文章编号:1004-0609(2011)10-2577-17
铝酸钠溶液分解过程的理论及技术研究进展
李小斌,赵东峰,王丹琴,阎 丽
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:铝酸钠溶液分解是碱法生产氧化铝过程的关键环节。总结铝酸钠溶液分解过程的理论及技术研究进展,论述铝酸钠溶液分解过程的热力学和宏观动力学、铝酸钠溶液的结构及其演变规律、氢氧化铝颗粒行为、分解体系固-液界面作用调控以及铝酸钠溶液分解技术等。认为铝酸钠溶液分解过程中有利于氢氧化铝析出的含铝离子是![]() ,调控溶液中其他复杂含铝离子结构向该类结构转变则有利于氢氧化铝析出;增大分解末期体系中最小颗粒尺寸是提高分解深度的关键;协同调控分解过程中溶液物理化学性质、离子结构和固-液界面作用的原理和方法是强化铝酸钠溶液分解技术研究的基础。
,调控溶液中其他复杂含铝离子结构向该类结构转变则有利于氢氧化铝析出;增大分解末期体系中最小颗粒尺寸是提高分解深度的关键;协同调控分解过程中溶液物理化学性质、离子结构和固-液界面作用的原理和方法是强化铝酸钠溶液分解技术研究的基础。
关键词:
中图分类号:TF821 文献标志码:A
Research progress in theory and technology of
gibbsite precipitation from sodium aluminate solution
LI Xiao-bin, ZHAO Dong-feng, WANG Dan-qin, YAN Li
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The precipitation of sodium aluminate solution is the key process for alumina refinery by alkaline method. The research progress in theory and technology of the precipitation process was summarized. Five important aspects, including the thermodynamics and macrokinetics, aluminium-bearing species present in the aluminate solution and the species evolution, the behavior of aluminum trihydroxide particles, the interaction regulation of the interface between gibbsite particles and aluminate solution and the strengthening techniques for gibbsite precipitation, were discussed. It is held that the aluminate species favoring gibbsite precipitation is ![]() and thus the ways of converting other complex aluminate species to
and thus the ways of converting other complex aluminate species to ![]() would increase the precipitation rate, and that the key of improving the precipitation rate is to increase the smallest particle size in the late period of precipitation. It is also held that it is the research fundamental of seeded precipitation to study the principle and method for coordinately regulating physicochemical properties of aluminate solution, ion structure and the interaction of the interface between gibbsite particle and aluminate solution.
would increase the precipitation rate, and that the key of improving the precipitation rate is to increase the smallest particle size in the late period of precipitation. It is also held that it is the research fundamental of seeded precipitation to study the principle and method for coordinately regulating physicochemical properties of aluminate solution, ion structure and the interaction of the interface between gibbsite particle and aluminate solution.
Key words: sodium aluminate solution; seeded precipitation; mechanism; technology; research progress
铝酸钠溶液分解过程是影响氧化铝生产效率及产品质量的关键环节,包括碳酸化分解和晶种分解两种方式。自1889—1892年Bayer提出拜耳法生产氧化铝技术近120年以来,氧化铝工业规模逐年扩大,特别是近20年来,氧化铝产能迅速增加,据统计,2008年全球氧化铝总产能约1亿t[1]。但作为拜耳法生产氧化铝技术中关键工序之一的晶种分解过程却一直未能有任何突破性进展,是全球氧化铝技术研究的热点问题之一[2]。分解过程中始终存在氢氧化铝晶种加入量大(晶种系数大于2)、分解时间长(30~70 h)、分解率低(45%~52%)和产出率低(一般每立方米溶液产出Al2O3小于90 kg)等问题。目前一般认为,这主要是由于铝酸钠溶液存在强烈的过饱和现象,完全不同于一般无机盐溶液。由于铝酸钠溶液与氢氧化铝晶体间的界面张力大,分解过程难以自发成核,必须加入大量晶种以缩短成核的诱导期,促进氢氧化铝析出。在拜耳法工业生产中,分解过程就是在添加大量氢氧化铝晶种的条件下进行的。而烧结法生产氧化铝则主要采用碳酸化分解技术,碳酸化分解具有分解时间短(2~8 h)、分解率高(90%~92%)等优点,但产品质量较难调控。
铝酸钠溶液分解过程存在的上述问题是全球氧化铝生产亟待突破的技术瓶颈,为此,本文作者对近年来国内外众多研究者关于铝酸钠溶液分解过程机理和技术所开展的大量研究工作从如下5个方面进行了回顾与总结:1) 铝酸钠溶液分解过程的热力学与宏观动力学。主要包括三水铝石以及硅等杂质在Na2O-Al2O3-H2O系中的溶解度、析出氢氧化铝的颗粒尺寸与其溶解度的关系、铝酸钠溶液晶种分解和碳酸化分解过程的热力学和宏观动力学;2) 铝酸钠溶液的离子结构及其演变规律。主要包括铝酸钠溶液结构与其性质间的关系、铝酸根阴离子的行为及其结构调控;3) 氢氧化铝颗粒的行为、调控原理与方法;4) 固-液界面作用调控;5) 铝酸钠溶液分解技术。主要包括生产冶金级砂状氧化铝产品的工业生产技术、强化晶种分解技术、析出一水软铝石的铝酸钠溶液分解技术以及分解技术的最新进展。
1 铝酸钠溶液分解过程的热力学与宏观动力学
氧化铝水合物在NaOH-NaAl(OH)4-H2O体系中的溶解度是碱法生产氧化铝的理论基础,全面掌握水合氧化铝在氢氧化钠溶液中的溶解度,对于研究铝酸钠溶液分解过程至关重要。铝酸钠溶液是氧化铝生产过程的中间产物,其结构十分复杂,在不同条件下具有不同的物理化学特性。由于工业铝酸钠电解质溶液组成复杂,理论模型和半经验公式至今仍然不能可靠地应用在该体系中。RUSSELL等[3],IKKATAI和OKADA[4],APPS和NEIL[5],POKROVSKII和HELGESON[6],VERDES等[7],SCHR?DLE等[8]研究了水合氧化铝在氢氧化钠溶液中的溶解度。由于实验数据的局限性,很多学者则试图通过体系热力学模型的研究,得到更为便利的溶解度计算方法,已有的该体系热力学模型研究方法主要分为两类:1) 基于测定NaOH-NaAl(OH)4-H2O体系物理化学性质获取热力学计算模型的方法。ZHOU等[9]测得NaOH-NaAl(OH)4- H2O体系在313.2 K时的渗透系数,通过数据拟合得到Pitzer模型参数,适用于ρNaOHT (ρNaOH+ρNaAl(OH)4)= 0.05~12 mol/kg,αK(ρNaOHT/ ρNaAl(OH)4)= 1.64~ 5.53的铝酸钠溶液体系;K?NIGSBERGER等[10]通过Zdanovskii法测量了包括氢氧化钠和拜耳法溶液中其他成分在50 ℃和100 ℃下的压力,并依据Pitzer方程建立了热力学预测模型;CAIANI等[11]实测了温度为50~250 ℃时含铝离子在过量NaOH溶液中的表观摩尔比热容,得到了相应的Pitzer模型;MAGALH?ES等[12]测量了25 ℃时离子强度达到6 mol/kg的NaOH/NaAl(OH)4溶液的比热容,并验证了Pitzer模型的适用性和准确性。2) 基于NaOH-NaAl(OH)4-H2O体系Al(OH)3溶解度数据获取热力学计算模型的方法。WESOLOWSKI[13]通过Pitzer方程对三水铝石的溶解度数据进行了拟合,得到了0~100 ℃范围内NaAl(OH)4在纯电解质溶液中的Pitzer模型参数和混合参数;李小斌等则引入铝酸钠溶液表观介电常数的概念,试图将Debye-Hückel模型应用于NaOH- NaAl(OH)4-H2O体系,并得出了适用条件及其参数取值[14],构建了NaOH-NaAl(OH)4-H2O体系电解质活度系数的计算模型[15-16]。
尽管关于铝酸钠溶液热力学的研究已有很多报道,但对于平衡常数值并未得到共识,特别是缺乏准确描述平衡常数的理论模型。文献[9-13]均利用实测数据得到了相应Pitzer模型和参数,在研究过程中很多学者遇到了平衡常数数据匮乏等问题,且大都通过实验数据或其他文献计算而得。然而,溶解度实测数据与活度系数计算数据之间存在一定偏差,由溶解度数据计算所得反应平衡常数往往不完全与实际平衡常数相等。如WESOLOWSKI[13]计算所得平衡常数值与RUSSELL等[3]的实验数据匹配较好,但明显大于文献[4, 6, 9]利用以往实验数据所得的计算值。由于以往数据的误差及理论依据不足,本文作者曾报道过计算三水铝石在氢氧化钠溶液中溶解反应平衡常数的经验方程[14]。目前,本文作者已完善了该模型相应的理论支撑,得到适用范围在25~100 ℃内的表达式:
![]()
![]() (1)
(1)
式中:T为热力学温度,K。利用该表达式计算所得 平衡常数与文献报道值[3, 5, 13, 17]能较好地吻合,且获得了新的NaAl(OH)4的Pitzer模型参数和NaOH- NaAl(OH)4-H2O体系的热力学混合参数,可用于预测三水铝石溶解于氢氧化钠溶液的平衡溶解度和计算活度因子,其理论推导思路和计算方法也可推广到其他1:1价型的电解质溶液体系。
今后,有关NaOH-NaAl(OH)4-H2O体系中Al(OH)3溶解度的热力学计算模型方面的研究,应结合工业铝酸钠溶液结构更复杂和其物理化学性质变化大的特点,明晰杂质的影响规律,从理论上解决计算模型对于工业铝酸钠溶液的适应性问题。
氧化硅、碳酸钠、氯化钠、硫酸钠、钾离子和有机物等杂质对工业铝酸钠溶液中Al(OH)3析出过程的影响很大,杂质的存在不仅影响溶液分解速率,而且影响分解产品的质量[18-20]。
碱法生产氧化铝的关键在于铝硅分离,氧化铝生产中硅主要以钠硅渣(即水合铝硅酸钠)形式去除,溶液中SiO2的平衡溶解度直接影响溶液脱硅、分解以及管道结疤等过程,是影响氧化铝生产技术经济指标的重要因素[21]。铝酸钠溶液中SiO2的平衡溶解度受碱浓度、氧化铝浓度、温度及杂质等因素的影响,ADAMSON等[22],CRESSWELL[23]和JAMIALAHMADI等[24]对铝酸钠溶液中SiO2的平衡浓度进行了实验研究,并提出了相应的SiO2平衡浓度经验公式,但由于实验者采用的研究条件不同,经验公式仅适用于特定温度、浓度等条件,计算结果相差较大;对此,JAMIALAHMADI等[25]提出了径向基函数神经网络(RBF),用以计算SiO2在铝酸钠溶液中的溶解度,并拓宽了适用范围;李小斌等则提出了组成类似化合物的热力学数据与其组成间存在线性关系[26]和复杂无机化合物热力学数据估算方法[27],并据此计算了4种钠硅渣的热力学数据,得到了SiO2在铝酸钠溶液中的平衡浓度[26]和铝酸钠溶液碳酸化分解过程SiO2平衡浓度的变化规律,认为SiO2进入到产品主要是其平衡浓度变化的结果[28]。
目前,对其他杂质在铝酸钠溶液中的溶解度研究甚少,研究表明[29]:碳酸钠对工业铝酸钠溶液的热力学性质也有重要影响,彭小奇等[30]建立了基于Bromley模型的NaOH-NaAl(OH)4-Na2CO3-H2O体系活度因子的计算模型。更多的研究主要集中在杂质对分解过程的影响方面。彭志宏等[20]研究了铝酸钠溶液中无机盐杂质对晶种分解过程的影响,当氯化钠浓度大于10 g/L、碳酸钠浓度大于10 g/L、硫酸钠浓度大于5 g/L时,均对铝酸钠溶液晶种分解产生不利影响,这与文献[31]的报道基本一致;van STRATEN等[32]对过饱和铝酸钠溶液中的锂离子、钠离子和钾离子进行了研究,认为25 ℃下有Na+和K+存在时,水合氧化铝的析出顺序为:无定形、拟薄水铝石、拜耳石,而溶液中的Li+因形成铝酸锂而加速拜耳石的结晶和生长。
总之,溶液中的杂质对分解过程的影响极为复 杂,而有关杂质在铝酸钠溶液中的热力学行为研究较少,应加强分解过程中碳酸盐、硫酸盐和硅酸盐等主要无机盐杂质和有机物的热力学行为及其交互作用规律等方面的研究,以利于分解过程效率和产品质量的控制。
铝酸钠溶液分解属固-液-(气)多相体系,体系中粒子(晶种/或产品)的群分布特征势必导致不同粒子在分解过程中热力学和动力学行为的不确定性。1871年,THOMSON首次在气-液体系中提出了粒子尺寸与溶解度的关系,1900年,OSTWALD和FREUNDLICH将该关系式应用于固-液体系,提出了Ostwald 熟化公 式[33],并指出颗粒尺寸与其溶解度成反比,温度恒定时,对于其平衡状态下的固-液混合体系,若固体颗粒半径小于临界晶核尺寸,则该颗粒将溶解。
对于铝酸钠溶液分解体系,随着体系中能量和质量的交换,氢氧化铝固体不断析出,固-液界面之间的物理化学性质的变化不容忽视。LI等[34]研究了Al(OH)3在铝酸钠溶液中的成核机理,从固-液界面性质出发,认为氢氧化铝颗粒的尺寸与其溶解度密切相关,且晶体的生长速率取决于溶液的过饱和度和NaOH浓度[35]。与之同时,LI等[36]也提出在铝酸钠溶液分解体系中,当其他条件相同时,大颗粒氢氧化铝晶体表观生长速率明显大于小颗粒氢氧化铝晶 体,相对于细颗粒而言,粗颗粒在溶液中的平衡溶解度较小,因而其对应的溶液过饱和度较大,即分解过程的推动力较大,这与文献[35]的结论基本一致。
目前,对过饱和铝酸钠溶液晶种分解过程中Al(OH)3析出机理的研究较多[18, 37-39],认识也基本一致。一般认为,由于三水铝石在铝酸钠溶液中的固-液界面张力大、自发成核活化能高(120~160 kJ/mol),导致氢氧化铝晶核难于自发形成,因此,晶种分解时须向精液中加入大量Al(OH)3晶种以降低溶液的稳定性,缩短诱导期,从而促进Al(OH)3的析出。而对有固-液-气三相参与反应的碳酸化分解过程的研究则相对较少,对其Al(OH)3析出机理尚未达成共识。文献中主要有两种观点,即溶液稳定性破坏机制(式(2))和酸碱中和直接作用机制(式(3)),
![]() =
=![]() (2)
(2)
![]() =
=![]() (3)
(3)
前者实际上与晶种分解过程Al(OH)3的析出机理相同,即认为由于碳酸化分解过程中通入的CO2气体中和溶液中游离的苛性碱,提高了溶液的过饱和度,降低了溶液的稳定性,从而导致Al(OH)3析出。但近年来进一步的研究表明,碳酸化分解和晶种分解过程Al(OH)3析出遵循同一机制。李小斌等[28]结合实验数据对上述两种机制进行了热力学计算(见图1)和分析,结果表明,按溶液稳定性破坏机制进行时,其反应的吉布斯自由能变比按酸碱中和直接作用机制的小很多,即热力学上碳酸化分解反应按溶液稳定性破坏机制进行反应的可能性更大些;同时,动力学研究表明,铝酸钠溶液碳酸化分解和晶种分解过程中Al(OH)3析出强烈地受到溶液过饱和度的影响,两者对过饱和度的级数在2~5之间,其表观活化能也基本相当,碳分过程表观活化能为75.115 kJ/mol[40],晶种分解过程表观活化能为30~121 kJ/mol[41-47];此外,碳酸化分解试验结果表明,随着碳酸化分解时间的延长,溶液苛性比值呈“锯齿”型曲线变化[28],从试验上进一步支持了碳酸化分解过程中Al(OH)3析出遵循溶液稳定性破坏机制这一观点。碳酸化分解和晶种分解过程Al(OH)3析出机理的同一性对碳酸化分解过程具有重要的实际应用价值,已有晶种分解的理论研究成果对烧结法碳酸化分解过程同样具有指导意义。
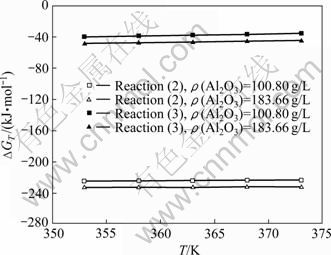
图1 式(2)和(3)的反应吉布斯自由能变?GT随温度的变化
Fig.1 Change of Gibbs free energy (?GT) for reactions (2) and (3) at different temperatures
2 铝酸钠溶液结构及其演变规律
铝酸钠溶液结构及其性质是碱法生产氧化铝的重要理论基础,只有揭示和掌握铝酸钠溶液结构及其性质变化的本质,找到过饱和铝酸钠溶液分解缓慢的原因,才能为提高氧化铝生产效率、改善产品质量和探索强化铝酸钠溶液分解新途径提供可靠的理论依据[48]。
2.1.1 铝酸钠溶液的结构
在铝酸钠溶液结构研究早期,一直未能解决铝酸钠溶液到底是胶体还是真溶液的问题[49],如今,铝酸钠溶液属于真溶液已经被广泛确定。关于铝酸根离子的结构,低浓度过饱和铝酸钠溶液中占绝对主要成分的是单核四面体铝酸根离子![]() ,这一说法目前已被红外、拉曼、核磁共振谱、溶液XRD等各种检测方法确定[50-51];然而关于高浓度铝酸钠溶液中是否存在
,这一说法目前已被红外、拉曼、核磁共振谱、溶液XRD等各种检测方法确定[50-51];然而关于高浓度铝酸钠溶液中是否存在![]() 之外的其他离子、这些离子的存在形式以及铝酸钠溶液中各种离子间的相互转化关系,至今仍不明晰。
之外的其他离子、这些离子的存在形式以及铝酸钠溶液中各种离子间的相互转化关系,至今仍不明晰。
单核铝酸根离子除![]() 外,CARREIRA等[52]研究认为在pH>12.5的铝酸钠溶液中铝酸根离子以
外,CARREIRA等[52]研究认为在pH>12.5的铝酸钠溶液中铝酸根离子以![]() 形态存在于溶液中;KRAUS等[53]也认为
形态存在于溶液中;KRAUS等[53]也认为![]() 会脱水产生
会脱水产生![]() 和
和![]() [52],从而解释蒸气压测量结果;电导率测量[54]及ab initio量子化学计算法[55]推测铝酸钠溶液中可能存在
[52],从而解释蒸气压测量结果;电导率测量[54]及ab initio量子化学计算法[55]推测铝酸钠溶液中可能存在![]() 离子,紫外、核磁及拉曼光谱法[56]推断在高苛性比的铝酸钠溶液中除
离子,紫外、核磁及拉曼光谱法[56]推断在高苛性比的铝酸钠溶液中除![]() 外还有少量
外还有少量![]() 离子存在,但其浓度很低。
离子存在,但其浓度很低。
NMR研究结果还揭示了在强碱性铝酸钠溶液中各种离子对的存在(如NaOH[50]和![]() [51])。GUTOWSKY等[57]认为,尽管
[51])。GUTOWSKY等[57]认为,尽管![]() 、OH-的静电半径都是1.40 ?,但溶液中Na+趋于与
、OH-的静电半径都是1.40 ?,但溶液中Na+趋于与![]() 、
、![]() 形成离子对而不是与OH-形成离子对,溶液中存在
形成离子对而不是与OH-形成离子对,溶液中存在![]() 、
、![]() 、
、![]() 、Na+OH-离子对及它们之间的化学平衡,其中
、Na+OH-离子对及它们之间的化学平衡,其中![]() 离子对是溶液的主要成分。同时ANDERSON和CASTET[58-59]通过对A12O3在碱性溶液中的溶解度和铝酸钠溶液热力学性质的研究,推论
离子对是溶液的主要成分。同时ANDERSON和CASTET[58-59]通过对A12O3在碱性溶液中的溶解度和铝酸钠溶液热力学性质的研究,推论![]() 和
和![]() 是铝酸钠溶液的主要成分,但关于Na+的配位状态及其与铝酸根形成离子对的方式(静电作用、氢键还是弱化学键)未曾报道。
是铝酸钠溶液的主要成分,但关于Na+的配位状态及其与铝酸根形成离子对的方式(静电作用、氢键还是弱化学键)未曾报道。
目前,存在较多争议的是聚合铝酸根离子的形态,其中包括二聚铝酸根离子和多聚铝酸根离子。拉曼光谱、核磁共振及电导率测量证明有二聚体离子![]() 的存在,WATLING[60]总结前人研究成果认为铝酸根离子既有四面体构型,也有八面体构型的铝酸根聚阴离子,且铝原子通过—O—,—OH— 桥连接起来。最近的研究指出,二聚铝酸根离子
的存在,WATLING[60]总结前人研究成果认为铝酸根离子既有四面体构型,也有八面体构型的铝酸根聚阴离子,且铝原子通过—O—,—OH— 桥连接起来。最近的研究指出,二聚铝酸根离子![]() ,
,![]() ,
,![]()
![]() [61]和(OH)3Al—O—
[61]和(OH)3Al—O—![]() ·OH-[54]也可能存在。CHEN等[62]通过量子化学计算,认为
·OH-[54]也可能存在。CHEN等[62]通过量子化学计算,认为![]() 是三水铝石析出的主要生长基元,SIPOS等[63]通过电势和光谱测量结果推论六个单体铝酸根离子
是三水铝石析出的主要生长基元,SIPOS等[63]通过电势和光谱测量结果推论六个单体铝酸根离子![]() 释放两个OH-后生成六聚体
释放两个OH-后生成六聚体![]() 。
。
总之,铝酸钠溶液中离子结构复杂,对此的认识并不深入,除Na+、OH-、![]() 以及二聚铝酸根离子(OH)3Al-O-
以及二聚铝酸根离子(OH)3Al-O-![]() )及它们形成的离子对,可以定性甚至定量的解释某些实验现象之外,其他铝酸根离子,如
)及它们形成的离子对,可以定性甚至定量的解释某些实验现象之外,其他铝酸根离子,如![]() 、
、![]() 或
或![]() 可能并不存在,被认为是生长基元的六聚体铝酸根离子,目前也只是通过计算的推测,尚未得到实验验证。
可能并不存在,被认为是生长基元的六聚体铝酸根离子,目前也只是通过计算的推测,尚未得到实验验证。
2.1.2 铝酸钠溶液的物理化学性质
铝酸钠溶液的表面张力、黏度、密度、折射率及电导率等物理化学性质得到了较多的研究。铝酸钠溶液的表面张力与温度成反比,氧化铝的浓度一定时,铝酸钠溶液的表面张力随碱浓度的增加而增加;苛性比一定时,铝酸钠溶液的表面张力随氧化铝浓度的增加而增加;同时,硅的存在使铝酸钠溶液的表面张力增加[64]。低温、高碱浓度、高苛性比及过饱和度较高的铝酸钠溶液黏度较大,反之则较小[65-66]。铝酸钠溶液黏度与温度、浓度及苛性比的定量关系为[65]: ln η=-1.554+1.139αk+0.018 72ρ-0.050 3T。铝酸钠溶液的电导率与溶液组成的关系也已经明晰,即铝酸钠溶液的电导率随苛性比的升高而增大,苛性比一定时,溶液的电导率随Na2O质量浓度的升高先升高后降低,并在100~120 g/L出现一个极大值[67]。
2.1.3 铝酸钠溶液的结构与物理化学性质之间的关系
通过铝酸钠溶液的物理化学性质与铝酸根离子的结构的关系,可以更有效地研究不同结构铝酸根离子之间的相互转化,但是关于这方面的研究仍然较少。王雅静等[64]结合拉曼光谱,定性解释了铝酸钠溶液表面张力和结构之间的关系,但是未对铝酸根离子结构形式作深入研究;HEFTER等[68]分析了NaOH+ NaAl(OH)4混合物体系的黏度,得出了混合电解质溶液体系的黏度与![]() 的含量之间的关系,但缺少对其他类型的铝酸根离子的分析;BARCZA等[54]曾通过研究溶液电导率,推测铝酸钠溶液中存在
的含量之间的关系,但缺少对其他类型的铝酸根离子的分析;BARCZA等[54]曾通过研究溶液电导率,推测铝酸钠溶液中存在![]() 、
、![]() ·OH-、
·OH-、![]() 、
、![]() 、
、![]() 。这些研究均未明晰铝酸钠溶液中铝酸根离子结构与其物理化学性质之间的关系。李小斌[67]等在解析铝酸钠溶液的结构与物理化学性质之间的关系方面作了有益的尝试,他们通过测定不同Na2O质量浓度和苛性比αk铝酸钠溶液的电导率,按Kohlrausch提出的强电解质溶液极限摩尔电导率的经验方程,计算离子的迁移数
。这些研究均未明晰铝酸钠溶液中铝酸根离子结构与其物理化学性质之间的关系。李小斌[67]等在解析铝酸钠溶液的结构与物理化学性质之间的关系方面作了有益的尝试,他们通过测定不同Na2O质量浓度和苛性比αk铝酸钠溶液的电导率,按Kohlrausch提出的强电解质溶液极限摩尔电导率的经验方程,计算离子的迁移数
![]() (C>0.001 mol/L)
(C>0.001 mol/L)
式中:![]() 为电解质溶液的极限摩尔电导率;t(X)为X离子的迁移数;c为浓度(mol/L),A为常数。
为电解质溶液的极限摩尔电导率;t(X)为X离子的迁移数;c为浓度(mol/L),A为常数。
计算了不同Na2O质量浓度下铝酸根阴离子的迁移数,结合铝酸钠溶液的红外光谱表征,研究了铝酸钠溶液的电导率与其结构间的内在关系。结果表明,铝酸钠溶液中铝酸根离子聚合度随溶液碱浓度的增大而增大,铝酸根离子的电迁移能力则随离子聚合度的增大而降低,且![]() 的电迁移能力最强,铝酸根离子迁移数的计算结果如表1和表2所列。该研究思路和结果将有助于更深入理解氧化铝生产过程溶液的作用规律,为强化分解过程技术研究提供新的思路。
的电迁移能力最强,铝酸根离子迁移数的计算结果如表1和表2所列。该研究思路和结果将有助于更深入理解氧化铝生产过程溶液的作用规律,为强化分解过程技术研究提供新的思路。
表1 铝酸钠溶液的极限摩尔电导率及铝酸根离子的离子迁移数
Table 1 Limiting molar electric conductivity and transference number of aluminate ions in aluminate solution
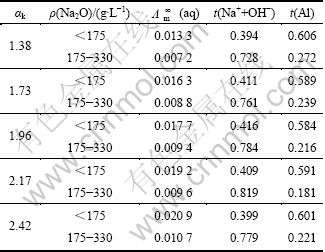
表2 高浓度下铝酸钠溶液的极限摩尔电导率及铝酸根离子的迁移数
Table 2 Limiting molar conductivity and transfer number of aluminate ions in aluminate solution at high concentration

2.2.1 分解过程铝酸根阴离子的行为
孙素琴等[69]用近红外傅立叶变化拉曼光谱仪原位跟踪了铝酸钠溶液碳酸化过程,发现代表![]() 离子的625 cm-1处的拉曼光谱峰在通入CO2分解过程中会逐步减弱;刘吉波[70]、尹建国等[71]分别研究了超声波处理铝酸钠溶液和加入阳离子聚丙烯酰胺强化种分过程,观察到这两种处理方法均使铝酸钠溶液中
离子的625 cm-1处的拉曼光谱峰在通入CO2分解过程中会逐步减弱;刘吉波[70]、尹建国等[71]分别研究了超声波处理铝酸钠溶液和加入阳离子聚丙烯酰胺强化种分过程,观察到这两种处理方法均使铝酸钠溶液中![]() 的浓度降低,并认为形成了高聚体离子,其中二聚体离子
的浓度降低,并认为形成了高聚体离子,其中二聚体离子![]() 等促使铝酸钠溶液结构向有利于分解的方向进行,但是工业实践中,铝酸钠溶液浓度较低、苛性比较小时,分解过程更容易进行,而此时溶液中以
等促使铝酸钠溶液结构向有利于分解的方向进行,但是工业实践中,铝酸钠溶液浓度较低、苛性比较小时,分解过程更容易进行,而此时溶液中以![]() 为主[72],上述研究观点并不能解释这个现象。COUNTER等[73]通过冷冻透射电镜研究了铝酸钠溶液种分过程,观察到四面体
为主[72],上述研究观点并不能解释这个现象。COUNTER等[73]通过冷冻透射电镜研究了铝酸钠溶液种分过程,观察到四面体![]() 聚集成有序的胶状粒子,最后生长成Al(OH)3晶体;WATLING[60]认为
聚集成有序的胶状粒子,最后生长成Al(OH)3晶体;WATLING[60]认为![]() 离子聚集成束,最终形成氢氧化铝;LI等[34]也认为铝酸钠溶液中四面体
离子聚集成束,最终形成氢氧化铝;LI等[34]也认为铝酸钠溶液中四面体![]() 和二聚体
和二聚体![]() 聚合成含铝阴离子,最终形成Al(OH)3晶体;但是到目前为止,究竟是四面体
聚合成含铝阴离子,最终形成Al(OH)3晶体;但是到目前为止,究竟是四面体![]() 还是聚合铝酸根离子
还是聚合铝酸根离子![]() 等引起铝酸钠溶液析出氢氧化铝仍不明晰。
等引起铝酸钠溶液析出氢氧化铝仍不明晰。
在LI等[74]最近的研究中,通过对种分实验中不 同分解时间的分解母液进行红外光谱跟踪测试,发现随着分解的进行,铝酸钠溶液的峰形以及各峰的强度都发生了变化,对880、720、635和530 cm-1处的吸收峰对应的铝酸根阴离子进行半定量计算,得出种分过程中各铝酸根阴离子的浓度随分解时间的变化规律,如图2所示。由于种分过程属于化学反应控制,说明铝酸钠溶液的分解主要是由720 cm-1和880 cm-1处对应的四面体铝酸根离子引起的,从而认为四面体铝酸根离子是导致铝酸钠溶液析出氢氧化铝的最主要因素。
2.2.2 固-液界面铝酸根离子结构
研究[34, 75]表明,三水铝石/溶液界面的物理化学性质与三水铝石析出的机理密切相关,但是,在晶体生长的界面层,采用光谱分析未能检测出显著的特征峰,这是由于参与晶体生长过程的胶束离子是在溶液本体中形成的。铝酸钠溶液晶种的表面性质对分解后期分解率的影响显著,只有附带分解母液的晶种能使铝酸钠溶液继续分解,而其他形式的晶种则很难促进铝酸钠溶液继续分解[76]。本文作者[74]采用ATR差减法半定量研究了铝酸钠溶液晶种分解过程中晶体表面附液层中的离子结构,通过种分过程中带附液的氢氧化铝红外谱图对氢氧化铝晶体的红外谱图进行差减而得到附液层中的离子结构。差减后的红外光谱图中主要是![]() ,即氢氧化铝晶体表面附液中主要为
,即氢氧化铝晶体表面附液中主要为![]() ,且其含量基本保持不变。
,且其含量基本保持不变。
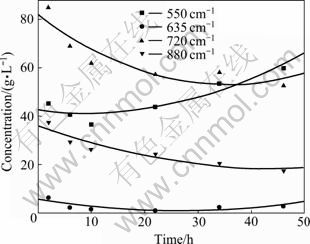
图2 分解过程中不同吸收峰处铝酸根阴离子的浓度变化规律
Fig.2 Concentration variation of aluminate ions during seeded precipitation process
采用压片法对不同分解时间取样抽滤后得到带附液的氢氧化铝进行红外光谱测试。用R(![]() /
/![]() )表征不同种分时间的晶体附液中
)表征不同种分时间的晶体附液中![]() 相对于二聚体
相对于二聚体![]() 的浓度变化(见图3),结果表明,随着分解时间的进行,R值不断增大,即随着分解进行氢氧化铝晶体表面附液中
的浓度变化(见图3),结果表明,随着分解时间的进行,R值不断增大,即随着分解进行氢氧化铝晶体表面附液中![]() 含量相对
含量相对![]() 含量而言略有增加。
含量而言略有增加。
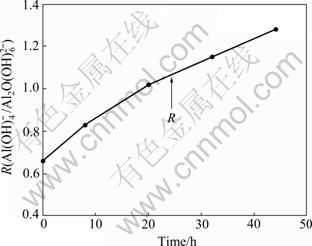
图3 分解过程中晶体附液中R(![]() /
/![]() )随时间的变化规律
)随时间的变化规律
Fig.3 Change of R(![]() /
/![]() ) with time during seeded precipitation process
) with time during seeded precipitation process
铝酸根离子从本体溶液扩散到晶体表面后,继续向边界层内扩散,其中,四面体铝酸根离子由于其电迁移能力较强,在边界层内更易扩散。然而,随着分解的进行,本体溶液中四面体铝酸根离子浓度越来越低,单体聚集成簇的驱动力越来越小,且簇的大小较难达到临界晶核尺寸,所以,分解后期即使附液层中四面体铝酸根阴离子浓度较高也较难析出氢氧化铝晶体。
目前,改变铝酸钠溶液离子结构的方式主要有:杂质、外场和水。杂质主要包括无机盐杂质(包括碳酸钠、硫酸钠、硅等)和添加剂(油酸、硬脂酸及醇类)。例如当溶液中1, 2辛二醇的浓度达到2 mmol/L时,溶液的两个侧峰峰形、强度发生了比较明显的变化,意味着溶液中多聚的铝酸根阴离子的形态可能发生了某种转变,使结晶过程变得困难[75]。外场包括电场、磁场及超声场等;刘吉波[70]认为,超声波作用下溶液中单体铝酸根离子![]() 浓度减少,二聚体离子
浓度减少,二聚体离子![]() 浓度增多,从而认为聚合体离子
浓度增多,从而认为聚合体离子![]() 等更有利于分解过程,但是,工业实践中铝酸钠溶液浓度较低、苛性比较小时,分解过程更容易进行,而此时溶液中以
等更有利于分解过程,但是,工业实践中铝酸钠溶液浓度较低、苛性比较小时,分解过程更容易进行,而此时溶液中以![]() 为主[50],上述研究观点并不能解释这个现象。最近,本文作者研究发现,铝酸钠溶液分解过程中,铝酸钠溶液蒸发一定浓度后,再加入水补回到蒸发前浓度时,溶液结构则有所变化。关于种分过程铝酸根离子结构的调控方法,还有待进一步的研究。
为主[50],上述研究观点并不能解释这个现象。最近,本文作者研究发现,铝酸钠溶液分解过程中,铝酸钠溶液蒸发一定浓度后,再加入水补回到蒸发前浓度时,溶液结构则有所变化。关于种分过程铝酸根离子结构的调控方法,还有待进一步的研究。
3 铝酸钠溶液分解过程中机理和氢氧化铝粒子的行为
铝酸钠溶液分解过程属于铝酸钠溶液-带电固体粒子的固-液两相体系,深入研究铝酸钠溶液分解过程的机理和氢氧化铝粒子的行为,寻找切实可行的调控固-液界面的有效途径,在提高铝酸钠溶液晶种分解过程的产出率的同时,保证分解产品质量,是氧化铝工业的迫切需要。氢氧化铝颗粒在铝酸钠溶液中的行为主要包括:成核、长大、附聚、破裂与磨损,这些现象往往同时发生,只是在不同条件下,发生的程度不同,且有主次之分。
成核又分为原生成核和次生成核,它会使最终产品的粒度细化。原生成核是指过饱和溶液中的均相成核。铝酸钠溶液中![]() 形成铝酸根离子群的缔合物不断长大,当其半径超过临界半径后,就可以成为晶核继续存在并长大成为晶体。对于均相成核的研究,HALFON等[43]选取0.01 μm作为临界核尺寸模拟了成核模型,但计算所得的结果小于三水铝石的晶格尺寸,大于微观粒子的观测值;利用HERRMANN等[77]报道的固-液界面张力数据,MYERSON和TOYOKURA 等[78]通过计算认为临界核尺寸为0.11 μm;张立川 等[79]通过测量铝酸钠溶液的电导率,认为氢氧化铝临界成核半径为1.5~2.0 μm;ROSSITER等[37]借助多角度激光扫描设备,计算得出三水铝石均相成核临界尺寸为(0.001 2±0.000 1) μm。上述不同的研究者得出的临界核尺寸不尽相同,这主要可归因于所采用的溶液浓度不同、方法各异以及实验误差等因素。研究溶液均相成核问题应将固-液界面性质(例如固体界面能、颗粒粒度、溶液表面张力等)考虑在内,而固-液界面能是随体系温度、浓度和颗粒性质等的变化而变化的,已有研究将宏观尺寸测得的界面性质用于计算微观的临界尺寸,且不考虑浓度和温度的影响,势必导致结果的差异。次生成核是在原始溶液过饱和度高、温度低、分解速度快而晶体表面积小的条件下先生成枝状结晶,在颗粒相互碰撞时发生破裂折断而脱离母晶转入溶液,从而产生新晶核的过程。BROWN[80]研究认为,晶种表面的微观磨蚀而产生的碎片是新晶核产生的主要源泉,晶核生成率与晶种粒度有关,晶种越粗,产生的新晶核数量越多;MISRA和WHITE等[41]认为在高搅拌速率下二次成核的效果才更显著;LI等[81]通过计算得出二次成核活化能为132 kJ/mol,且该过程与溶液过饱和度和温度密切相关,与钠离子浓度无关,这与文献[18]的结论相符;HALFON等[43]将成核率与晶种表面空缺数联系起来,得到了二次成核速率方程。总之,高过饱和度、低温、低种子比、低位错密度等条件有利于二次成核,在实际生产中要合理的控制这些条件。
形成铝酸根离子群的缔合物不断长大,当其半径超过临界半径后,就可以成为晶核继续存在并长大成为晶体。对于均相成核的研究,HALFON等[43]选取0.01 μm作为临界核尺寸模拟了成核模型,但计算所得的结果小于三水铝石的晶格尺寸,大于微观粒子的观测值;利用HERRMANN等[77]报道的固-液界面张力数据,MYERSON和TOYOKURA 等[78]通过计算认为临界核尺寸为0.11 μm;张立川 等[79]通过测量铝酸钠溶液的电导率,认为氢氧化铝临界成核半径为1.5~2.0 μm;ROSSITER等[37]借助多角度激光扫描设备,计算得出三水铝石均相成核临界尺寸为(0.001 2±0.000 1) μm。上述不同的研究者得出的临界核尺寸不尽相同,这主要可归因于所采用的溶液浓度不同、方法各异以及实验误差等因素。研究溶液均相成核问题应将固-液界面性质(例如固体界面能、颗粒粒度、溶液表面张力等)考虑在内,而固-液界面能是随体系温度、浓度和颗粒性质等的变化而变化的,已有研究将宏观尺寸测得的界面性质用于计算微观的临界尺寸,且不考虑浓度和温度的影响,势必导致结果的差异。次生成核是在原始溶液过饱和度高、温度低、分解速度快而晶体表面积小的条件下先生成枝状结晶,在颗粒相互碰撞时发生破裂折断而脱离母晶转入溶液,从而产生新晶核的过程。BROWN[80]研究认为,晶种表面的微观磨蚀而产生的碎片是新晶核产生的主要源泉,晶核生成率与晶种粒度有关,晶种越粗,产生的新晶核数量越多;MISRA和WHITE等[41]认为在高搅拌速率下二次成核的效果才更显著;LI等[81]通过计算得出二次成核活化能为132 kJ/mol,且该过程与溶液过饱和度和温度密切相关,与钠离子浓度无关,这与文献[18]的结论相符;HALFON等[43]将成核率与晶种表面空缺数联系起来,得到了二次成核速率方程。总之,高过饱和度、低温、低种子比、低位错密度等条件有利于二次成核,在实际生产中要合理的控制这些条件。
附聚是铝酸钠溶液中氢氧化铝颗粒长大的一种重要方式,一般认为[41, 82-84],该基本可以分为物理絮凝和结晶附聚两个步骤。MARCHAL等[85]认为附聚结果取决于颗粒的流体动力学碰撞频率、颗粒间相互作用以及晶体生长的结合能力;SAKAMOTO和KANEHARA等[86]认为氢氧化铝晶粒的附聚主要取决于“粘结剂”氢氧化铝的析出速度和为牢固稳定絮凝物所需的“粘结剂”数量之间的平衡。许多学者先后提出了不同的附聚模型,HALFON等[84]认为粒子附聚与原始尺寸无关,提出氢氧化铝晶体的附聚速率A的表达式为
A=a(c)X(m, t) (4)
式中:X为单位体积、单位时间内粒径在m~m+dm间粒子的二元碰撞数目;a(c)为相关系数。而STEEMSON等[87]则提出了临界附聚尺寸的概念,认为附聚主要发生在粒径相近的细粒子之间,超过某一临界尺寸的颗粒之间不会发生附聚;SEYSSIECQ等[82]在总结了不同附聚模型的基础上,认为附聚速率Ai可以表示为
![]() (5)
(5)
式中:Ni为某一体积区间的总粒子数;Kai, j为附聚参数;ZHOU等[88]研究了铝酸钠溶液碳酸化分解过程中Al(OH)3颗粒附聚问题,以分解过程颗粒数的消失速率表征附聚速率,从理论上推导出附聚模型,并得到实验结果的支持,认为附聚速率与溶液过饱和度的变化速率、颗粒数、颗粒尺寸等因素相关。上述研究表明,高温、高过饱和度、低种子比、加入一定量的细晶种等有利于附聚。一般认为附聚发生在分解初期(5~9 h),本文作者提出[76],在分解后期亦存在附聚现象,铝酸钠溶液晶种分解后期晶种表面界面层溶液结构对分解速率有重大影响。因而,调控利于附聚的因素,充分利用附聚得到粒度适中、强度较高的砂状氧化铝产品对工业生产至关重要。
晶体长大是氢氧化铝直接从铝酸钠溶液中析出的重要途径。目前,较一致的观点是[89-91],氢氧化铝晶体表面存在不同的生长速率,因而晶体的生长上具有形貌多样性;关于Al(OH)3晶体生长的微观机理[92-93],研究表明氢氧化铝晶体的(001)面本身具有一种垂直于该表面的静电引力,使得该表面处于较高的能级状态,易于吸附![]() 离子,使晶体长大。关于晶体生长速率,一般用单位时间三水铝石直径的增大值来表示,晶体生长相关动力学模型[41-47]反映的就是所有粒径粒子的生长而引起的质量增加的综合作用,较为一致的结论是:晶种分解过程在经典的动力学领域被视为二级反应,分解速率与溶液过饱和度成正比。然而,多数研究忽略了晶体尺寸的影响因素,为了更真实地描述Al(OH)3生长的特征与规律,必须提出与晶体尺寸相关的晶体生长速率的计算模型,并结合体系组成与温度制度,得出不同尺寸晶体生长的动力学参数。LI等[36]认为,虽然晶种分解是一个包含成核、附聚与晶体生长等多种物理化学变化的复杂过程,但它们综合作用的结果都反映在分解过程的粒子数密度的变化率上,由此提出了通过对分解过程的群分布颗粒粒度分布的变化进行数据处理计算长大速率的方法,并得出颗粒长大速率与尺寸正相关的结论,这一结论与工业实践相吻合。
离子,使晶体长大。关于晶体生长速率,一般用单位时间三水铝石直径的增大值来表示,晶体生长相关动力学模型[41-47]反映的就是所有粒径粒子的生长而引起的质量增加的综合作用,较为一致的结论是:晶种分解过程在经典的动力学领域被视为二级反应,分解速率与溶液过饱和度成正比。然而,多数研究忽略了晶体尺寸的影响因素,为了更真实地描述Al(OH)3生长的特征与规律,必须提出与晶体尺寸相关的晶体生长速率的计算模型,并结合体系组成与温度制度,得出不同尺寸晶体生长的动力学参数。LI等[36]认为,虽然晶种分解是一个包含成核、附聚与晶体生长等多种物理化学变化的复杂过程,但它们综合作用的结果都反映在分解过程的粒子数密度的变化率上,由此提出了通过对分解过程的群分布颗粒粒度分布的变化进行数据处理计算长大速率的方法,并得出颗粒长大速率与尺寸正相关的结论,这一结论与工业实践相吻合。
氢氧化铝晶体的破裂与磨蚀主要是由于在搅拌过程中颗粒间及颗粒与器壁、搅拌浆等固体物质的相互碰撞所引起的,它属于一种机械现象[48],该过程所产生的细粒子在分解过程中作为二次成核的晶核而存在。一般在工业分解槽中的搅拌强度不会导致显著的颗粒破裂。
总之,铝酸钠溶液分解产品的产量和质量与其对应的分解过程动力学密切相关,其中的任何一个过程受到影响,都可能对整个的工艺产生较大的影响,甚至可能影响到最终产品的质量。在实际的生产过程中对这些过程要很好地进行控制与调整。
4 分解体系固-液界面作用调控
LI等[76]研究了不同类型晶种对高苛性比铝酸钠溶液分解率的影响,指出分解后期晶种表面界面层溶液结构对分解速率有重要影响,该结论在后来的研究中[74]也得到了证明,由此可见,在铝酸钠溶液分解过程中,固-液界面作用的影响是必然的。结合上述铝酸钠溶液结构与性能的关系、Al(OH)3颗粒在分解体系的溶解度及其与颗粒尺寸之间的关系、粒子在分解过程中的行为,研究调控固-液界面作用的原理与方法,对于缓和产品质量与分解率之间的矛盾具有重要意义[94],引起了业界广泛重视。有关分解体系界面作用调控的研究主要从两个方面进行。
晶种的表面性质对铝酸钠溶液种分分解率的影响显著,目前制备活化晶种的主要方法有以下几种[95]:1) 热活化。采用工业氢氧化铝焙烧窑窑灰作晶种;2) 机械活化。采用球磨机等设备研磨工业晶种使其表面化学键断裂,晶体缺陷增加而改变固体的热力学性质和参与化学反应的能力;3) 湿法活化处理。用离子交换树脂处理分解原液,可显著缩短种分诱导期,降低表观活化能提高分解速度,同时可制得具有较大比表面积和大量活性点的氢氧化铝晶种;4) 铝酸钠溶液分解制备活性晶种。有报道向铝酸钠溶液中添加诱发剂分解制取活性晶种,铝酸钠溶液冷冻法制取活性晶种,铝酸钠溶液自发分解制取活性晶种。
上述活化晶种的方法实际上是增大晶种的比表面积,增加活性点,强化溶液与晶体间的传质;晶种活化对铝酸钠溶液分解过程及产品粒度的影响也被广泛研究[96-99],但大多随种分进行晶种表面都会快速失去活性,或者对产品的粒度及纯度有影响,而且活化晶种的处理往往操作困难,处理时间长,有些活化方法设备投资大,因此均未能得到工业应用。
添加剂具有界面吸附、定向排列、胶束生成以及由此产生的界面张力下降等性质,在种分过程中采用添加剂主要有两个目的,即改善产品质量和提高分解率。有关采用添加剂改善产品质量的研究比较深入,其中,CGM(Crystal growth modificater)已得到工业应用,但对采用添加剂提高分解率的研究至今还没有突破性进展。PAULAIME等[94]和SEYSSIECQ等[100]的研究表明,有机添加剂在与它接触的界面上产生选择定向吸附,一方面增加了晶种表面的润湿性,另一方面降低了铝酸钠溶液的界面张力,此结论在李小斌 等[101]的研究中也得到证实;彭志宏等[102]研究了有机添加剂对种分过程的影响,指出一定量的非离子表面活性剂可改变Al(OH)3颗粒表面的Zeta电位,同时使铝酸钠溶液的表面张力降低20~30 mN/m,从而可大幅度降低溶液的稳定性,加快晶体生长速度;ROE和PERISHO[103]、OWEN和DAVIS[104]、CHEN等[105]、吴玉胜等[106]和薛红等[107]的研究表明,一些有机聚合物和部分表面活性剂可以强化分解,其中,15-冠-5-醚可能是由于有机物分子吸附到晶种表面,降低了铝酸钠溶液的稳定性,强化了成核过程[96]。
添加剂的强化作用是通过固-液界面间完成的,然而这些添加剂与晶种或者溶液之间的相互作用关系尚不明确,对添加剂提高分解率的效果和作用机理尚无共识。添加剂与晶体间的固-液界面作用机理主 要包括:1) 吸附理论,BRONSWIJK等[108-109]研究发现,一些有机添加剂吸附在固体表面属于化学吸附,且符合兰缪尔等温式;2) 双电层理论,氢氧化铝晶种表面带正电荷,其表面紧密吸附着带负电荷的OH-,形成双电层结构,带负电荷的氢氧化铝胶团粒子间互相排斥,析晶困难,加入阴离子表面活性剂后,反离子进入滑动面,压缩双电层,使ζ电势变小,从而提高分解率;3) 形成分子间化合物[100],某些有机物与生长基元之间形成了一种分子化合物,这些有机添加剂导致形成的分子化合物只能选择性的嵌入到特定的晶面上,因而改变了晶体的结晶行为。比如不同的多元羧酸可以改变晶体的生长形貌,其中,草酸会导致晶体主要在(101)和(112)晶面生长,从而导致晶体成片状结构,DL-苹果酸, 酒石酸和羟基丙二酸会使晶体在(100)和(101)晶面生长,从而导致晶体成针状结构。
5 铝酸钠溶液分解技术
为适应现代铝电解工业普遍采用的干法净化技术,要求氧化铝企业生产砂状氧化铝。国外冶金级砂状氧化铝生产技术主要有以美铝公司为首的低碱浓度、低αk条件下的二段分解技术和以法铝公司为代表的高碱浓度、较高αk条件下的一段分解技术。一段分解流程较简单,便于掌握和控制,但通常氧化铝产品的强度较小,且分解过程易出现周期性细化,常需在晶种分解过程中加入结晶助剂[110]。而二段分解产品粒度较均匀,强度高,但种子需分成粗、细两部分,附聚段结束后溶液需中间降温,工业生产控制难度较大。国内晶种分解生产砂状氧化铝主要以法铝的一段分解技术为原型,而碳酸化分解生产砂状氧化铝则采用中南大学开发的“稳定碳分梯度、细颗粒快速附聚、粗颗粒缓慢修饰”的中州模式[111]。
冶金级砂状氧化铝产品除了需满足其物理性能指标外,对其化学纯度同样有严格的要求。冶金级产品化学纯度主要取决于产品中杂质碱(Na2O)、硅(SiO2)和铁(Fe2O3)的含量。其中,杂质碱、硅含量主要由SiO2在溶液中的平衡溶解度所决定[26],因此,无论是晶种分解过程还是碳酸化分解过程,控制分解原液的硅量指数是保证分解产品中杂质碱、硅的含量符合要求的前提[112]。而产品中杂质铁含量的控制是一个棘手的问题,我国拜耳法处理高硫一水硬铝石矿的生产实践表明,当矿石中硫含量大于0.7%时,产品中铁含量将严重超标(高达0.1%,质量分数)。一般认为,溶液中未能过滤的铁以微小氧化铁水合物颗粒[113]或以羟基铁酸根配合离子![]() 形态存在;尤其是在高硫铝土矿溶出过程中,黄铁矿转变为硫离子和亲水的胶体水合氧化铁和亚铁硫化物[114-117],这些胶粒因为硫离子的分散作用而在溶液中变得稳定,难以滤除。此外,硫离子的存在还能促进铁转变为溶解度更大的硫铁配合物Na2[FeS2(OH)2]?2H2O,使铝酸钠溶液中铁含量大幅度增加[118-119]。但总体而言,目前这方面的研究还较少,对于铝酸钠溶液中铁的存在形式、影响因素、析出机理及其抑制措施等急待深入研究。
形态存在;尤其是在高硫铝土矿溶出过程中,黄铁矿转变为硫离子和亲水的胶体水合氧化铁和亚铁硫化物[114-117],这些胶粒因为硫离子的分散作用而在溶液中变得稳定,难以滤除。此外,硫离子的存在还能促进铁转变为溶解度更大的硫铁配合物Na2[FeS2(OH)2]?2H2O,使铝酸钠溶液中铁含量大幅度增加[118-119]。但总体而言,目前这方面的研究还较少,对于铝酸钠溶液中铁的存在形式、影响因素、析出机理及其抑制措施等急待深入研究。
为了强化铝酸钠溶液的分解过程,曾试图通过改变溶液体系以实现氢氧化铝结晶过程的强化。这类方法主要有离子膜电解法、萃取法、醇法和NaHCO3法等。其中,离子膜电解法[120]是在电场和离子膜的复合作用下,阴极析出H2同时产生OH-,并与阳极区迁移过来的Na+结合,在电解槽阴极区产生浓NaOH溶液,而阳极则析出O2同时产生H+;阳极区含H+的料浆送入分解槽进行晶种分解,产出氢氧化铝。其中,电解的作用类似于碳酸化分解中通入的CO2。该法具有溶液分解速率大、分解率和产出率高等优点,但对离子膜的耐碱性要求甚高,因此该法难以工业应用。萃取法[121]是通过脂肪醇或烷基酚从正常的拜耳法循环母液中萃取Na(K)OH,使母液苛性比降至1.4~1.5,再一次进行晶种分解,以提高拜耳法循环中氧化铝的产出率。萃取油相用水反萃后获得不含铝的NaOH浓溶液,用于循环溶出铝土矿或先溶出赤泥中的氧化铝再溶出铝土矿,从而强化晶种分解过程。但该法存在严重腐蚀设备、后处理工艺复杂、废液排放量多等缺点。醇法[122-124]是通过向铝酸钠溶液中加入乙醇改变铝酸钠溶液的性质,降低溶液的稳定性,增加氢氧化铝结晶驱动力,达到加速铝酸钠溶液分解的目的。ZHANG等[125]的研究表明,添加乙醇强化分解的原因可能是由于乙醇与水化铝酸根离子中的水能形成稳定的氢键,从而促进氢氧化铝的成核过程。该法主要存在醇难以循环利用的问题。此外,向铝酸钠溶液中加入NaHCO3,中和溶液中的游离苛性碱,降低溶液的稳定性,亦可强化溶液的分解过程[126-127],但由于碳酸钠溶液难以循环,导致其实际应用价值不大。
为降低氢氧化铝焙烧工序能耗和整个拜耳法生产氧化铝的能耗,BALLAS等[128-131]自20世纪80年代以来最先对铝酸钠溶液分解析出一水软铝石进行了探讨,随后许多学者相继开展了该方面的研究。PANIAS和PASPALIARIS[132]和李小斌等[133]分别对Na2O- Al2O3-H2O系进行了热力学计算和分析,结果表明,从过饱和铝酸钠溶液析出一水软铝石的温度取决于溶液组成,适当条件下可在50 ℃下析出一水软铝石物相;PANIAS的理论研究[133]表明,降低溶液pH和增加一水软铝石晶种量均有利于提高一水软铝石的分解效率。在试验研究方面,1)常压下、85 ℃以上时,在铝酸钠溶液中添加一水软铝石晶种可析出一水软铝石,这似乎与SKOUFADIS等[134]的动力学研究结果不一致;2) 分解效率与溶液中氧化铝的过饱和度有关,低过饱和度有利于一水软铝石的析出[135];3) 添加细颗粒、大晶种量的一水软铝石晶种,可提高溶液的分解率[135];4) 添加酒石酸等有机添加剂[136],在同等分解条件下可降低一水软铝石的析出温度。此外,在分解温度为120~130 ℃下,向铝酸钠溶液中加入一水软铝石凝胶可制备高纯一水软铝石粉体[137];而向铝酸钠溶液中加入H2O2水溶液,溶液中98%的铝将以一水软铝石的形式析出[138]。迄今为止,虽然在铝酸钠溶液析出一水软铝石方面开展了大量研究,但由于同等条件下一水软铝石析出速率相比三水铝石的小2个数量级,且一水软铝石似乎不发生颗粒间的附聚,导致产品非常细,不适于流态化焙烧[139],因此,目前铝酸钠溶液析出一水软铝石的研究离大规模工业应用还相差甚远。此外,适用于未来低温(<860 ℃)熔盐电解的高活性、易溶氧化铝的制备研究亦应是铝酸钠溶液分解技术的重要方向之一[48]。
基于溶液离子结构演变[67, 74]、添加剂作用机理[101]和颗粒附聚机理[88]等相关理论研究,中南大学在国家“973计划”项目的资助下先后提出了“固-液界面协同调控强化铝酸钠溶液晶种分解”技术和“成核、附聚-再附聚、长大”碳酸化分解技术。前者是通过外场、晶种促进铝酸钠本体溶液中含铝组元向有利氢氧化铝生长基元转变,借助高效结晶助剂改变固-液界面性质,强化生长基元向界面扩散、吸附和叠合,同时通过强化颗粒附聚消除细粒子以提高体系的过饱和度,从而在一定程度上有效解决了分解率与分解产品质量间的矛盾。图4所示为不同分解率下晶种分解产品的SEM像,结果表明,采用耦合调控强化分解技术不仅分解率高,而且产品粒度较粗、分布均匀。而后者则是基于溶液碳酸化分解过程中氢氧化铝成核、附聚和长大子过程间的互匹配规律,在成核、附聚段,控制适宜的溶液过饱和度变化速率和搅拌强度,使细颗粒充分附聚,获得粒度适中且分布均匀的高活性晶种;在再附聚、长大段,控制较高过饱和度以提供较粗颗粒附聚所需粘结剂,并控制较小的搅拌速率,使较粗粒子再次发生附聚形成更大颗粒,然后在长大过程中,通过改变分解条件,使颗粒在低过饱和度下完成晶体长大,获得物理性能优良的砂状产品(见图5)。
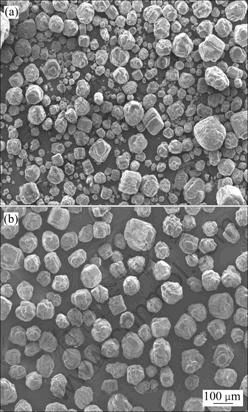
图4 现行分解工艺与固-液界面调控强化分解技术所得氢氧化铝产品的SEM像
Fig.4 SEM images of gibbsite products obtained by current industrial precipitation technology and strengthening precipitation technology by regulating solid/liquid interfacial properties: (a) Products of traditional technology, precipitation ratio of 51%; (b) Products of new technology, precipitation ratio of 60%
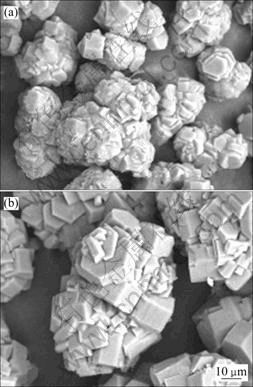
图5 不同分解率下获得的Al(OH)3颗粒的SEM像
Fig.5 SEM images of Al(OH)3 obtained at different carbonation rates: (a) Precipitation time of 60 min, precipitation ratio of 88.44% for preparing seed; (b) Precipitation time of 360 min, precipitation ratio of 91.25% for products
6 结语
铝酸钠溶液分解是从铝土矿提取氧化铝过程中至关重要的工序之一,直接影响产品的物理和化学性能以及分解过程的效率。碳酸化分解以前存在的产品质量相对较差的问题,通过试验研究和工业生产实践,现已经基本上得到解决;而对于晶种分解过程存在的分解效率低的问题,虽然在宏观上的热力学、动力学、颗粒行为、强化分解技术以及微观上的离子形态及其演变与调控等方面已开展大量的试验研究工作,但至今始终未能有任何实质性突破。要实现铝酸钠溶液的强化分解,解决当前氧化铝工业上的技术瓶颈问题,则首先必须在分解理论上寻求突破。本文作者认为,铝酸钠溶液分解过程中有利于氢氧化铝结晶析出的含铝离子种类的确定、其他含铝离子与该有利结晶析出离子间的相互转变规律及其调控手段、体系颗粒分布调控以及氢氧化铝晶体与铝酸钠溶液界面性质的调控将是未来铝酸钠溶液分解过程理论研究的重要方向。
REFERENCES
[1] 李旺兴. 氧化铝生产理论与工艺[M]. 长沙: 中南大学出版社,2010: 9.
LI Wang-xing. Theory and technics of alumina production[M]. Changsha: Central South University Press, 2010: 9.
[2] 陈启元. 有色金属基础理论研究: 新方法与新进展[M]. 北京: 科学出版社,2005: 88-95.
CHEN Qi-yuan. Fundamental theoretical research of non-ferrous metals—New methods and new progresses[M]. Beijing: Science Press, 2005: 88-95.
[3] RUSSELL A S, EDWARDS J D, TAYLOR C S. Solubility and density of hydrated aluminas in NaOH solutions[J]. Journal of Metals, 1955, 7: 1123-1128.
[4] IKKATAI T, OKADA N. Extractive metallurgy of aluminum[M]. New York: Interscience, 1963: 159-173.
[5] APPS J A, NEIL J M. Chemical modeling of aqueous solutions (Ⅱ)[M]. Washington DC: American Chemical Society, 1990: 414-428.
[6] POKROVSKII V A, HELGESON H G. Thermodynamic properties of aqueous species and the solubilities of minerals at high pressures and temperatures: The system Al2O3-H2O-NaCl[J]. American Journal of Science, 1995, 295: 1255-1342.
[7] VERDES G, GOUT R, CASTET S. Thermodynamic properties of the aluminate ion and of bayerite boehmite, diaspore and gibbsite[J]. European Journal of Mineralogy, 1992, 4(4): 767-792.
[8] SCHR?DLE S, K?NIGSBERGER E, MAY P M, HEFTER G. Heat capacities of aqueous sodium hydroxide/aluminate mixtures and prediction of the solubility constant of boehmite up to 300 ℃[J]. Geochimica et Cosmochimica Acta, 2010, 74(8): 2368-2379.
[9] ZHOU Jun, CHEN Qi-yuan, LI Jie, YIN Zhou-lan, ZHOU Xia, ZHANG Ping-min. Isopiestic measurement of the osmotic and activity coefficients for the NaOH-NaAl(OH)4-H2O system at 313.2 K[J]. Geochimica et Cosmochimica Acta, 2003, 67(18): 3459-3472.
[10] K?NIGSBERGER E, K?NIGSBERGER L C, HEFTER G, MAY P M. Zdanovskii’s rule and isopiestic measurements applied to synthetic Bayer liquors[J]. Journal of Solution Chemistry, 2007, 36(11/12): 1619-1634.
[11] CAIANI P, CONTI G, GIANNI P, MATTEOLI E. Apparent molar heat capacity and relative enthalpy of aqueous sodium hydroxoaluminate between 323 and 523 K[J]. Journal of Solution Chemistry, 1989, 18(5): 447-461.
[12] MAGALH?ES M C F, K?NIGSBERGER E, MAY P M, HEFTER G. Heat capacities of concentrated aqueous alkaline aluminate solutions at 25 ℃[J]. Journal of Chemical and Engineering Data, 2002, 47(4): 960-963.
[13] WESOLOWSKI D J. Aluminum speciation and equilibria in aqueous solution: I. The solubility of gibbsite in the system Na-K-Cl-OH-Al(OH)4 from 0 to 100 ℃[J]. Geochimica et Cosmochimica Acta, 1992, 56(3): 1065-1091.
[14] LI Xiao-bin, LU Wei-jun, FENG Gang-tao, LIU Gui-hua, PENG Zhi-hong, ZHOU Qiu-sheng, MENG Yun. The applicability of Debye-Hücklel model in NaAl(OH)4-NaOH-H2O system[J]. The Chinese Journal of Process Engineering, 2005, 5(5): 525-528.
[15] LI Xiao-bin, LU Wei-jun, LIU Gui-hua, PENG Zhi-hong, ZHOU Qiu-sheng, MENG Yun, REN Wan-neng. Activity coefficient calculation model for NaOH-NaAl(OH)4-H2O system[J]. Transactions of Nonferrous Metals Society of China, 2005, 15(4): 908-912.
[16] 李小斌, 任万能, 刘桂华, 彭志宏, 周秋生. NaOH-NaAl(OH)4-H2O体系活度系数的计算模型[J]. 中南大学学报: 自然科学版, 2006, 37(3): 493-497.
LI Xiao-bin, REN Wan-neng, LIU Gui-hua, PENG Zhi-hong, ZHOU Qiu-sheng. Activity coefficient calculation model for NaOH-NaAl(OH)4-H2O system[J]. Journal of Central South University: Science and Technology, 2006, 37(3): 493-497.
[17] CHANG B T. Determination of thermodynamic properties of gibbsite from its solubility data in NaOH solutions[J]. Bulletin of the Chemical Society of Japan, 1981, 54: 1960-1963.
[18] LI J, PRESTIDGE C A, ADDAI-MENSAH J. The influence of alkali metal ions on homogeneous nucleation of Al(OH)3 crystals from supersaturated caustic aluminate solutions[J]. Journal of Colloid and Interface Science, 2000, 224(2): 317-324.
[19] OKU O, YAMADA. The dissolution rate of quarts and the rate of desilication in Bayer liquor[C]//Warrendale, PA: TMS Light Metals, 1971: 31-45.
[20] 彭志宏, 邓永春, 周秋生, 刘桂华, 李小斌. 无机盐杂质对铝酸钠溶液晶种分解率的影响[J]. 矿冶工程, 2010, 30(1): 317-324.
PENG Zhi-hong, DENG Yong-chun, ZHOU Qiu-sheng, LIU Gui-hua, LI Xiao-bin. Influence of inorganic salt impurities on seed decomposition rate in sodium aluminate solutions[J]. Mining and Metallurgical Engineering, 2010, 30(1): 317-324.
[21] 彭志宏, 王浩宇, 刘桂华, 周秋生, 李小斌. 二氧化硅在铝酸钠溶液中的反应行为[J]. 矿冶工程, 2009, 29(6): 57-60.
PENG Zhi-hong, WANG Hao-yu, LIU Gui-hua, ZHOU Qiu-sheng, LI Xiao-bin. Reaction behavior of silica in sodium aluminate solution[J]. Mining and Metallurgical Engineering, 2009, 29(6): 57-60.
[22] ADAMSON A N, BLOORE E J, CARR A R. Basic principles of Bayer process design[J]. Journal of Extractive Metallurgy of Aluminum, 1963, 1: 23-58.
[23] CRESSWELL P J. Factors affecting desilication of Bayer process liquor[C]//BARTO A C T. The 12th Australian Chemical Engineering Conference, Chemeca 84. Melbourne: Institute of Chemical Engineering, 1984: 285-292.
[24] JAMIALAHMADI M, M?LLER-STEINHAGEN H. Thermodynamic relationships for the solubility of silica in Bayer process liquor[J]. Aluminum Jahrgang, 1992, 68(3): 230-233.
[25] JAMIALAHMADI M, M?LLER-STEINHAGEN H. Determining silica solubility in Bayer process liquor[J]. Journal of the Minerals, Metals and Materials Society, 1998, 50(11): 44-49.
[26] 李小斌, 刘桂华, 彭志宏, 翟玉春. 铝酸钠溶液中二氧化硅的平衡浓度[J]. 东北大学学报, 2002, 23(3): 251-254.
LI Xiao-bin, LIU Gui-hua, PENG Zhi-hong, ZHAI Yu-chun. Equilibrium concentration of SiO2 in aluminate solution[J]. Journal of Northeastern University, 2002, 23(3): 251-254.
[27] 李小斌, 李永芳, 刘祥民, 刘桂华, 彭志宏, 翟玉春. 复杂硅酸盐矿物Gibbs自由能和焓的一种近似计算法[J]. 硅酸盐学报, 2001, 29(3): 232-237.
LI Xiao-bin, LI Yong-fang, LIU Xiang-min, LIU Gui-hua, PENG Zhi-hong, ZHAI Yu-chun. A simple method of estimation of Gibbs free energy and enthalpy of complicate silicates[J]. Journal of the Chinese Ceramic Society, 2001, 29(3): 232-237.
[28] 李小斌, 刘祥民, 苟中入, 彭志宏, 刘桂华, 周秋生, 丁安平, 李 明, 刘业翔. 铝酸钠溶液碳酸化分解的热力学[J]. 中国有色金属学报, 2003, 13(4): 1005-1010.
LI Xiao-bin, LIU Xiang-min, GOU Zhong-ru, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng, DING An-ping, LI Ming, LIU Ye-xiang. Thermodynamics of carbonization of sodium aluminate solution[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(4): 1005-1010.
[29] REYNOLDS J G, CARTER R. Density model for sodium hydroxide-sodium aluminate solutions[J]. Hydrometallurgy, 2007, 89(3/4): 233-241.
[30] 彭小奇, 宋国辉, 宋彦坡, 张建智, 刘振国. NaOH-NaAl(OH)4-Na2CO3-H2O体系活度因子的计算模型[J]. 中国有色金属学报, 2009, 19(7): 1332-1337.
PENG Xiao-qi, SONG Guo-hui, SONG Yan-po, ZHANG Jian-zhi, LIU Zhen-guo. Calculation model of activity coefficients for NaOH-NaAl(OH)4-Na2CO3-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(7): 1332-1337.
[31] 谢雁丽. 强化铝酸钠溶液分解及粗化产品氢氧化铝粒度的研究[D]. 沈阳: 东北大学, 2000: 60-66.
XIE Yan-li. Studies on the strengthening of seeded precipitation of caustic aluminate solution and coarsening the product particle size[D]. Shenyang: Northeastern University, 2000: 60-66.
[32] van STRATEN H A, SCHOONEN M A A, BRUYN P L D. Precipitation from supersaturated aluminate solution. Ⅲ. Influence of alkali ions with special reference to Li+[J]. Journal of Colloid and Interface Science, 1985, 103(2): 493-507.
[33] MULLIN J W. Crystallization[M]. 4th ed. Oxford: Butterworth- Heinemann, 2001: 108-110.
[34] LI Hui-xin, ADDAI-MENSAH J, THOMAS J C, GERSON A R. The crystallization mechanism of Al(OH)3 from sodium aluminate solutions[J]. Journal of Crystal Growth, 2005, 279(3/4): 508-520.
[35] LI Hui-xin, ADDAI-MENSAH J, THOMAS J C, GERSON A R. The influence of Al(Ⅲ) supersaturation and NaOH on the rate of crystallization of Al(OH)3 precursor particles from sodium aluminate solutions[J]. Journal of Colloid and Interface Science, 2005, 286(2): 511-519.
[36] LI Xiao-bin, LIU Zhi-jian, XU Xiao-hui, ZHOU Qiu-sheng, LIU Gui-hua. Model of apparent crystal growth rate and kinetics of seeded precipitation from sodium aluminate solution[J]. Journal of Central South University of Technology, 2005, 12(6): 662-666.
[37] ROSSITER D S, FAWELL P D, ILIEVSKI D, PARKINSON G M. Investigation of the unseeded nucleation of gibbsite, Al(OH)3, from synthetic Bayer liquors[J]. Journal of Crystal Growth, 1998, 191(3): 525-536.
[38] 李 洁, 陈启元, 尹周澜. 过饱和铝酸钠溶液中氢氧化铝自发成核动力学规律的研究[J]. 高等学校化学学报, 2003, 24(9): 1652-1656.
LI Jie, CHEN Qi-yuan, YIN Zhou-lan. Studies on the kinetics of unseeded nucleation of aluminum trihydroxide from supersaturated sodium aluminate solutions[J]. Chemical Journal of Chinese Universities, 2003, 24(9): 1652-1656.
[39] S?HNEL O. Estimation of electrolyte-crystal-aqueous-solution interfacial tension[J]. Journal of Crystal Growth, 1983, 63(1): 174-176.
[40] 李小斌, 陈 滨, 周秋生, 刘桂华, 彭志宏, 刘祥民. 铝酸钠溶液碳酸化分解过程动力学[J]. 中国有色金属学报, 2004, 14(5): 848-853.
LI Xiao-bin, CHEN Bin, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong, LIU Xiang-min. Kinetics of carbonization decomposition of sodium aluminate solution[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(5): 848-853.
[41] MISRA C, WHITE E T. Kinetics of crystallization of aluminium trihydroxide from seeded caustic aluminate solutions[J]. Chemical Engineering Progress Symposium Series, 1971, 67: 53-65.
[42] KING W R. Some studies in alumina trihydrate precipitation kinetics[C]//Light Metals. Chicago, IL: TMS Light Metals, 1973: 551-563.
[43] HALFON A, KALIAGUINE S. Alumina trihydrate crystallization. Part Ⅰ. Secondary nucleation and growth rate kinetics[J]. The Canadian Journal of Chemical Engineering, 1976, 54(3): 160-167.
[44] WHITE E T, BATEMAN S H. Effect of caustic concentration on the growth rate of Al(OH)3 particles[C]//Light Metals. Phoenix, Arizona: TMS Light Metals, 1988: 157-162.
[45] VEESLER S, BOISTELLE R. Growth kinetics of hydrargillite Al(OH)3 from caustic soda solutions[J]. Journal of Crystal Growth, 1994, 142(1/2): 177-183.
[46] FARHADI F, BABAHEIDARY M B. Mechanism and estimation of Al(OH)3 crystal growth[J]. Journal of Crystal Growth, 2002, 234(4): 721-730.
[47] 周秋生, 李小斌, 彭志宏, 刘桂华, 赵清杰, 吴 洁. 高浓度铝酸钠溶液晶种分解动力学[J]. 中南大学学报: 自然科学版, 2004, 35(4): 557-561.
ZHOU Qiu-sheng, LI Xiao-bin, PENG Zhi-hong, LIU Gui-hua, ZHAO Qing-jie, WU Jie. Kinetics of seeded precipitation from sodium aluminate solution with high concentration[J]. Journal of Central South University: Science and Technology, 2004, 35(4): 557-561.
[48] ANICH I, BAGSHAW T, MARGOLIS N, SKILLINGBERG M. Alumina technology roadmap[C]//Light Metals. Warrendale, PA: TMS Light Metals, 2002: 193-198.
[49] MAHIN E G, INGRAHAM D C, STEWART O J. The constitution of aluminates[J]. Journal of the American Chemical Society, 1913, 35(1): 30-39.
[50] Moolenaar R J, Evans J C, McKeever L D. Structure of the aluminate ion in solutions at high pH[J]. Journal of Physical Chemistry, 1970, 74(20): 3629-3636.
[51] Lippincott E R, Psellos J E, Tobin M C. The Raman spectra and structures of aluminate and zincate ions[J]. Journal of Chemical Physics, 1952, 20: 536-541.
[52] Carreira L A, Maroni V A, Swaine J W, Plumb R C. Raman and Infrared Spectra and Structures of the aluminate ions[J]. Journal of Chemical Physics, 1966, 45(6): 2216-2220.
[53] Kraus I P, Derevyankin V A, Kuznetsov S I. Kinetics of formation and the solubility of hydrated sodium aluminosilicate in aluminate solutions[J]. Tsvetnaya Metally, 1968, 41(1): 43-46.
[54] Barcza L, Pálfalvi-Rózsahegyi M. The aluminate lye as a system of equilibria[J]. Materials Chemistry and Physics, 1989, 21(4): 345-356.
[55] Gale J D, Rohl A L, Watling H R, Parkinson G M. Theoretical investigation of the nature of aluminum-containing species present in alkaline solution[J]. Journal of Physical Chemistry B, 1998, 102(50): 10372-10382.
[56] Watling H R, Fleming S D, van Bronswijk W, Rohl A L. Ionic structure in caustic aluminate solutions and the precipitation of gibbsite[J]. Journal of the Chemical Society, Dalton Transactions, 1998, 23: 3911-3918.
[57] Gutowsky H S, Saika A. Dissociation chemical exchange, and the proton magnetic resonance in some aqueous electrolytes[J]. Journal of Physical Chemistry, 1953, 21(10): 1688-1694.
[58] Anderson G M, Castet S. Reaction of quartz and corundum with aqueous chloride and hydroxide solutions at high temperatures and pressure[J]. American Journal of Science, 1967, 265: 1227-1232.
[59] Anderson G M, Castet S. Feldspar solubility and the transport of aluminum under metamorphic condition[J]. American Journal of Science, 1983, 238-A: 283-297.
[60] Watling H. Spectroscopy of concentrated sodium aluminate solution[J]. Journal of Applied Spectroscopy, 1998, 52(2): 250-258.
[61] Zámbó J. Structure of sodium aluminate liquors, molecular model of the mechanism of their decomposition[C]//Light Metals. New Orleans, Louisiana: TMS, 1986, 2: 199-215.
[62] CHEN Qi-yuan, LI Jie, Yin Zhou-lan, Zhang Ping-min. Decomposition of supersaturated sodium aluminate solution[J]. Transactions of Nonferrous Metals Society of China, 2003, 13(3): 649-654.
[63] SIPOS P, CAPEWELL S G, MAY P M, HEFTER G T, LAURENCY G, LUKukács F, Roulet R. Spectroscopic studies of the chemical speciation in concentrated alkaline aluminate solutions[J]. Journal of the Chemical Society, Dalton Transactions, 1998, 18: 3007-3012.
[64] 王雅静, 翟玉春, 田彦文, 韩跃新, 刘连利. 铝酸钠溶液结构改变时的表面张力变化[J]. 过程工程学报, 2003, 3(2): 121-124.
WANG Ya-jing, ZHAI Yu-chun, TIAN Yan-wen, HAN Yue-xin, LIU Lian-1i. Variation of surface tension of sodium aluminate solution with electrolytic microstructure[J]. The Chinese Journal of Process Engineering, 2003, 3(2): 121-124.
[65] 成琼文, 李小斌, 彭志宏, 刘桂华, 周秋生. 铝酸钠溶液的黏度[J]. 中南大学学报: 自然科学版, 2005, 36(2): 229-233.
CHENG Qiong-wen, LI Xiao-bin, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng. Viscosity of sodium aluminate solution[J]. Journal of Central South University: Science and Technology, 2005, 36(2): 229-233.
[66] LI J, PRESTIDGE C A, ADDAI-MENSAH J. Viscosity, density, and refractive index of aqueous sodium and potassium aluminate solutions[J]. Journal of Chemical and Engineering Data, 2000, 45(4): 665-671.
[67] 李小斌, 王丹琴, 梁 爽, 刘桂华, 彭志宏, 周秋生. 铝酸钠溶液的电导率与结构的关系[J]. 高等学校化学学报, 2010, 31(8): 1651-1655.
LI Xiao-bin, WANG Dan-qin, LIANG Shuang, LIU Gui-hua, PENG Zhi-hong, ZHOU Qiu-sheng. Relationship between electric conductivity and ion structure of sodium aluminate solution[J]. Chemical Journal of Chinese Universities, 2010, 31(8): 1651-1655.
[68] HEFTER G, MAY P M, SIPOS P, STANLEY A. Viscosities of concentrated electrolyte solutions[J]. Journal of Molecular Liquids, 2003, 103/104: 261-273.
[69] 孙素琴, 胡鑫尧, 洪 梅, 陈念贻, 张声俊. 铝酸钠溶液碳酸化过程的红外光谱研究[J]. 光谱与光谱分析, 1996, 16(4): 21-23.
SUN Su-qin, HU Xin-yao, HONG Mei, CHEN Nian-yi, ZHANG Sheng-jun. Study on the carbonation process of sodium aluminate solution with FT-IR spectroscopy[J]. Spectroscopy and Spectral Analysis, 1996, 16(4): 21-23.
[70] 刘吉波. 超声波强化铝酸钠溶液分解过程机理的研究[D]. 长沙: 中南大学, 2004: 73-77.
LIU Ji-bo. Research on decomposition mechanism of sodium aluminate solution intensifying by ultrasonic wave[D]. Changsha: Central South University, 2004: 73-77.
[71] 尹建国, 陈启元, 尹周澜, 胡慧萍. 阳离子聚丙烯酰胺强化铝酸钠溶液种分附聚过程的机理[J]. 中国有色金属学报, 2008, 18(专辑1): 134-138.
YIN Jian-guo, CHEN Qi-yuan, YIN Zhou-lan, HU Hui-ping. Mechanism of cationic polyacrylamide enhancing seeded agglomeration of sodium aluminate liquors[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(Special 1): 134-138.
[72] RADNAI T, MAY P M, HEFTER G T, SIPOS P. Structure of aqueous sodium aluminate solutions: A solution X-ray diffraction study[J]. Journal of Physical Chemistry A, 1998, 102(40): 7841-7850.
[73] COUNTER J A, ADDAI-MENSAH J, RALSTON J. The formation of Al(OH)3 crystals from supersaturated sodium aluminate solutions revealed by cryovitrification-transmission electron microscopy[J]. Journal of Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999, 154(3): 389-398.
[74] LI Xiao-bin, WANG Dan-qin, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong. Concentration variation of aluminate ions during the seeded precipitation process of gibbsite from sodium aluminate solution[J]. Hydrometallurgy, 2011, 106(1/2): 93-98.
[75] PANIAS D. Role of boehmite/solution interface in boehmite precipitation from supersaturated sodium aluminate solutions[J]. Hydrometallurgy, 2004, 74(3/4): 203-212.
[76] LI Xiao-bin, FENG Gang-tao, ZHOU Qiu-sheng, PENG Zhi-hong, LIU Gui-hua. Phenomena in late period of seeded precipitation of sodium aluminate solution[J]. Transactions of Nonferrous Metals Society of China, 2006, 16(4): 947-950.
[77] HERRMANN E, STIPETIC J. Thermodynamic properties of Al(OH)3 in Bayer liquors[J]. Inorganic Chemistry, 1950, 262: 258-287. (in France)
[78] MYERSON A S, TOYOKURA K. Crystallization as a separations process[M]. Washington DC: American Chemical Society, 1990: 329-343.
[79] 张立川, 陈启元, 尹周澜. 过饱和铝酸钠溶液中Al(OH)3均相成核机理[J]. 中国有色金属学报, 2008, 18(8): 1560-1565.
ZHANG Li-chuan, CHEN Qi-yuan, YIN Zhou-lan. Mechanism of homogeneous nucleation in supersaturated sodium aluminate solutions[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(8): 1560-1565.
[80] BROWN N. Secondary nucleation of aluminum trihydroxide in seeded caustic aluminate solutions[J]. Journal of Crystal Growth, 1972, 16(2): 163-169.
[81] LI J, PRESTIDGE C A, ADDAI-MENSAH J. Secondary nucleation of gibbsite crystals from synthetic Bayer liquors: effect of alkali metal ions[J]. Journal of Crystal Growth, 2000, 219(4): 451-464.
[82] SEYSSIECQ I, VEESLER S, BOISTELLE R, LAM?RANT J M. Agglomeration of gibbsite Al(OH)3 crystals in Bayer liquors. Influence of the process parameters[J]. Chemical Engineering Science, 1998, 53(12): 2177-2185.
[83] SEYSSIECQ I, VEESLER S, MANGIN D, KLEIN J P, BOISTELLE R. Modelling gibbsite agglomeration in a constant supersaturation crystallizer[J]. Chemical Engineering Science, 2000, 55(23): 5565-5578.
[84] HALFON A, KALIAGUINE S. Aluminina trihydrate crystallization. Part Ⅱ: A model of agglomeration[J]. The Canadian Journal of Chemical Engineering, 1976, 54: 168-172.
[85] MARCHAL P, DAVID R, KLEIN J P, VILLERMAUS J. Crystallization and precipitation engineering Ⅰ. An efficient method for solving population balance in crystallization with agglomeration[J]. Chemical Engineering Science, 1988, 43(1): 59-67.
[86] SAKAMOTO K, KANEHARA M. Agglomeration of crystalline particles of gibbsite during the precipitation in sodium aluminate solution[J]. Japanese Chemical Engineering Society, 1971, 35: 481-487.
[87] STEEMSON M L, WHITE E T, MARSHALL R J. Mathematical model of the precipitation section of a Bayer plant[C]//Light Metals. LosAngeles, California: TMS, 1984: 237-253.
[88] ZHOU Qiu-sheng, PENG Dian-jun, PENG Zhi-hong, LIU Gui-hua, LI Xiao-bin. Agglomeration of gibbsite particles from carbonation process of sodium aluminate solution[J]. Hydrometallurgy, 2009, 99(3/4): 163-169.
[89] FLEMING S, ROHL A, LEE M, GALE J, PARKINSON G. Atomistic modeling of gibbsite: Surface structure and morphology[J]. Journal of Crystal Growth, 2000, 209(1): 159-166.
[90] TEPPEN B J, RASMUSSEN K, BERTSCH P M, MILLER D M, SCH?FER L. Molecular dynamics modeling of clay minerals. 1. Gibbsite, kaolinite, pyrophyllite, and beidellite[J]. Journal of Physical Chemistry B, 1997, 101(9): 1579-1587.
[91] VEESLER S, ROURCE S, BOISTELLE R. About supersaturation and growth rates of hydragillite Al(OH)3 in alumina caustic solutions[J]. Journal of Crystal Growth, 1993, 130(3/4): 411-415.
[92] ADDAI-MENSAH J, RALSTON J. The influence of interfacial structuring on gibbsite interactions in synthetic Bayer liquors[J]. Journal of Colloid and Interface Science, 1999, 215(1): 124-130.
[93] ADDAI-MENSAH J, PRESTIDGE C A, RALSTON J. Interparticle forces, interfacial structure development and agglomeration of gibbsite particles in synthetic Bayer liquors[J]. Minerals Engineering, 1999, 12(6): 655-669.
[94] PAULAIME A, SEYSSIECQ I, VEESLER S. The influence of organic additives on the crystallization and agglomeration of gibbsite[J]. Powder Technology, 2003, 130(1/3): 345-351.
[95] 谢雁丽, 李尚明, 毕诗文, 杨毅宏, 王 志. 强化铝酸钠溶液晶种分解过程的研究[J]. 轻金属, 2000(7): 18-24.
XIE Yan-li,Li Shang-ming,BI Shi-wen,YANG Yi-hong, WANG Zhi. Study of intensified seed precipitation process of sodium aluminate solution[J]. Light Metals, 2000(7): 18-24.
[96] Zeng Ji-shu, Yin Zhou-lan, Chen Qi-yuan. Intensification of precipitation of gibbsite from seeded caustic sodium aluminate liquor by seed activation and addition of crown ether[J]. Hydrometallurgy, 2007, 89(1/2): 107-116.
[97] Misra C. Industrial alumina chemicals[M]. Washington DC: American Chemical Society Monograph, 1986: 1-165.
[98] Andrija F. Improved method for recovering alumina from aluminate liquor. Germany Patent, 1123184[P]. 1968-08-14.
[99] Chen Qi-yuan, Yin Jian-guo, Yin Zhou-lan. Effect of mechanically activated seeds on the agglomeration process of supersaturated sodium aluminate liquors[C]//Light Metals, Orlando, Florida: TMS Light Metals, 2007: 157-161.
[100] SEYSSIECQ I, VEESLER S, P?PE G, BOISTELLE R. The influence of additives on the crystal habit of gibbsite[J]. Journal of Crystal Growth, 1999, 196(1): 174-180.
[101] 李小斌, 阎 丽, 周秋生, 彭志宏, 刘桂华. 醚类添加剂B35对铝酸钠溶液晶种分解过程的影响[J]. 中国有色金属学报, 2011, 21(2): 459-464.
LI Xiao-bin, YAN Li, ZHOU Qiu-sheng, PENG Zhi-hong, LIU Gui-hua. Effect of ethers additive B35 on seeded precipitation of sodium aluminate solution[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(2): 459-464.
[102] 彭志宏, 刘燕庭, 周秋生, 刘桂华, 李小斌. 非离子型表面活性剂对铝酸钠溶液晶种分解的影响[J]. 中国有色金属学报, 2008, 18(10): 1909-1913.
PENG Zhi-hong, LIU Yan-ting, ZHOU Qiu-sheng, LIU Gui-hua, LI Xiao-bin. Effect of nonionic surfactant on seeded precipitation of sodium aluminate solution[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(10): 1909-1913.
[103] ROE W J, PERISHO J L. Use of polymers in alumina precipitation in the Bayer process of bauxite beneficiation. US Patent, 4608237[P]. 1986-08-26.
[104] Owen D O, Davis D C. Use of surfactants in alumina precipitation in the Bayer process. United States Patent, 0286034[P]. 1988-03-31.
[105] Chen Feng, Zhang Ban-yan, Bi Shi-wen, Xie Yan-li. Effect of anionics-oily additive on seed precipitation from sodium aluminate solution[J]. Journal of Northeastern University, 2004, 25(6): 606-609.
[106] 吴玉胜, 于海燕, 杨毅宏, 毕诗文. 添加剂对铝酸钠溶液晶种分解过程附聚及二次成核的影响[J]. 化工学报, 2005, 56(12): 2434-2439.
WU Yu-sheng, YU Hai-yan, YANG Yi-hong, BI Shi-wen. Effects of additives on agglomeration and secondary nucleation in seed precipitation in sodium aluminate solution[J]. Journal of Chemical Industry and Engineering, 2005, 56(12): 2434-2439.
[107] 薛 红, 毕诗文, 谢雁丽, 王希慧, 姜小凯, 刘铸战. 添加剂强化拜耳法铝酸钠溶液分解[J]. 中国有色金属学报, 1998, 8(增刊2): 415-417.
XUE Hong, BI Shi-wen, XIE Yan-li, WANG Xi-hui, JIANG Xiao-kai, LIU Zhu-zhan. The intensifying effect of additives on the seeded precipitation of gibbsite from sodium aluminate solution[J]. The Chinese Journal of Nonferrous Metals, 1998, 8(Supple 2): 415-417.
[108] van Bronswijk W, Watling H R, Yu Z. A study of the adsorption of acyclicpolyols on hydrated alumina[J]. Journal of Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999, 157(1/3): 85-94.
[109] Smith P G, WatlinG H R, Crew P. The effects of model organic compounds on gibbsite crystallization from alkaline aluminate solutions: Polyols[J]. Journal of Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1996, 111(1/2): 119-130.
[110] WATLING H, LOH J, GATTER H. Gibbsite crystallization inhibition Ⅰ. Effects of sodium gluconate on nucleation, agglomeration and growth[J]. Hydrometallurgy, 2000, 55(3): 275-288.
[111] 刘祥民. 烧结法生产砂状氧化铝的理论与工艺研究[D]. 长沙: 中南大学, 2004: 91-92.
LIU Xiang-min. Study on the fundamentals and technology of sandy alumina production by sinter process[D]. Changsha: Central South University, 2004: 91-92.
[112] 李小斌, 周小淞, 周秋生, 彭殿军, 刘 伟. 高浓度铝酸钠溶液碳酸化分解产品中Na2O含量的控制[J]. 过程工程学报, 2008, 8(5): 945-948.
LI Xiao-bin, ZHOU Xiao-song, ZHOU Qiu-sheng, PENG Dian-jun, LIU Wei. Control of Na2O content in aluminum hydroxide from carbonization of sodium aluminate solution with high alumina concentration[J]. The Chinese Journal of Process Engineering, 2008, 8(5): 945-948.
[113] CALDEIRA C L,CIMINELLI V S T, DIAS A, OSSEO-ASARE K. Pyrite oxidation in alkaline solutions: Nature of the product layer[J]. International Journal of Mineral Processing, 2003, 72(1/4): 373-386.
[114] 孙水裕, 王淀佐, 龙翔云. 硫化矿-溶液界面电子转移的前线分子轨道理论讨论[J]. 北京矿冶研究总院学报, 1994, 3(1): 34-39.
SUN Shui-yu, WANG Dian-zuo, LONG Xiang-yun. Frontier molecular orbital theory consideration for alectron transfer process across sulfide mineral-solution interface[J]. Journal of Bgrimm, 1994, 3(1): 34-39.
[115] 曲启恒, 秦美云, 薛红进. 硫化碱中铁的存在形式及除铁机理探讨[J]. 无机盐工业, 1998, 30(1): 27-28.
QU Qi-heng, QIN Mei-yun, XUE Hong-jin. Study on existing form and removing mechanism of iron in sodium sulfide[J]. Chinese Journal of Inorganic Chemicals Industry, 1998, 30(1): 27-28.
[116] AHLBERG E. The surface oxidation of pyrite in alkaline solution[J]. Journal of Applied Electrochemistry, 1990, 20(6): 1033-1039.
[117] RIMSTIDT J D, VAUGHAN D J. Pyrite oxidation: A state-of- the-art assessment of the reaction mechanism[J]. Geochimica et Cosmochimica Acta, 2003, 67(5): 873-880.
[118] GRACHEV V V, KUZNETSOV S. Influence of different sulfur compounds on the solubility of iron in alkaline and aluminate solutions[J]. Nonferrous Metallurgy, 1974(3): 63-67. (in Russian)
[119] KUZNETSOV S, GRACHEV V V, TYURIN N G. Interaction of iron and sulfur in alkaline aluminate solutions[J]. Zhurnal Prikladnoi Khimii, 1975, 48(4): 748-750. (in Russian)
[120] LI Yuan-gao, CHEN Qi-yuan, WANG Song-sen, YIN Zhou-lan, ZHANG Ping-min. Preparation of Al(OH)3 by ion membrane electrolysis and precipitation of sodium aluminate solution with seeds[J]. Transactions of Nonferrous Metals Society of China, 2008, 18(4): 974-979.
[121] 陈念贻, 陆文聪, 张良苗, 顾松青, 齐利娟, 白万全. 萃取拜耳法及其物理化学基础研究[J]. 中国稀土学报, 2006, 24(专辑): 78-81.
Chen Nian-yi, LU Wen-cong, Zhang Liang-miao, Gu Song-qing, Qi Li-juan, Bai Wan-quan. Liquid-liquid extraction modified Bayer process and its physico-chemical basis[J]. Journal of the Chinese Rare Earth Society, 2006, 24(Special Issue): 78-81.
[122] Andrija F. Improved method for recovering alumina from aluminate liquor. Germany Patent, 1 123 184[P]. 1968-08-14.
[123] Wilhelmy R B. Control of form of crystal precipitation of aluminum hydroxide using co-solvents and varying caustic concentration. United States Patent, 4 900 537[P]. 1990-02-13.
[124] 王 雪, 郑诗礼, 张 懿. 甲醇溶析铝酸钠制备氢氧化铝[J]. 过程工程学报, 2008, 8(1): 72-77.
Wang Xue, Zheng Shi-li, Zhang Yi. Preparation of aluminum hydroxide with dissolution of sodium aluminate in methanol-water solvent[J]. The Chinese Journal of Process Engineering, 2008, 8(1): 72-77.
[125] Zhang Ying, Zheng Shi-li, Du Hao, Xu Hong-bin, Wang Shao-na, Zhang Yi. Improved precipitation of gibbsite from sodium aluminate solution by adding methanol[J]. Hydrometallurgy, 2009, 98(1/2): 38-44.
[126] LI Yan, Zhang Yi-fei, Yang Chao, Zhang Yi. Precipitating sandy aluminium hydroxide from sodium aluminate solution by the neutralization of sodium bicarbonate[J]. Hydrometallurgy, 2009, 98(1/2): 52-57.
[127] Li Yan, Zhang Yi-fei, Chen Fang-fang, Yang Chao, Zhang Yi. Polymorphic transformation of aluminum hydroxide precipitated from reactive NaAl(OH)4-NaHCO3 solution[J]. Crystal Growth and Design, 2011, 11(4): 1208-1214.
[128] BALLAS D, COPPIETERS A, KALAITZOGLOU K, KONTOPOULOS A, PANOU G, RIGAS G. Process for production of monohydrate alumina from supersaturated aluminate solutions. European Patent, 996590[P]. 1998-12-30.
[129] PANIAS D, PASPALIARIS I, AMANATIDIS A, SCHMIDT H W, HOLLNAGEL A. Boehmite process: An alternative technology in alumina production[C]//Light Metals. Warrendale, PA: TMS, 2001: 97-103.
[130] PANIAS D, KRESTOU A. Effect of synthesis parameters on precipitation of nanocrystalline boehmite from aluminate solutions[J]. Powder Technology, 2007, 175(3): 163-173.
[131] PANIAS D, ASIMIDIS P, PASPALIARIS I. Solubility of boehmite in concentrated sodium hydroxide solutions: Model development and assessment[J]. Hydrometallurgy, 2001, 59(1): 15-29.
[132] PANIAS D, PASPALIARIS I. Thermodynamic determination of the stability area of boehmite (γ-AlOOH) in Al2O3-Na2O-H2O and Al2O3-H2O systems[J]. Erzmetall, 1999, 52(11): 585-595.
[133] 李小斌, 潘 军, 刘桂华, 彭志宏, 周秋生. 从铝酸钠溶液中析出一水软铝石的实验研究[J]. 中南大学学报: 自然科学版, 2006, 37(1): 25-30.
LI Xiao-bin, PAN Jun, LIU Gui-hua, PENG Zhi-hong, ZHOU Qiu-sheng. Boehmite precipitation from sodium aluminate solution[J]. Journal of Central South University: Science and Technology, 2006, 37(1): 25-30.
[134] SKOUFADIS C, PANIAS D, PASPALIARIS I. Kinetics of boehmite precipitation from supersaturated sodium aluminate solutions[J]. Hydrometallurgy, 2003, 68(1/3): 57-68.
[135] DASH B, TRIPATHY B C, BHATTACHARYA I N, DAS S C, MISHRA C R, PANI B S. Effect of temperature and alumina/caustic ratio on precipitation of boehmite in synthetic sodium aluminate liquor[J]. Hydrometallurgy, 2007, 88(1/4): 121-126.
[136] DASH B, TRIPATHY B C, BHATTACHARYA I N, DAS S C, MISHRA C R, MISHRA B K. Precipitation of boehmite in sodium aluminate liquor[J]. Hydrometallurgy, 2009, 95(3/4): 297-301.
[137] MISRA C, SIVAKUMAR T J. Boehmite production by precipitation from sodium aluminate solution at elevated temperatures. United States Patent, 4595581[P]. 1986-06-17.
[138] EL-KATATNY E A, HALAWY S A, MOHAMED M A, ZAKI M I. A novel synthesis of high-area alumina via H2O2- precipitated boehmite from sodium aluminate solutions[J]. Journal of Chemical Technology and Biotechnology, 1998, 72(4): 320-328.
[139] LOH J, VERNON C, LOAN M, BRODIE G. Boehmite and gibbsite precipitation[C]//Light Metals. San Francisco, CA: TMS, 2005: 203-208.
(编辑 龙怀中)
基金项目:国家重点基础研究发展计划资助项目(2005CB6237)
收稿日期:2011-04-30;修订日期:2011-07-30
通信作者:李小斌,教授,博士;电话:0731-88830454;传真:0731-88830453;E-mail: X.B.Li@csu.edu.cn
李小斌教授简介
李小斌,1962年出生,博士,中南大学教授,博士生导师,享受国务院政府特殊津贴。中国有色金属学会轻金属学术委员会委员、氧化铝专业委员会副主任。长期从事氧化铝生产技术与理论、工业结晶和冶金废渣金属资源化等领域的研究。先后承担了国家“八五”、“九五”重点科技攻关项目、国家“973”项目课题、国家自然科学基金项目以及中国铝业、ALCOA(美国铝业公司)等企业技术开发项目,取得了多项研究成果。先后在国内外公开发表学术论文160余篇;申报国家发明专利12项,已获授权发明专利9项,多项技术在工业生产中得以应用;主持的“强化烧结法生产氧化铝新工艺”获2006年度国家科技发明二等奖和2003年度国家发明专利金奖,作为主要骨干参与的“铝资源高效利用与高新能铝材制备的理论与技术”获2007年度国家科技进步一等奖。由于对企业的突出贡献,2003年获中国铝业公司首届科技大会科技合作奖。


