Trans. Nonferrous Met. Soc. China 26(2016) 2102-2108
Synthesis of Cu2ZnSnS4 thin film from mixed solution of Cu2SnS3 nanoparticles and Zn ions
Zheng-fu TONG1, Jia YANG1, Chang YAN2, Meng-meng HAO1, Fang-yang LIU1,2, Liang-xing JIANG1, Yan-qing LAI1, Jie LI1, Ye-xiang LIU1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Australian Centre for Advanced Photovoltaics, School of Photovoltaic and Renewable Energy Engineering, University of New South Wales, Sydney, NSW 2052, Australia
Received 7 August 2015; accepted 3 May 2016
Abstract:
The Cu2ZnSnS4 thin film was prepared by a facile solution method without vacuum environment and toxic substance. The formation mechanism of the film was studied by transmission electron microscopy (TEM), X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), and Raman scattering measurements. Through cyclic voltammetry and photo-electricity tests, the electrocatalytic activity of the prepared film as the counter electrode of dye-sensitized solar cell was also studied. The results show that the mixed precursor solution mainly consists of Cu2SnS3 nanoparticles and Zn ions. After 550 °C annealing process on the precursor film prepared from the mixed solution, Cu2ZnSnS4 thin film is obtained. Besides, it is found that the prepared Cu2ZnSnS4 thin film has the electrocatalytic activity toward the redox reaction of I3-/I- and the dye-sensitized solar cell with the prepared Cu2ZnSnS4 thin film as the counter electrode achieves the efficiency of 1.09%.
Key words:
Cu2ZnSnS4 thin film; Cu2SnS3 nanoparticle; Zn ion; electrocatalytic; dye-sensitized solar cells;
1 Introduction
Copper zinc tin sulfide (Cu2ZnSnS4) is the I2-II-IV-VI4 quaternary compound semiconductor. All constituents in Cu2ZnSnS4 are abundant in the crust of the earth (Cu: 50×10-6, Zn: 75×10-6, Sn: 2.2×10-6, S: 260×10-6) and are nontoxic. It not only has an appropriate energy gap (1.5 eV) and high absorption coefficient (over 1×104 cm-1 in visible wavelength region) for solar cell application [1], but also has an excellent catalytic activity for a variety of chemical and electrochemical reactions [2,3]. So, it has attracted much attention in these years.
Many researches have reported the preparation of Cu2ZnSnS4 thin films via vacuum and non-vacuum methods such as evaporation [4], sputtering [5], pulsed laser deposition [6], sol–gel [7], successive ionic layer adsorption and reaction [8], electrodeposition [9], and particle-ink coating method [10-12]. Compared with vacuum methods, non-vacuum methods have the advantage of low fabrication cost. TANAKA et al [7] first prepared Cu2ZnSnS4 thin films by spin-coating a sol-gel precursor solution containing Cu, Zn and Sn metal salts in 2-methoxyethanol solvent. SCRAGG et al [13] made Cu2ZnSnS4 thin films on Mo substrates using sequential electrodeposition of constituents metals Cu, Zn and Sn. SHINDE et al [14] reported a novel chemical successive ionic layer adsorption and reaction (SILAR) technique for Cu2ZnSnS4 thin films formation by sequential immersion of the substrate into the cationic precursor solutions of CuSO4, ZnSO4, SnSO4 and the anionic precursor solution of thioacetamide. TODOROV et al [15] prepared Cu2ZnSnS4 thin films using an hybrid solution-particle approach where the slurry consists of Cu-Zn-Sn chalcogenide in hydrazine. GUO et al [10] reported the synthesis of Cu2ZnSnS4 nanoparticles in oleyamine. ZHOUG et al [11] synthesized high quality Cu2ZnSn(S,Se)4 film from the aqueous nanoink which consists of the Cu/Zn sulfide nanoparticles with Sn2S64– and Sn2S76– as self-component ligands.
Despite many methods have been adopted to prepare the Cu2ZnSnS4 film, the preparation of the Cu2ZnSnS4 film from mixed solution of Cu2SnS3 nanoparticles and Zn ions has not been studied. As the preparation of the mixed (precursor) solution is simple and does not need vacuum environment, high temperature and any extremely toxic substance such as hydrazine and toluene, it is a low-cost and environmentally-friendly technology which is appropriate for industrial production. The formation mechanism of Cu2ZnSnS4 thin films from the mixed solution is studied. Moreover, the prepared Cu2ZnSnS4 thin film is applied as the counter electrode in dye-sensitized solar cell (DSSC).
2 Experimental
A typical synthesis process for the preparation of Cu2ZnSnS4 thin films from the mixed solution of Cu2SnS3 nanoparticles and Zn ions is as follows. First, 0.068 g CuCl2·2H2O, 0.048 g SnCl2·2H2O and 0.055 g ZnCl2 are mixed and dissolved in 50 mL ethylene glycol at 165 °C. Then, 0.25mL mercapto-acetic acid is dropped into the solution and the color of the solution changes from green to black quickly. After stirring for 0.5 h, the precursor solution of nanoparticles and metal ions is obtained. Second, the precursor solution is drop-coated onto a Mo or glass substrate. Finally, the prepared precursor thin film is annealed in sulfur atmosphere at 550 °C to obtain the Cu2ZnSnS4 nanoparticals thin films. The Cu2ZnSnS4 thin films deposited on Mo substrates are used as counter electrodes in dye-sensitized solar cells (DSSC) which are assembled according to Refs. [16,17].
The nano-porous TiO2 films are fabricated by pasting the colloid containing 25 nm TiO2 nanoparticles on the fluorine-doped tin oxide (FTO) substrates, followed by sintering at 500 °C for 30 min. The sintered films are then dipped in a N719 solution for 12 h to allow the dye to adsorb on the innersurfaces of the nano-porous films. Then, the films are rinsed in ethanol and dried. Finally, iodine-based electrolyte (the electrolyte solution consists of 0.04 mol/L LiI, 0.03 mol/L I2, 0.1 mol/L GuSCN (Guanidinium thiocyanate), 0.5 mol/L TBP(4-tert-Butylpyridine), 1 mol/L PMII (1-Methyl-3-propyl imidazolium iodide) in acetonitrile and propylene carbonate (V:V=1:1)) is dropped onto the films and these films are assembled with the Cu2ZnSnS4 thin film as counter electrodes to obtain DSSC samples.
The nanoparticles of the mixed precursor solution are purified by centrifugation. Then, the precipitated nanoparticles are collected and studied by transmission electron microscopy (TEM). The crystallographic properties of the prepared thin films are characterized by X-ray diffraction (XRD, Rigaku3014) and Raman spectrometer (LabRAM ARAMIS). The surface morphology and the composition of the films are analyzed by a field-emission scanning electron microscope (SEM, FEI Quanta-200) equipped with an energy dispersive X-ray spectroscopy system (EDS, EDAX-GENSIS60S). The cyclic voltammetry (CV) study is carried out in a three-electrode system on PARSTAT 4000 (AMETEK.) with a pure graphite plate as the counter electrode, a Ag/Ag+ electrode as the reference electrode and the prepared Cu2ZnSnS4 film as the working electrode in an acetonitrile solution containing 10 mmol/L LiI, 1 mmol/L I2, and 0.1 mmol/L LiClO4 at a scan rate of 50 mV/s. The current density- voltage (J-φ) characteristics of the DSSC with the prepared Cu2ZnSnS4 thin film as the counter electrode are measured using a Keithley 2400 source meter under simulated sunlight (100 mW/cm2, AM 1.5).

Fig. 1 TEM images (a, b) and SAED pattern (c) of Cu2SnS3 nanoparticles
3 Results and discussion
3.1 Characteristics of mixed precursor solution
To study the composition of the mixed precursor solution, the solution is centrifuged and the precipitate is collected and analyzed by TEM. Figure 1(a) shows the low-magnification TEM image of the nanoparticles with irregular shape. It indicates that the crystallinity of the nanoparticles is low which may be due to the low prepared temperature for the precursor solution. Despite of this, it would not affect the preparation of the Cu2ZnSnS4 thin film finally. The high-resolution TEM (HRTEM) image (Fig. 1(b)) illustrates the nanoparticles surface and lattice fringes with interplanar spacings of 3.13 and 3.34 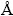 that can be ascribed to the
that can be ascribed to the 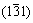 and
and 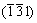 planes of Cu2SnS3, respectively. The selected area electron diffraction (SAED) pattern shown in Fig. 1(c) matches with the structure of Cu2SnS3 (JCPDS: 35-0684), as indicated via the diffraction spots corresponding to
planes of Cu2SnS3, respectively. The selected area electron diffraction (SAED) pattern shown in Fig. 1(c) matches with the structure of Cu2SnS3 (JCPDS: 35-0684), as indicated via the diffraction spots corresponding to 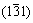 ,
,  and (304) planes. Figure 2 shows the XRD pattern of the film consisted of precipitated nanoparticles. The peaks at 26.89°, 28.9° and 47.79° are corresponding to
and (304) planes. Figure 2 shows the XRD pattern of the film consisted of precipitated nanoparticles. The peaks at 26.89°, 28.9° and 47.79° are corresponding to 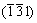 ,
, 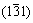 and
and 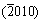 planes of Cu2SnS3 (JCPDS:35-0684), respectively. This result is in accord with the TEM results. To further confirm the composition of the mixed precursor solution, the EDS measurements are performed on the films prepared by the precursor solution and precipitated nanoparticles, respectively. The main composition of the film prepared by the precipitated nanoparticles is 34.66% Cu, 14.22% Sn and 47.64% S (mole fraction). Besides, there is a little amount of Cl (3.01%) and Zn (0.77%) which could be considered as the ion residues that are not washed off completely. The mole ratio of Cu/Sn/S is about 2:1:3 matching the composition ratio of Cu2SnS3. The main composition of the film prepared by the mixed precursor solution is 21.76% Cu, 10.92% Sn, 49.99% S, 6.69% Cl and 10.76% Zn. The content of Zn is the dominating difference between two films. This indicates that the precursor solution may mainly consist of two parts. One is nanoparticles Cu2SnS3 and the other is Zn ions in solvent.
planes of Cu2SnS3 (JCPDS:35-0684), respectively. This result is in accord with the TEM results. To further confirm the composition of the mixed precursor solution, the EDS measurements are performed on the films prepared by the precursor solution and precipitated nanoparticles, respectively. The main composition of the film prepared by the precipitated nanoparticles is 34.66% Cu, 14.22% Sn and 47.64% S (mole fraction). Besides, there is a little amount of Cl (3.01%) and Zn (0.77%) which could be considered as the ion residues that are not washed off completely. The mole ratio of Cu/Sn/S is about 2:1:3 matching the composition ratio of Cu2SnS3. The main composition of the film prepared by the mixed precursor solution is 21.76% Cu, 10.92% Sn, 49.99% S, 6.69% Cl and 10.76% Zn. The content of Zn is the dominating difference between two films. This indicates that the precursor solution may mainly consist of two parts. One is nanoparticles Cu2SnS3 and the other is Zn ions in solvent.
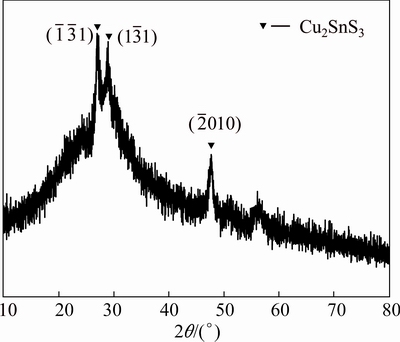
Fig. 2 XRD pattern of film prepared by precipitated nanoparticles
3.2 Annealing process of prepared film
Figure 3(a) shows the XRD patterns of films prepared by the precursor solution sulfurized at 350, 450 and 550 °C. From the XRD patterns, we can observe the peaks at 28.38°, 47.45° and 55.95°. As the structures of Cu2ZnSnS4 and Cu2SnS3 are very similar, so they have the close XRD peaks at these positions and it is difficult to distinguish the origin of these peaks only by XRD measurement. Thus, the films are further examined using Raman technology. After being sulfurized at 350 °C, from the Raman spectrum of the same film (Fig. 3(b)), there are two obvious peaks at about 336 and 338 cm-1 corresponding to Cu2SnS3 and Cu2ZnSnS4, respectively [18,19]. This indicates that the peaks at 28.38°, 47.45° and 55.95° of the corresponding XRD pattern belong to the planes of Cu2SnS3 (JCPDS: 35-0684) and Cu2ZnSnS4 (JCPDS: 26-0575). The generation of Cu2ZnSnS4 is probably due to the Zn atoms diffusion into Cu2SnS3 lattice in the sulfur atmosphere which agrees with other reports [20]. But as the sulfurized temperature is low, Cu2SnS3 cannot be totally reacted and translated to Cu2ZnSnS4 [21]. After being sulfurized at 450 °C, the XRD peaks of the prepared film at 28.38°, 47.45° and 55.95° become intense compared with those of film sulfurized at 350 °C. This is because higher annealing temperature improves the crystallization of the film. Emerging peaks at 15.13° and 31.86° corresponding to the (001) plane of SnS2 (JCPDS:23-0677) and the (200) plane of Cu2S (JCPDS:30-0505), respectively, are also observed. Moreover, from the Raman spectrum of the film (Fig. 3(c)), it is observed that the peak at 338 cm-1 corresponding to Cu2ZnSnS4 appears and the peak at 336 cm-1 corresponding to Cu2SnS3 disappears. This indicates that the XRD peaks at 28.38°, 47.45° and 55.95° are corresponding to Cu2ZnSnS4 only. Besides, a new peak at 313 cm-1 corresponding to SnS2 is observed [22]. According to the results of XRD patterns and Raman spectra, it indicates that the decomposition of the residual Cu2SnS3 introduces other two second phases such as Cu2S and SnS2. The formation mechanism of Cu2SnS3 has been reported according to the reversible reaction [23,24]:
Cu2S+SnS2 Cu2SnS3 (1)
Cu2SnS3 (1)
But as SnS2 is instable, it would easily decompose into SnS which vanishes as gas above 400 °C [25,26]. This leads the reaction above reversed towards the decomposition reaction of Cu2SnS3 which introduces the second phases Cu2S and SnS2. Utilizing the EDS measurement, it is found that Zn/Sn mole ratio of sample sulfurized at 450 °C increases to 1.07:1 compared with 0.99:1 of the precursor film. The increase of Zn/Sn mole ratio indicates Sn loss, which verifies our speculation. After being sulfurized at 550 °C, the XRD peaks at 18.2°, 28.5°, 32.9°, 47.3°, 56.1°, 69.16° and 76.48° of the prepared film become more intense. The sharp and strong major peak is an indication of the good crystalline quality of the film. From the Raman spectrum of the same film (Fig. 3(d)), an obvious major peak at 338 cm-1 and a minor peak at 289 cm-1 corresponding to Cu2ZnSnS4 can be clearly seen and no other characteristic peaks of impurity phases are observed [19,27]. So, the seven main XRD peaks mentioned above reflected the (101), (112), (200), (220), (312), (008) and (332) planes of kesterite Cu2ZnSnS4. The disappearance of the second phases Cu2S and SnS2 is likely due to the reaction of Cu2S, SnS2 and Zn atoms in sulfur atmosphere at relatively high temperature forming the single Cu2ZnSnS4 phase which is similar with the hybrid solution-particle approach for the preparation of Cu2ZnSnS4 using Cu-Zn-Sn chalcogenide [28]. The composition ratio of the film sulfurized at 550 °C is n(Cu)/n(Zn+Sn)=0.92:1, n(Zn)/n(Sn)=1.1:1 (25.06% Cu, 14.34% Zn, 13.01% Sn and 47.59% S) approaching the composition ratio of the high-quality Cu2ZnSnS4 thin film.
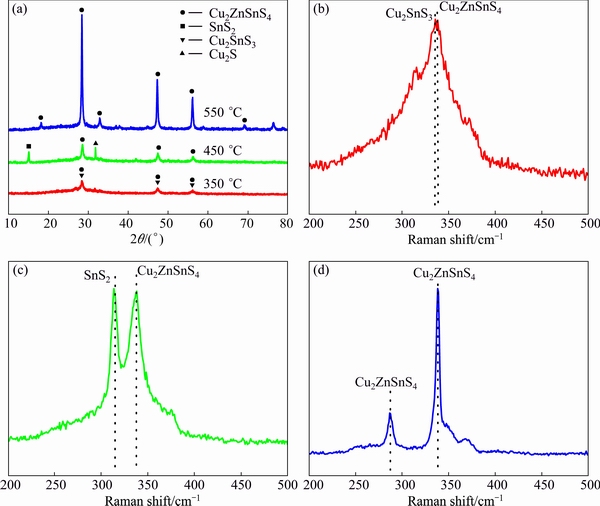
Fig. 3 XRD patterns (a) and Raman spectra (b-d) of films prepared by precursor solution sulfurized at 350 °C (b), 450 °C (c) and 550 °C (d)
Figure 4 shows the SEM images of Cu2ZnSnS4 thin films after being sulfurized at 350, 450 and 550 °C. After being sulfurized at 350 °C, no obvious large particles can be observed because the film is poorly crystalline and there are some residual organics undecomposed on the surface of the film. After being sulfurized at 450 °C, obvious nanostructures are observed which mean the improvement of the crystalline and the decomposition of the residual organics in the film. Despite of it, the surface morphology of the film is not uniform. The particles are in different shapes like spherical and sheet, and different sizes ranging from 150 nm to 2 μm. This is because of the hybrid phases of the film with SnS2, Cu2S and Cu2ZnSnS4, which is proved by the XRD patterns and Raman spectra. After sulfurization at 550 °C, the surface morphology of the film (Fig. 4(c)) becomes uniform and the size of the particles is around 300 nm.
According to the analysis above, we can speculate the formation process of the Cu2ZnSnS4 thin film by the facile solution method, as shown in Fig. 5. During the solution reaction process, CuCl2·2H2O, SnCl2 and mercapto-acetic acid would react to form Cu2SnS3 nanoparticles. In the sulfurized process at 350 °C, Cu2SnS3 nanoparticles, Zn atoms and S vapor would react to form Cu2ZnSnS4 and partial Cu2SnS3 unreacted exists in the film. When the sulfurized temperature rises up to 450 °C, Cu2SnS3 unreacted would decompose into SnS2 and Cu2S. In the sulfurized process at 550 °C, SnS2 and Cu2S would react with Zn atoms forming Cu2ZnSnS4 and the film of pure Cu2ZnSnS4 is achieved.
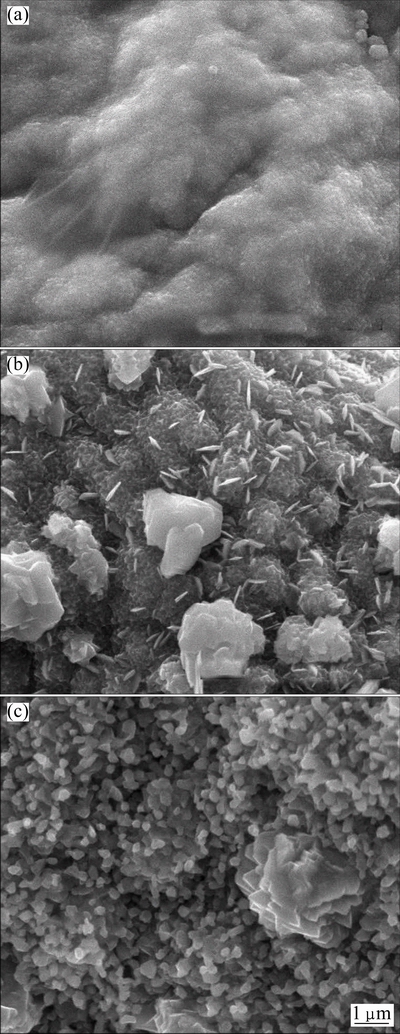
Fig. 4 SEM images of Cu2ZnSnS4 thin films after being sulfurized at 350 °C (a), 450 °C (b) and 550 °C (c)
3.3 Characteristics of electrocatalytic activity of prepared Cu2ZnSnS4 thin film
Figure 6(a) shows the cyclic voltammetry of the I3-/I- system with the Cu2ZnSnS4 thin film working electrode. One pair of oxidation and reduction peaks (Ox/Red) is observed which could be described by [29]
I3-+2e 3I- (2)
3I- (2)
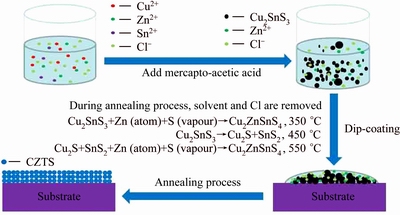
Fig. 5 Formation mechanism of Cu2ZnSnS4 film from mixed solution of Cu2SnS3 nanoparticles and Zn ions
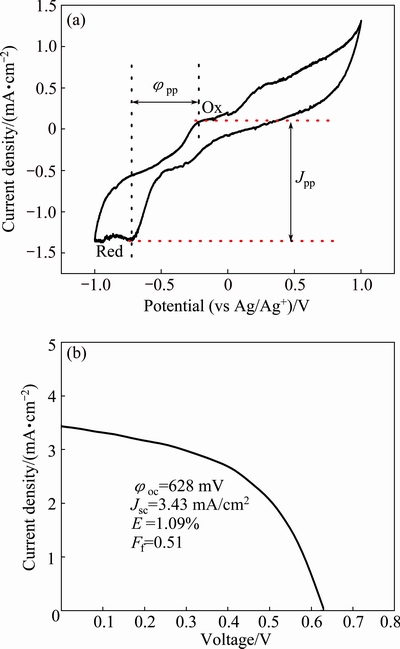
Fig. 6 Cyclic voltammetry of Cu2ZnSnS4 thin film counter electrode in acetonitrile solution of 10 mmol/L LiI, 1 mmol/L I2, and 0.1 mmol/L LiClO4 at scan rate of 50 mV/s (a), and J-φ characteristics of DSSCs using Cu2ZnSnS4 thin film counter electrode (b)
From Eq. (2), we can observe that the voltage separation (φpp) and currents density separation (Jpp) of the two peaks(Ox/Red) are 522 mV and 1.44 mA/cm2, respectively, which indicate that the Cu2ZnSnS4 thin film has the electrocatalytic activity toward the redox reaction of I3-/I- [30]. It is well known that the counter electrode (CE) is an indispensable component in DSSC, which catalyzes the redox couple (I3-/I-) regeneration after electron injection [31-33]. Since the Cu2ZnSnS4 thin film has the electrocatalytic activity toward the redox reaction of I3-/I-, it is used as the counter electrode in DSSC. Figure 6(b) illustrates the photocurrent-voltage curves of the DSSC with the Cu2ZnSnS4 thin film counter electrode. The open-circuit voltage (φoc), short-circuit photocurrent density (Jsc), fill factor (Ff) and efficiency (E) of the cells are 628 mV, 3.43 mA/cm2, 0.51 and 1.09%, respectively. This indicates that the Cu2ZnSnS4 thin film is an alternative material for the counter electrode of DSSC. Despite that the device with Cu2ZnSnS4 thin film as counter electrode could operate under simulated sun light, its efficiency is low due to the weak Jsc. It is well know that Cu2ZnSnS4 is a semiconductor and the prepared film is quite thick because of the dipping coating method, so, the electron transfer at counter electrode is limited. This leads to that the performance of the corresponding device is not as good as that of DSSC devices with the traditional conductive material platinum [34]. Despite of it, because of low cost, non-toxicity and abondent constituents of the prepared Cu2ZnSnS4 thin film, it is still a competitive material for the counter electrode of DSSC.
4 Conclusions
1) Without any vacuum equipment and extremely toxic substance, the mixed precursor solution composed of Cu2SnS3 nanoparticles and Zn ions is prepared.
2) According to the analysis of the annealing process, 550 °C is an appropriate temperature to prepare pure Cu2ZnSnS4 film from mixed precursor solution without any second phases.
3) The prepared Cu2ZnSnS4 thin film has the electrocatalytic activity toward the redox reaction of I3-/I-.
4) The Cu2ZnSnS4 thin film can be applied as counter electrode of the DSSC which shows an efficiency of 1.09% with Jsc of 3.43 mA/cm2, φoc of 628 mV and Ff of 0.51.
References
[1] SURYAWANSHI M P, AGAWANE G L, BHOSALE S M, SHIN S W, PATIL P S, KIM J H, MOHOLKAR A V. CZTS based thin film solar cells: A status review [J]. Materials Science and Technology, 2013, 28(1): 98-109.
[2] WANG Lu, WANG Wen-zhong, SUN Song-mei. A simple template-free synthesis of ultrathin Cu2ZnSnS4 nanosheets for highly stable photocatalytic H2 evolution [J]. Journal of Materials Chemistry, 2012, 22(14): 6553-6555.
[3] XIN X K, HE M, HAN W, JUNG J, LIN Z Q. Low-cost copper zinc tin sulfide counter electrodes for high-efficiency dye-sensitized solar cells [J]. Angewandte Chemie International Edition, 2011, 50(49): 11739-11742.
[4] KATAGIRI H, SASAGUCHI N, HANDO S, HOSHINO S, OHASHI J, YOKOTA T. Preparation and evaluation of Cu2ZnSnS4 thin films by sulfurization of EB evaporated precursors [J]. Solar Energy Materials and Solar Cells, 1997, 49(1): 407-414.
[5] CHALAPATHY R, JUNG G S, AHN B T. Fabrication of Cu2ZnSnS4 films by sulfurization of Cu/ZnSn/Cu precursor layers in sulfur atmosphere for solar cells [J]. Solar Energy Materials and Solar Cells, 2011, 95(12): 3216-3221.
[6] SEKIGUCHI K, TANAKA K, MORIYA K, UCHIKI H. Epitaxial growth of Cu2ZnSnS4 thin films by pulsed laser deposition [J]. Physica Status Solidi (C), 2006, 3(8): 2618-2621.
[7] TANAKA K, MORITAKE N, UCHIKI H. Preparation of Cu2ZnSnS4 thin films by sulfurizing sol–gel deposited precursors[J]. Solar Energy Materials and Solar Cells, 2007, 91(13): 1199-1201.
[8] SUN Kai-wen, SU Zheng-hua, YAN Chang, LIU Fang-yang, CUI Hong-tao, JIANG Liang-xing, SHEN Yan-song, HAO Xiao-jing, LIU Ye-xiang. Flexible Cu2ZnSnS4 solar cells based on successive ionic layer adsorption and reaction method [J]. RSC Adv, 2014, 4(34): 17703-17708.
[9] AHMED S, REUTER K B, GUNAWAN O, GUO L, ROMANKIW L T, DELIGIANNI H. A high efficiency electrodeposited Cu2ZnSnS4 solar cell [J]. Adv Energy Mater, 2012, 2(2): 253-259.
[10] GUO Q J, FORD GRAYSON M, YANG W C, WALKER BRYCE C, STACH ERIC A, HILLHOUSE HUGH W, AGRAWAL R. Fabrication of 7.2% efficient CZTSSe solar cells using CZTS nanocrystals [J]. Journal of the American Chemical Society, 2010, 132(49): 17384-17386.
[11] ZHONG Jie, XIA Zhe, ZHANG Cheng, LI Bing, LIU Xin-sheng, CHENG Yi-bing, TANG Jiang. One-pot synthesis of self-stabilized aqueous nanoinks for Cu2ZnSn(S,Se)4 solar cells [J]. Chem Mater, 2014, 26(11): 3573-3578.
[12] WANG W, WINKLER M T, GUNAWAN O, GOKMEN T, TODOROV T K, ZHU Y, MITZI D B. Device characteristics of CZTSSe thin-film solar cells with 12.6% efficiency [J]. Adv Energy Mater, 2014, 4(7): 1301465.
[13] SCRAGG J J, DALE P J, PETER L M, ZOPPI G, FORBES I. New routes to sustainable photovoltaics: Evaluation of Cu2ZnSnS4 as an alternative absorber material [J]. Physica Status Solidi (B), 2008, 245(9): 1772-1778.
[14] SHINDE N M, DUBAL D P, DHAWALE D S, LOKHANDE C D, KIM J H, MOON J H. Room temperature novel chemical synthesis of Cu2ZnSnS4 (CZTS) absorbing layer for photovoltaic application [J]. Materials Research Bulletin, 2012, 47(2): 302-307.
[15] TODOROV T K, REUTER K B, MITZI D B. High-efficiency solar cell with earth-abundant liquid-processed absorber [J]. Advanced Materials, 2010, 22(20): E156-E159.
[16] TONG Zheng-fu, PENG Tao, SUN Wei-wei, LIU Wei, GUO Shi-shang, ZHAO Xing-zhong. Introducing an intermediate band into dye-sensitized solar cells by W6+ doping into TiO2 nanocrystalline photoanodes [J]. J Phys Chem C, 2014, 118(30): 16892-16895.
[17] LI Yue-ying, HAO Hong-shun, WANG Li-jun, GUO Wei-hua, SU Qing, QIN Lei, GAO Wen-yuan, LIU Gui-shan, HU Zhi-qiang. Preparation and photoelectric properties of Ho3+-doped titanium dioxide nanowire downconversion photoanode [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 3974-3979.
[18] FERNANDES P A, SALOM P M P, CUNHA A F D A. A study of terenary Cu2SnS3 and Cu3SnS4 thin films prepared by sulphurizing stacked metal precursors [J]. Journal of Physics D: Applied Physics, 2010, 43(21): 215403.
[19] NAGAOKA A, YOSHINO K, TANIGUCHI H, TANIYAMA T, MIYAKE H. Preparation of Cu2ZnSnS4 single crystals from Sn solutions [J]. J Cryst Growth, 2012, 341(1): 38-41.
[20] HAN J , SHIN S W, GANG M G, KIM J H , LEE J Y. Crystallization behaviour of co-sputtered Cu2ZnSnS4 precursor prepared by sequential sulfurization processes [J]. Nanotechnology, 2013, 24(9): 095706.
[21] NARAYANA T, SUBBAIAH Y P V, PRATHAP P, REDDY Y B K, REDDY K T R. Influence of sulfurization temperature on physical properties of Cu2ZnSnS4 thin films [J]. J Renew Sustain Ener, 2013, 5(3): 031606.
[22] SHI Wei-dong, HUO Li-hua, WANG Hai-shui., ZHANG Hong-jie, YANG Jian-hui, WEI Ping-hui. Hydrothermal growth and gas sensing property of flower-shaped SnS2 nanostructures [J]. Nanotechnology, 2006, 17(12): 2918-2924.
[23] BOUAZIZ M, AMLOUK M, BELGACEM S. Structural and optical properties of Cu2SnS3 sprayed thin films[J]. Thin Solid Films, 2009, 517(7): 2527-2530.
[24] BECERRA R A, CORREA J M, SUAREZ H, GORDILLO G. One-step diffusion membrane assisted CBD synthesis and characterization of Cu2SnS3 thin films [J]. Journal of Physics: Conference Serises, 2014, 480(1): 012008.
[25] SHARMA R C, CHANG Y A. The S-Sn (Sulfur-Tin) system [J]. Journal of Phase Equilibria, 1986, 7(3): 269-273.
[26] PIACENTE V, FOGLIA S, SCARDALA P. Sublimation study of the tin sulphides SnS2, Sn2S3 and SnS [J]. Journal of Alloys and Compounds, 1991, 177(1): 17-30.
[27] FONTANE X, CALVO-BARRIO L, IZQUIERDO-ROCA V, SAUCEDO E, PEREZ-RODRIGUEZ A, MORANTE J R, BERG D M, DALE P J, SIEBENTRITT S. In-depth resolved Raman scattering analysis for the identification of secondary phases: Characterization of Cu2ZnSnS4 layers for solar cell applications [J]. Appl Phys Lett, 2011, 98(18): 181905.
[28] KIM J, HIROI H, TODOROV T K, GUNAWAN O, KUWAHARA M, GOKMEN T, NAIR D, HOPSTAKEN M, SHIN B, LEE Y S, WANG W, SUGIMOTO H, MITZI D B. High efficiency Cu2ZnSn(S,Se)(4) solar cells by applying a double In2S3/CdS emitter [J]. Adv Mater, 2014, 26(44): 7427-7431.
[29] WU Ji-huai, LI Qing-hua, FAN Le-qing, LAN Zhang, LI Pin-jiang, LIN Jiang-ming, HAO San-chun. High-performance polypyrrole nanoparticles counter electrode for dye-sensitized solar cells [J]. Journal of Power Sources, 2008, 181(1): 172-176.
[30] ROY-MAYHEW J D, BOZYM D J, PUNCKT C, AKSAY I A. Functionalized graphene as a catalytic counter electrode in dye-sensitized solar cells [J]. ACS Nano, 2010, 4(10): 6203-6211.
[31] BELLA F, GERBALDI C, BAROLO C, GRATZEL M. Aqueous dye-sensitized solar cells [J]. Chem Soc Rev, 2015, 44(11): 3349-3862.
[32] CHEN Ai-lian, CHENG Qian, LI Zhi-na. Preparation of inverted opal macroporous carbon material and its application in dye-sensitized solar cells as counter electrode [J]. Chinese Journal of Nonferrous Metals, 2014, 24(4): 981-986. (in Chinese)
[33] LIN Jia-peng, ZHANG Hai-yan, CHEN Yi-ming, WEI Ai-xiang, LIU Chuan-biao, CHEN Yu-ting. Electrochemical performance of carbon nano-composite counter electrode for dye-sensitized solar cells [J]. Chinese Journal of Nonferrous Metals, 2010, 20(9): 1753-1757. (in Chinese)
[34] SUGATHAN V, JOHN E, SUDHAKAR K, Recent improvements in dye sensitized solar cells: A review [J]. Renewable and Sustainable Energy Reviews, 2015, 52(12): 54-64.
利用Cu2SnS3纳米晶和Zn离子混合溶液制备Cu2ZnSnS4薄膜
童正夫1,杨 佳1,颜 畅2,郝萌萌1,刘芳洋1,2,蒋良兴1,赖延清1,李 劼1,刘业翔1
1. 中南大学 冶金与环境学院,长沙 410083;
2. Australian Centre for Advanced Photovoltaics, School of Photovoltaic and Renewable Energy Engineering, University of New South Wales, Sydney, NSW 2052, Australia
摘 要:在非真空条件下,选用无毒原材料,采用溶液法制备Cu2ZnSnS4薄膜。利用透射电镜、X射线衍射、扫描电镜、能谱以及拉曼等研究手段,对Cu2ZnSnS4薄膜的形成机理进行分析。通过循环伏安及光电测试,探讨Cu2ZnSnS4薄膜作为染料敏化太阳能电池对电极的催化性能。结果表明:采用溶液法制备的Cu2ZnSnS4混合前驱体溶液主要由Cu2SnS3纳米晶和Zn离子组成,将其滴涂成膜后,经过550 °C退火,最终可以得到Cu2ZnSnS4薄膜;制备的Cu2ZnSnS4薄膜对氧化还原对I3-/I-具有一定的催化作用,将其应用于染料敏化太阳能电池的对电极取得了1.09%的光电转化效率。
关键词:Cu2ZnSnS4薄膜;Cu2SnS3纳米晶;Zn离子;电催化;染料敏化太阳能电池
(Edited by Mu-lan QIN)
Foundation item: Projects (51204214, 51272292, 51222403) supported by the National Natural Science Foundation of China
Corresponding author: Liang-xing JIANG; Tel: +86-731-88876454; E-mail: lxjiang@csu.edu.cn
DOI: 10.1016/S1003-6326(16)64282-6
Abstract: The Cu2ZnSnS4 thin film was prepared by a facile solution method without vacuum environment and toxic substance. The formation mechanism of the film was studied by transmission electron microscopy (TEM), X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), and Raman scattering measurements. Through cyclic voltammetry and photo-electricity tests, the electrocatalytic activity of the prepared film as the counter electrode of dye-sensitized solar cell was also studied. The results show that the mixed precursor solution mainly consists of Cu2SnS3 nanoparticles and Zn ions. After 550 °C annealing process on the precursor film prepared from the mixed solution, Cu2ZnSnS4 thin film is obtained. Besides, it is found that the prepared Cu2ZnSnS4 thin film has the electrocatalytic activity toward the redox reaction of I3-/I- and the dye-sensitized solar cell with the prepared Cu2ZnSnS4 thin film as the counter electrode achieves the efficiency of 1.09%.


