Trans. Nonferrous Met. Soc. China 23(2013) 3470-3475
Removal of phosphorus from metallurgical grade silicon by Ar-H2O gas mixtures
Feng LI, Peng-fei XING, Da-gang LI, Yan-xin ZHUANG, Gan-feng TU
School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 24 January 2013; accepted 25 June 2013
Abstract:
The removal of phosphorus in metallurgical grade silicon (MG-Si) by water vapor carried with high purity argon was examined. The effect of the nozzle types, refining time, refining temperature, refining gas temperature and refining gas flow rate on the phosphorus removed was investigated by the self-designed gas blowing device. The optimal refining conditions are nozzle type of holes at bottom and side, refining time of 3 h, refining temperature of 1793 K, refining gas temperature of 373 K, refining gas flow rate of 2 L/min. Under these optimal conditions, the phosphorus content in MG-Si is reduced from 94×10-6 initially to 11×10-6 (mass fraction), which indicates that gas blowing refining is very effective to remove phosphorus in MG-Si.
Key words:
metallurgical grade silicon; gas blowing; phosphorus; thermodynamics;
1 Introduction
In the past decade, the increasing demand for solar grade silicon (SOG-Si) has triggered intensive research to develop a low-cost process to produce SOG-Si [1]. The metallurgical refining methods are being investigated to instead the commercial high-cost process (i.e. Siemens process) [2,3]. The secondary refining technology, which is an important step in purifying from metallurgical silicon (MG-Si) to SOG-Si, can effectively remove some of the impurities in the MG-Si and it has been widely investigated.
Phosphorus in silicon is difficult to be removed by zone refining and unidirectional solidification since its segregation coefficient in silicon is 0.35 [4,5]. It has been reported that phosphorus can be removed by plasma refining [6], electron beam melting [7,8], vacuum treatment [9], slag refining [10,11], acid leaching [12], direct electrolytic reduction [13]. However, an optimal refining process to remove phosphorus from the molten silicon through metallurgical processes has not been found.
The MG-Si is usually secondarily refined by gas blowing, which can pre-remove some of impurities such as Al, Ca, Mg, B and P in silicon to a certain degree. To our knowledge, the phosphorus removal using the Ar-H2O gas blowing refining has not been investigated. In this work, the phosphorus removal was realized by introducing Ar-H2O gas mixtures into the molten silicon. The effect of the nozzle types, refining time, refining temperature, refining gas temperature and refining gas flow rate was investigated. The mechanism of phosphorus removal from silicon was discussed. Additionally, a detailed analysis of the thermodynamics and kinetics for phosphorus removal was carried out.
2 Experimental
Initial phosphorus content in MG-Si is 94×10-6. The schematic drawing of experimental devices is shown in Fig. 1. A medium frequency induction furnace (MFIF) was used to melt silicon. The inlet pipe was used to introduce the high purity argon (99.999%) into a water vapor generator whose temperature can be controlled at a given value. The refining gas temperature was regulated by controlling the temperature of the water vapor generator. The refining gas flow rate was adjusted by controlling the Ar gas flow rate.
7 kg silicon was put into a high purity graphite crucible with an inner diameter of 160 mm and a height of 250 mm and heated to a given temperature (1793, 1813, 1833, 1853, 1873 K). The refining gas was then introduced into the molten silicon at different temperature (353, 358, 363, 368, 373 K) and kept for a certain refining time (1.5, 2, 2.5, 3, 3.5 h). The refining gas flow rate was 0.5, 1, 1.5, 2 and 2.5 L/min, respectively.
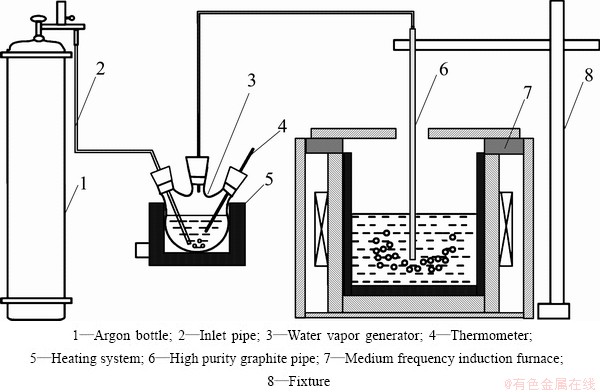
Fig. 1 Schematic drawing of experimental devices
The dephosphorization ratio would be influenced by nozzle types in the refining process, because the nozzle types could affect the effective reaction time and contact area between the refining gas and the molten silicon. Therefore, three different nozzle types were designed in this work. Figure 2 shows the schematic of three different nozzle types. Type A nozzle has one big hole with a diameter of 8 mm at the bottom of pipe. Type B nozzle has 7 small holes at the bottom of the pipe which are beneficial to forming small diameter bubbles. Type C nozzle has 15 small holes at the bottom and side of the high purity graphite pipe and the bubbles with small size are formed and uniformly distribute in the molten silicon.
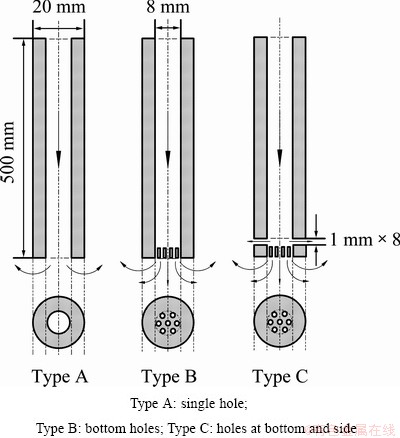
Fig. 2 Schematic drawing of nozzle types
A number of samples were taken from the furnace after gas blowing refining experiment. Phosphorus contents were analyzed using inductively coupled plasma atomic emission spectrometer (ICP-AES). The microstructure was observed by a scanning electron microscopy (SEM) equipped with energy dispersive spectrum (EDS).
3 Results and discussion
3.1 Effect of nozzle types on phosphorus removal
Figure 3 shows the effect of the nozzle type on phosphorus removal in MG-Si, when the refining temperature is 1793 K, the refining gas temperature is 373 K and the refining gas flow rate is 2 L/min. In general, the dephosphorization ratio increases with the increase of refining time for all of the three nozzle types. When type A nozzle is used, the dephosphorization ratio increases from 47.77% to 58.19% with the refining time increasing from 1.5 h to 3.5 h. When type B nozzle is used, the dephosphorization ratio increases from 59.57% to 78.62% with the refining time increasing from 1.5 h to 3.5 h. When type C nozzle is used, the dephosphorization ratio increases from 66.38% to 88.51% with the refining time increasing from 1.5 h to 3.5 h. It is clear that type C nozzle has the highest dephosphorization ratio, type A nozzle has the lowest dephosphorization ratio and type B nozzle has moderate dephosphorization ratio.
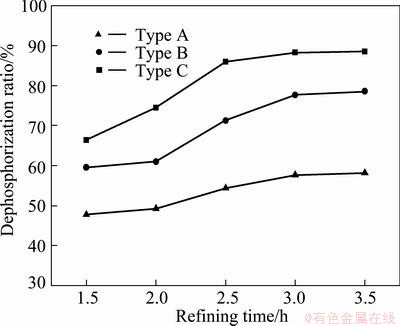
Fig. 3 Effect of nozzle types on dephosphorization ratio
When type A nozzle is used, the Ar bubble formed in the molten silicon is bigger and rises faster, which results in a shorter effective reaction time and a smaller specific contact area between the refining gas and the molten silicon and thereby a limited effect on phosphorus removal. Type C nozzle is beneficial to simultaneously forming many smaller Ar bubbles with uniform distribution in the molten silicon, which could enhance the melt mixing and the specific contact area between the bubbles and the molten silicon. Meanwhile, the smaller bubble has slower floatation speed, meaning that the time of Ar bubble passing through the molten silicon is longer [15]. Therefore, type C nozzle has the largest dephosphorization ratio and is used in all the experiments later.
When type C nozzle is used, the dephosphorization behavior is enhanced by increasing the refining time. It means that longer refining time is helpful for removing phosphorus in the system. However, with further increase of the refining time, the dephosphorization ratio increases slowly. Further longer refining time than 3 h is not necessary. The optimal refining time is 3 h.
3.2 Effect of refining temperature on phosphorus removal
Figure 4 shows the dephosphorization ratio at different refining temperature. It is clear that the higher temperature has disadvantage over phosphorus removal. The dephosphorization ratio reaches 88.30% at the refining temperature of 1793 K. The dephosphorization ratio decreases slightly with the increase of refining temperature between 1793 K and 1833 K. However, at the temperature between 1833 K and 1873 K, the dephosphorization ratio reduces rapidly. The dephosphorization ratio reaches 38.48% at the refining temperature of 1873 K. It can be concluded that the temperature is a main factor for the dephosphorization of the molten silicon. The optimal refining temperature is 1793 K.
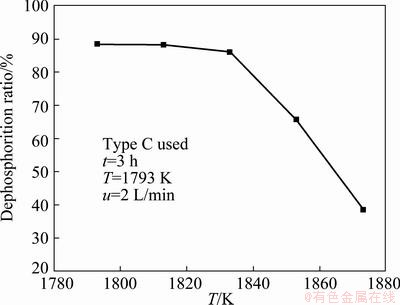
Fig. 4 Dephosphorization ratio of silicon at different refining temperature
3.3 Effect of refining gas temperature on phosphorus removal
Figure 5 shows the dephosphorization ratio at different refining gas temperature. It is clear that higher refining gas temperature is beneficial to removing phosphorus from the molten silicon. When the refining gas temperature is 353 K, the dephosphorization ratio is only 15.32%. However when the refining gas temperature is 358 K, the dephosphorization ratio reaches 17.47%. With further increase of the refining gas temperature, the dephosphorization ratio reaches 88.30% at the refining gas temperature of 373 K. With the increase of the refining gas temperature, the water vapor content in the refining gas also increases, which is beneficial to removal of phosphorus in the molten silicon. The optimal refining gas temperature is 373 K.
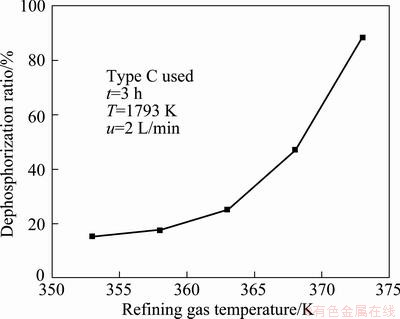
Fig. 5 Dephosphorization ratio of silicon at different refining gas temperature
3.4 Effect of refining gas flow rate on phosphorus removal
Figure 6 shows the effect of refining gas flow rate on phosphorus removal. It is obvious that the dephosphorization ratio increases from 23.76% at the refining gas flow rate of 0.5 L/min to 88.30% at the refining gas flow rate of 2 L/min and then reduces to 80.43% at refining gas flow rate of 2.5 L/min.
With the increase of the refining gas flow rate, the total amount of water vapor in the refining gas per unit time increases too, which is beneficial to removal of phosphorus in the molten silicon. However, when the refining gas flow rate is too fast, the dephosphorization ratio decreases because it is easy to form chain bubble flow, the contact area between the bubble and the molten silicon becomes small [15]. Therefore, the optimal refining gas flow rate is 2 L/min.
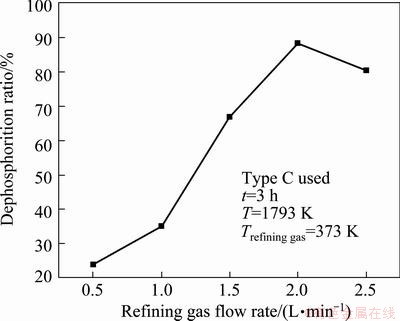
Fig. 6 Dephosphorization ratio of silicon at different refining gas flow rate
3.5 Effect of optimal condition on phosphorus removal
From the results above it can be found that the optimal refining conditions are nozzle type of holes at bottom and side, refining time of 3 h, refining temperature of 1793 K, refining gas temperature of 373 K and refining gas flow rate of 2 L/min. ICP-AES analysis shows that the silicon obtained at such an optimal refining process has a lower content of phosphorus (11×10-6). However, metal impurities still remain in the silicon obtained at the optimal conditions. Figure 7(a) shows the SEM image of the sample before the gas refining, showing that there are a large number of white inclusions in the MG-Si. The impurities are segregated in the white inclusions. The chemical compositions in the white inclusion (position A) in Fig. 7(a) are 11.019% Al, 48.963% Si, 4.857% Ca, 4.426% Ti, 18.642% Fe, 12.093% Ni (mass fraction). The dark matrix (position B) contains Si 100.00%. As shown in Fig. 7(b), the white inclusions in the silicon are reduced substantially after the gas blowing refining though the inclusion has similar compositions (10.330% Al, 47.685% Si, 3.863% Ca, 2.663% Ti, 18.774% Fe, 7.513% Ni, 9.172% O) as the initial MG-Si. The metal impurities in the silicon have been enriched in the grain boundary.
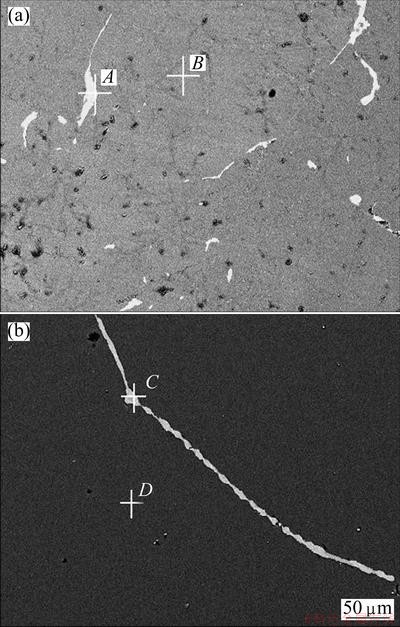
Fig. 7 SEM images of MG-Si before (a) and after refining (b)
3.6 Thermodynamic analysis of phosphorus removal
Discharging temperature of MG-Si is about 2073 K, slag-off and casting are carried out at the temperature from 1723 K to 1773 K. Therefore, the secondary refining temperature can be selected in the range from 1773 K to 1973 K [14]. The reactions involved in the process of phosphorous removal are listed in Table 1.
Table 1 Reaction equations involved in the process of phosphorous removal

The water vapor in the refining gas introduced into the molten silicon reacts with silicon to produce silicon oxide and H2. Part of H2 is dissolved into the molten silicon. The hydrogen in the molten silicon can be expressed as [H]. The [H] reacts with the impurity [P] in the molten silicon to form PH3 gas which can be taken out from the molten silicon with Ar bubble.
Figure 8 shows the relationship between Gibbs free energy change and temperature. Equations. (1) and (2) would take place in the temperature range from 1793 K to 1873 K, meanwhile, the reaction 3[H]+[P]=2PH3(g) will happen in the molten silicon.. Therefore, phosphorus can be removed from the molten silicon through a series of reactions.
3.7 Kinetic analysis of phosphorus removal
Complex chemical reactions exist in the gas blowing refining process of silicon. The water vapor in the refining gas reacts with the molten silicon to produce silicon oxide and H2 in the temperature range from 1793 to 1873K. Part of H2 is dissolved into the molten silicon to form [H], then [H] reacts with [P] in molten silicon to produce PH3 which diffuses into the Ar bubble and is taken out from the molten silicon by the Ar bubble. The detailed gas blowing refining process is shown in Fig. 9. During the gas refining process, the diffusion of PH3 through the bubble boundary layer determines its migration from the molten silicon to Ar bubbles. The diffusion coefficient is a limited value at a given temperature, and PH3 in the Ar bubble is difficult to reach its equilibrium value in such a short time. Therefore, the increase of the specific contact area between the Ar bubble and the molten silicon will be good for the removal of the [P] in the molten silicon.
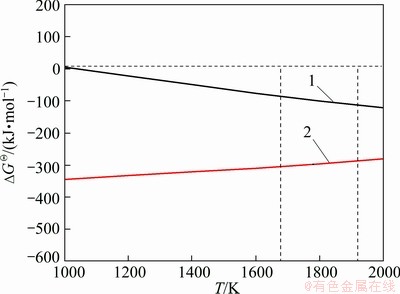
Fig. 8 Diagram of ΔGθ-T for involved reactions of phosphorous removal
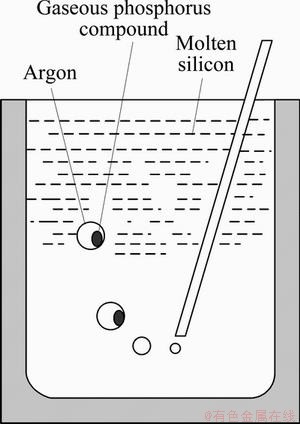
Fig. 9 Schematic drawing of reaction process by gas blowing refining
In the gas blowing refining process, assume that PH3 concentration is very small in the Ar bubble inside. The interface gas concentration is equal to zero, the linear concentration gradient can be approximated as Δc/Δx=(c-0)/δ. Therefore, the dynamics of removal of PH3 by Ar bubble could be expressed as [15]
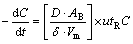 (3)
(3)
where D is the diffusion coefficient of PH3 molecules; AB is the average surface area of bubble; δ is boundary layer thickness; Vm is the molten silicon volume; u is the refining gas flow velocity; tR is the average residence time of bubble; C is the gas instantaneous concentration in the molten silicon. Equation (3) is integrated to get expression as follows:
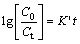 (4)
(4)
where C0 is the gaseous initial instantaneous concentration in the molten silicon; Ct is the gaseous final instantaneous concentration in the molten silicon, K′ is the constant expressed as follows:
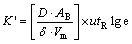 (5)
(5)
From Eqs. (4) and (5), it is known that in the gas blowing refining process, using the type C nozzle which can increase the average surface area of bubble, prolonging the refining time and increasing the refining gas flow rate are beneficial to degassing.
4 Conclusions
1) The phosphorus in MG-Si could be removed using the gas bowing refining. The phosphorus content in MG-Si could be reduced from 94×10-6 initially to 11×10-6 at the optimal conditions: nozzle type of holes at bottom and side, refining time of 3 h, refining temperature of 1793 K, refining gas temperature of 373 K and refining gas flow rate of 2 L/min.
2) Phosphorus content in MG-silicon is effectively reduced by gas blowing refining. The water vapor in the refining gas which is introduced into the molten silicon reacts with silicon to produce silicon oxide and H2. Part of H2 is dissolved into the molten silicon. The [H] reacts with impurity [P] in the molten silicon to form PH3 gas which can be taken out from the molten silicon with the Ar bubble.
References
[1] BRAGA A F B, MOREIRA S P, ZAMPIERI P R, BACCHIN J M G, MEI P R. New processes for the production of solar-grade polycrystalline silicon: A review [J]. Solar Energy Materials and Solar Cells, 2008, 92(4): 418-424.
[2] MORITA K, MIKI T. Thermodynamics of solar grade silicon refining [J]. Intermetallics, 2003, 11(11-12): 1111-1117.
[3] LI Feng, XING Peng-fei, ZHAO Pei-yu, TU Gan-feng. Impurity remove of carbonized rice husk for preparing high purity silicon [J]. Journal of Northeastern University: Natural Science, 2012, 33(9): 1319-1322. (in Chinese)
[4] GRIBOV B G, ZINOV’EV K V. Preparation of high-purity silicon for solar cells [J]. Inorganic Materials, 2003, 39(7): 653-662.
[5] KAZUHIRO H, NORIYOSHI Y, YOSHIEI K. Evaporation of phosphorus in molten silicon by an electron beam irradiation method [J]. Materials Transactions, 2004, 45(3): 844-849.
[6] DELANNOY Y, ALEMANY C, LI K I, PROULX P, TRASSY C. Plasma-refining process to provide solar grade Silicon [J]. Solar Energy Materials and Solar Cells, 2002, 72(1-4): 69-75.
[7] PIRES J C S, BRAGA A F B, MEI P R. Profile of impurities in polycrystalline silicon samples purified in an electron beam melting furnace [J]. Solar Energy Materials and Solar Cells, 2003, 79(3): 347-355.
[8] IKEDA T, MEEDA M. Purification of metallurgical grade silicon by electron beam button melting [J]. ISIJ International, 1992, 32(5): 635-642.
[9] ZHENG Song-sheng, SAFARIAN J, SEOK S, KIM S, MERETE T, LUO Xue-tao. Elimination of phosphorus vaporizing from molten silicon at finite reduced pressure [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 697-702.
[10] EUN Jin-jung, BYUNG Moon-moon, DONG Joon-min. Quantitative evaluation for effective removal of phosphorus for SoG-Si [J]. Solar Energy Materials and Solar Cells, 2011, 95(7): 1779-1784.
[11] LUO Da-wei, LIU Ning, LU Yi-ping, ZHANG Guo-liang, LI Ting-ju. Removal of boron from metallurgical grade silicon by electromagnetic induction slag melting [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(5): 1178-1184.
[12] MA Xiao-dong, ZHANG Jian, WU Ya-ping, LI Ting-ju. Research on hydrometallurgical purification of metallurgical grade silicon under ultrasonic field [J]. Journal of Functional Materials, 2008, 39(7): 1071-1073. (in Chinese)
[13] YASUDA K, NOHIRA T, HAGIWARA R, OGATA Y H. Direct electrolytic reduction of solid SiO2 in molten CaCl2 for the production of solar grade silicon [J]. Electrochimica Acta, 2007, 53(1): 106-110.
[14] Edition group of practical industrial silicon technology. Practical industrial silicon technology [M]. Beijing: Chemical Industry Press, 2005: 118-119. (in Chinese)
[15] CHEN Cun-zhong. Non-ferrous metal smelting and casting [M]. Beijing: Metallurgy Industry Press, 2003: 39-42. (in Chinese).
冶金级硅吹Ar-H2O混合气精炼除磷
李 峰,邢鹏飞,李大纲,庄艳歆,涂赣峰
东北大学 材料与冶金学院,沈阳 110004
摘 要:通过吹高纯氩气和水蒸汽混合气体的方法除去冶金级硅中的杂质磷。采用自行设计的吹气精炼装置,研究喷嘴类型、精炼时间、精炼温度、精炼气温度、精炼气流速等因素对除磷效果的影响。研究表明:使用侧壁和底部多孔型喷嘴,精炼时间3 h,精炼温度1793 K,精炼气温度373 K,精炼气流速2 L/min作为最优吹气精炼条件时,冶金级硅熔体中的磷元素质量分数由94×10-6降低到11×10-6。表明吹气精炼是一种有效的去除冶金级硅中磷的方法。
关键词:冶金级硅;吹气;磷;热力学
(Edited by Chao WANG)
Foundation item: Project (51074043) supported by the National Natural Science Foundation of China; Project (2011BAE03B01) supported by the National Key Technology R&D Program of China
Corresponding author: Peng-fei XING; Tel: +86-24-83683673; E-mail: xingpf@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(13)62890-3
Abstract: The removal of phosphorus in metallurgical grade silicon (MG-Si) by water vapor carried with high purity argon was examined. The effect of the nozzle types, refining time, refining temperature, refining gas temperature and refining gas flow rate on the phosphorus removed was investigated by the self-designed gas blowing device. The optimal refining conditions are nozzle type of holes at bottom and side, refining time of 3 h, refining temperature of 1793 K, refining gas temperature of 373 K, refining gas flow rate of 2 L/min. Under these optimal conditions, the phosphorus content in MG-Si is reduced from 94×10-6 initially to 11×10-6 (mass fraction), which indicates that gas blowing refining is very effective to remove phosphorus in MG-Si.


