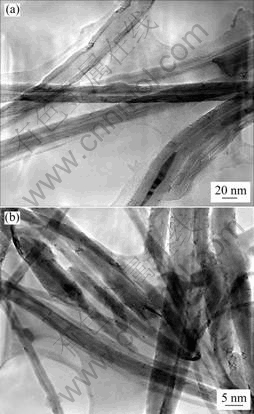
Fig.2 TEM image of MWNTs obtained from pyrolysis at (a) 900 ℃ and (b) 850 ℃ of 20% ferrocene solution in hexane during 30 min
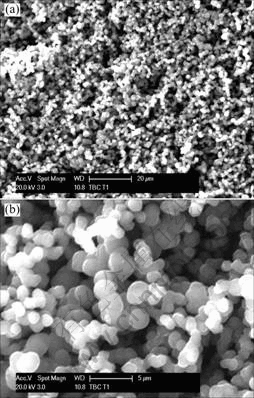
Fig.3 SEM image of sample obtained from pyrolysis at 800 ℃
Raman spectroscopy has been applied to all the samples obtained at different reaction temperatures. The Raman spectra of CNTs with different temperatures are shown in Fig.4. All the spectra indicate the presence of G-band (at about 1 590 cm-1) and D-band (at about 1 320 cm-1) with different G/D. The G-band is representative of the amount of graphitization associated with the nanotube growth, while the D-band represents the amount of defects (open ends, disorder, amorphous deposit, etc). It is observed that the G/D ratio of as-grown nanotubes depends on the reaction temperature. For the sample synthesized at 900 ℃, the G/D ratio is 1.81. G/D values of samples obtained at 850 ℃ and 800 ℃ are 1.18 and 0.77, respectively. The broad D-band is an indication of the formation of defective MWNTs and graphitic impurities. The narrow D-band and higher G/D observed at 900 ℃ synthesis indicate that the amounts of graphitic impurities and side wall defects are much lower than those at the other temperatures.
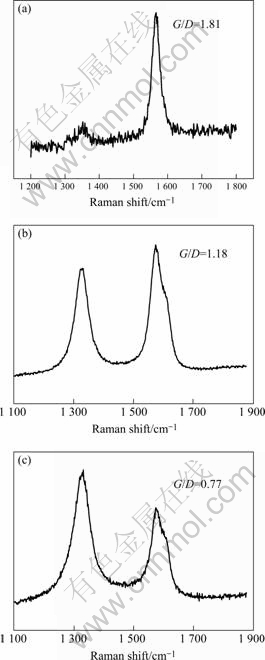
Fig.4 Raman spectra of aligned CNTs obtained at different reaction temperatures: (a) 900℃; (b) 850℃; (c) 800℃
4 Conclusions
Well-aligned high-density carbon nanotubes were synthesized by aerosol method. A hollow and multi-walled structure was observed with diameter ranging from 15 to about 50 nm, leading to an aspect ratio over 200. The quality of the product is dependent on the pyrolysis temperature, carrier gas flow rate and the catalyst precursor concentration. The optimum temperature for the growth of MWNTs is 900 ℃. A flow rate of 1 000 mL/min of argon and a concentration of 20 g/L are found to be ideal for the growth of MWNTs. It is observed that the G/D ratio of as-grown nanotubes depends on the reaction temperature. For the sample synthesized at 900 ℃, the G/D ratio is 1.81. The G/D ratios of samples obtained at 850 ℃ and 800 ℃ are 1.18 and 0.77, respectively. Any significant changes in diameter are not observed when the concentration is changed. The procedure described can be scaled up for large-scale production.
References[1] IIJIMA S. Helical microtubules of graphitic carbon[J]. Nature, 1991, 354: 56.
[2] COLLINS P G, ZETTLE A, BANDO H, et al. Nanotube nanodevice[J]. Science, 1997, 278: 100-103.
[3] TANS S J, DEVORET M H, DAI H, et al. Individual single-wall carbon nanotubes as quantum wires[J]. Nature, 1997, 386: 474.
[4] KONG J, FRANKLIN N R, ZHOU C W, et al. Nanotube molecular wires as chemical sensors[J]. Science, 2000, 287: 622-625.
[5] KIRSTEN EDGAR, JOHN L. Aerosol-based synthesis of carbon nanotubes[J]. Spencer Current Applied Physics, 2004, 4: 121-124.
[6] IAIA A, MARTY C, NAUD V, LOISEAU A, DI MUOIO E, FOURNIER T, BONNOT A M. Oriented growth of suspended single wall carbon nanotube by hot filament CVD[J]. Thin Solid Films, 2006, 501: 221-223.
[7] ANASTASIOS JOHN HART, ALEXANDER H. Slocum. Rapid growth and flow-mediated nucleation of millimeter-scale aligned carbon nanotube: Structures from a thin-film catalyst[J]. J Phys Chem B, 2006, 110, 8250-8257.
[8] LIANG Z, TAN Y Q, DANIEL E.. Controlling the growth of vertically oriented single-walled carbon nanotubes by varying the density of CoAMo catalyst particles[J]. Chemical Physics Letters, 2006, 422: 198-203.
[9] CHIUA C C, TAIA N H, YEHB M K, CHENB B Y, TSENGA S H, CHANGC Y H. Tip-to-tip growth of aligned single-walled carbon nanotubes under an electric field[J]. Journal of Crystal Growth, 2006, 290: 171-175.
[10] LANGLET M, JOUBERT J C. Chemistry of advanced materials—a chemistry for the 21st century (IUPAC Monograph)[M]. Oxford: Blackwell Scientic Publishers, 1993: 55.
[11] RAJU A R, HEMANTKUMAR N, AND RAO C N R. Oriented films of LaNiO3 and other members of the Lan+1NinO3n+1 series, LaCuO3- delta. and Pb(Zr0.52Ti0.48)O3, obtained by nebulized spray pyrolysis[J]. Chem Mater, 1995, 7: 225-231.
[12] AIYER H N, RAJU A R, SUBBANNA G N. Epitaxial nature of the films of LaNiO3, Pb(Zr0.5Ti0.5)O3, and La0.95Mn0.95O3 obtained by nebulized spray pyrolysis[J]. Chem Mater, 1997, 9: 755-760.
[13] GLERUP M, KANZOW H, ALMAIRAC R, CASTIGNOLLES M. Synthesis of multi-walled carbon nanotubes and nano-bres using the aerosol method with metal-ions as the catalyst precursors[J]. Chemical Physics Letters, 2003, 377: 293-29.
Foundation item: Projects(50772033; 50372020) supported by the National Natural Science Foundation of China
Corresponding author: CHEN Xiao-hua; Tel: +86-731-8821610; E-mail: hudacxh62@yahoo.com.cn


