
Hydrothermal synthesis and characterization of Eu-doped GaOOH/α-Ga2O3/β-Ga2O3 nanoparticles
QUAN Yu(全 玉)1, LIU Su-qin(刘素琴)1, HUANG Ke-long(黄可龙)1,
FANG Dong(方 东)1, ZHANG Xue-ying(张学英)2, HOU Hua-wei(侯华卫)2
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Henan Branch of China Aluminium Company, Zhengzhou 450041, China
Received 19 January 2010; accepted 13 April 2010
Abstract:
Eu-doped GaOOH nanoparticles with size of 5-8 nm were prepared by hydrothermal method using sodium dodecylbenzene sulfonate (SDBS) as surfactant. Eu-doped α-Ga2O3 and β-Ga2O3 were further fabricated by annealing GaOOH?Eu and then characterized by X-ray diffraction(XRD), transmission electron microscopy (TEM) and photoluminescence (PL). The TEM results show that monodisperse Eu3+-doped GaOOH nanoparticles form and then transform into Eu3+-doped α-Ga2O3 and β-Ga2O3 through annealing the GaOOH?Eu nanoparticles at 600 and 900 °C, respectively. PL studies indicate that GaOOH?Eu has the highest intensity at 618 nm. Luminescence quenching is observed at higher Eu3+concentration in all samples.
Key words:
hydrothermal method; GaOOH; Ga2O3; Eu-doped nanoparticle; photoluminescence;
1 Introduction
Gallium oxide (Ga2O3) is an important wide-band- gap compound, which has long been known to have excellent conduction and luminescence properties[1]. Great efforts have been made to investigate their applications as optoelectronic devices, as well as a candidate for a high-temperature stable gas sensor transparent conductor and supported selective catalysts[2-4]. Synthesis of nanometer-sized phosphors has attracted much attention owing to their size-dependent electrical and optical properties originating from the quantum confinement. The rare earth ion Eu3+-doped Ga2O3 phosphors are of great interest because the major emission band is centered near 618 nm red light, which is one of the three primary colors. Many studies on structure and morphology of Ga2O3 were reported[5-10]. However, optical properties of nanocrystalline Ga2O3 doped by Eu3+ have not been studied extensively, and the optical properties of Eu3+-doped GaOOH has not been reported.
Gallium oxides with different morphologies have been synthesized by many high temperature methods: thermal evaporation, arc discharging, laser ablation, chemical vapor deposition, carbon thermal reduction and catalyst assisted methods[11-14]. These methods require heat treatment at high temperatures for several hours and some of them need subsequent grinding. This may damage the phosphor surface, resulting in the loss of emission intensity. Hydrothermal method is one of the most promising solutions due to the obvious advantages, such as economics, energy efficiency, and environmental friendliness. It is well known that the particle size and distribution, phase homogeneity, and morphology could be well controlled by surfactant. Nonionic and cationic surfactants were used to obtain the gallium oxide and it is noted that the above surfactants have limited effect on the morphology of resulted GaOOH crystals[15]. In many published literatures, sodium dodecylbenzene sulfonate (SDBS) is one of the most popular anionic surfactants to fabricate the nanomaterials[16]. In our work, SDBS was used as the surfactant to synthesize Eu-doped GaOOH nanoparticles by hydrothermal method. Then, calcination of the GaOOH nanoparticles at 600 °C and 900 °C in air yielded gallium oxide (α-Ga2O3, β-Ga2O3) crystals[17-18]. The optical properties of the samples were investigated as well.
2 Experimental
In a typical procedure, Ga(NO3)3?·nH2O was dissolved in a deionized water to form a solution with 0.01 mol/L Ga3+, the solution was added with SDBS, and the molar ratio of surfactants to Ga3+ is 1?10. To prepare GaOOH?Eu3+ phosphor, an appropriate amount of Eu2O3 (99.99%) was dissolved in HNO3 and heated to form a clear solution. The Ga(NO3)3 with SDBS and Eu(NO3)3 solution were mixed by constant stirring, then a desired amount of aqueous ammonia was added to the clear solutions to make pH of the solution as 8. The solution was poured into a Teflon-lined autoclave apparatus for a hydrothermal treatment at 170 °C for 10 h. The obtained solid products (GaOOH?Eu3+) were then heated in an electric furnace at 600 °C or 900 °C for 5 h in air, and α-Ga2O3?Eu3+/β-Ga2O3?Eu3+ were obtained, respectively.
All the samples were characterized by powder X-ray diffraction (XRD) on a MXPAHF X-ray diffractometer with Cu Kα radiation (λ=1.790 21 ?). The morphological characterization of the samples was carried out by transmission electron microscopy (JEM-3010; JESL, Tokyo, Japan). For transmission electron microscopy, the samples were separated by ultrasonically dispersion in 1 mL of ethanol, then a drop of the solution was placed on a Cu grid covered with carbon film. The excitation and photoluminescence(PL) spectra measurements were performed on a Hitachi F-2500 fluorescence spectrophotometer equipped with a Xe lamp as the excitation light source.
3 Results and discussion
The typical XRD patterns of samples obtained after hydrothermal treatment are shown in Fig.1. The diffraction peak of Fig.1(a) can be assigned to
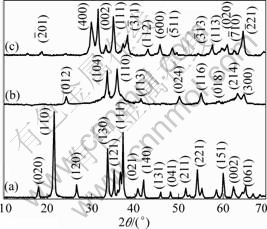
Fig1 XRD patterns of obtained GaOOH (a), α-Ga2O3 (b) and β-Ga2O3 (c)
orthorhombic GaOOH(JCPDS 060180). The peaks are sharp and narrow, indicating identical crystal structure. After a subsequent heat treatment at 600 and 900 °C in air, the GaOOH?Eu3+ crystals transform to pure hexahedral α-Ga2O3?Eu3+ (JCPDS 060503) (Fig.1(b)) and monoclinic β-Ga2O3?Eu3+ (JCPDS 411103) (Fig.1(c)) crystals), respectively, indicating that the doped Eu3+ ions cannot affect the phase purity in our experiments.
The TEM image of the as-synthesized GaOOH powders is shown in Fig.2(a), which indicates that the product comprises mainly nanoparticles with the diameter ranging from 5 to 8 nm. Further information about the microstructure of the as-synthesized GaOOH nanoparticles is provided by SAED pattern (Fig.2(b)). The linearly spotted SAED pattern reveals that the GaOOH nanoparticles are single crystals. The measured interplanar spacing corresponds to the (002) crystal plane of orthorhombic structure. EDS analysis taken from different parts of the monodispersed nanocrystalline GaOOH?Eu3+ reveals Ga, Eu and O species in the sample together with a minor fraction of Cu (from the TEM grid) in addition to some carbon content (surface contaminant) (see Fig.3).
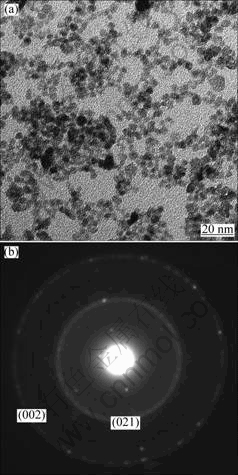
Fig.2 TEM image (a) and SAED pattern (b) of monodispersed nanocrystalline GaOOH?Eu3+
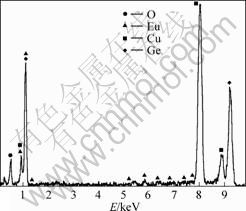
Fig.3 EDS spectrum of monodispersed nanocrystalline GaOOH?Eu3+
It is well known that surfactant molecules can form micelles in aqueous solution when the concentration is above the so-called critical micelle concentration. The aggregates are generally round globular micelles. These surfactant micelles may serve as nanoparticle reactor where the GaOOH crystals are limited in them to form the nanoparticles.
Fig.4(a) displays the TEM image of α-Ga2O3?Eu3+ products converted from GaOOH?Eu3+ annealed at 600 °C. The exactly same morphologies indicate the inheritance of nanoparticles. Corresponding XRD characterization (Fig.1(b)) indicates the complete conversion of GaOOH to α-Ga2O3. Compared with the size of the GaOOH?Eu3+ nanoparticles, the particles are all agglomerates with particle sizes ranging from 50 to 70 nm. Fig.4(b) shows the diffraction rings in the SAED pattern of the α-Ga2O3?Eu3+, and (110) and (117) planes of α-Ga2O3 could be identified. An individual α-Ga2O3 corresponding SAED pattern indicates that α-Ga2O3 nanoparticles are single-crystalline in structure. Fig.4(c) displays the TEM image of β-Ga2O3?Eu3+ products converted from GaOOH?Eu3+ annealed at 900 °C. It is worthy to note that even after high temperature treatment, the Ga2O3 still maintains the same morphology. Fig.4(d) shows the HRTEM images of the β-Ga2O3?Eu3+ nanoparticles. The value of fringes corresponds to the (201) and (110) planes of the β-Ga2O3 nanoparticles. This is further confirmed by the starry electron diffraction (ED) pattern as shown in the inset of Fig.4(d).
The excitation and emission spectra of GaOOH:Eu3+, α-Ga2O3?Eu3+, β-Ga2O3?Eu3+ excited at 394 nm are
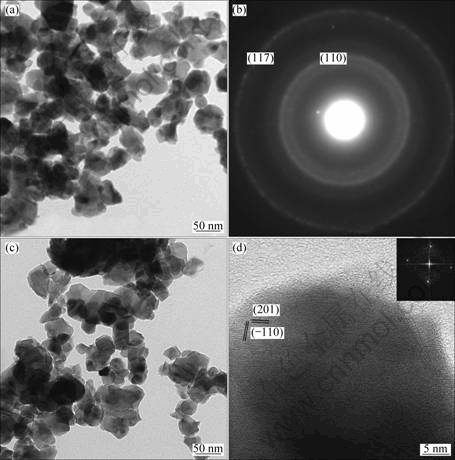
Fig. 4 TEM image (a) and SAED pattern of α-Ga2O3?Eu3+ (b), TEM image (c) and HRTEM of β-Ga2O3?Eu3+ (d)
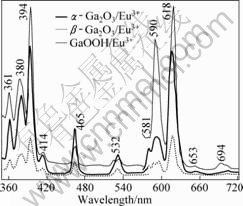
Fig.5 Emission/excitation spectra of GaOOH?Eu3+, α-Ga2O3?Eu3+ and β-Ga2O3?Eu3+ (Eu3+ 7%, mole fraction)
illustrated in Fig.5. A number of sharp excitation peaks in the region from 350 to 550 nm which are associated with the f-f transition of Eu3+, and the emission bands of GaOOH?Eu3+correspond to 5D0-7F0 (around 581 nm), 5D0-7F1 (around 590 nm), 5D0-7F2 (around 618 nm), 5D0-7F3 (around 653 nm), and 5D0-7F4 (around 694 nm) transitions of Eu3+[19]. Two broad emission bands of α-Ga2O3?Eu3+ and β-Ga2O3?Eu3+ centered at 591 and 614 nm could be assigned to 5D0-7F1 and 5D0-7F2 transitions for Eu3+, respectively. Of the two samples, the emission spectra of these samples are similar in shape, and the bands differ only in their relative intensities. The GaOOH?Eu3+ has a high emission intensity, and to our best knowledge, the photoluminescence properties of GaOOH?Eu3+ have not been reported.
In Fig.5, the emission intensity of the GaOOH is very high. This phenomenon could be explained according to the crystal structure of GaOOH. The crystal structure of GaOOH was reported to be analogous to the diaspore (α-AlOOH) type, consisting of double chains of an edge-shared octahedron. The structure of GaOOH?Eu itself is asymmetry, and it is reported[20] that the 5D0-7F2 emission of Eu3+ belongs to hypersensitive transitions, which is strongly influenced by the outside surroundings. The relative intensity of these depends on the local symmetry of Eu3+ ions, and a lower symmetry local site will result in a higher emission intensity. α-Ga2O3 has the corundum structure with R?3C symmetry. The oxygen ions are approximately hexagonal close packed and the gallium ions occupy two-thirds of the octahedral sites. The crystalline structure can be described in terms of GaO6 octahedra[21]. The monoclinic phase of β-Ga2O3 has C2/m symmetry. The Ga atoms have tetrahedral (GaO4) and octahedral coordinations in the lattice. The two kinds of Ga3+ ions exist in equal quantity: one in tetrahedral site (Td point symmetry, without inversion center) coordinated by four oxygen atoms, the other in octahedral site (Oh point symmetry, with an inversion center) coordinated by six oxygen atoms. It is easier for Eu3+ to replace the octahedral Ga3+ than the tetrahedral Ga3+ in the host lattices[22], so, the percentage of Eu in the host lattice of α-Ga2O3?Eu3+ is higher than that of β-Ga2O3?Eu3+. It is likely that the more the unequal replacement of Ga3+, the lower the symmetry of Eu3+ site in host lattices. A lower symmetry local site (without inversion symmetry center) will result in a higher emission intensity and R/O value, thus the emission intensity of α-Ga2O3?Eu3+ is higher than that of β-Ga2O3?Eu3+.
Fig.6 shows the emission spectra of the GaOOH?Eu3+,
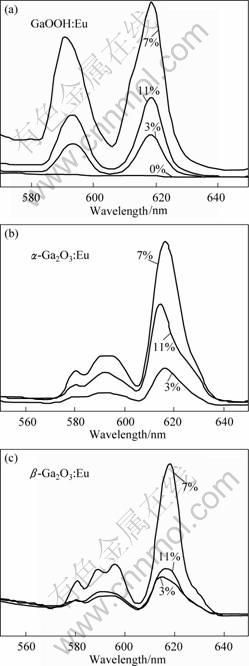
Fig.6 Emission spectra of GaOOH?Eu3+ (a), α-Ga2O3?Eu3+ (b), β-Ga2O3?Eu3+ (c) with doping different Eu3+ contents
α-Ga2O3?Eu3+and β-Ga2 O3?Eu3+ with different doping contents (3%, 7 % and 11%, mole fraction). From the results, the variation trend is the same of the three samples. The integrated intensity increases gradually as the doping content increases to 7%, and the concentration quenching effect appears when the doping content reaches 11%. This results from the fact that more Eu3+ ions are incorporated into the host lattice at a higher doping content (7%) and prominent energy migration between the Eu3+ ions takes place. The emission intensity of 5D0→7Fj (j = 0-4) depends on the amount of Eu2O3. In this case, the pairing or aggregation of activator atoms at high content may change a fraction of the activators into quenchers and induce the quenching effect. The migration of excitation by resonant energy transfer between the Eu3+ activators can sometimes be so efficient that it may carry the energy to a distant killer or to a quenching center existing on the surface of the crystal.
4 Conclusions
1) The particle size of GaOOH:Eu3+ nanoparticles is about 5-8 nm. The morphologies and sizes of α-Ga2O3?Eu3+ and β-Ga2O3?Eu3+ nanoparticles fabricated by the nanoparticles remain unchanged.
2) The red emissions peak 5D0-7F2 (around 618 nm) from Eu3+ ions are observed under an excitation of 394 nm in all the samples. Luminescence quenching is observed in the nanoparticles as the amount of Eu2O3 increases to 11%.
3) The GaOOH?Eu3+ has high emission intensity, and the emission intensity of α-Ga2O3?Eu3+ is superior to that of β-Ga2O3?Eu3+.
References
[1] BINET L, GOURIER D. Origin of the blue luminescence of β-Ga2O3 [J]. J Phys Chem Solids, 1998, 59: 1241-1249.
[2] OGITA M, HIGO K, NAKANISHI Y, HATANAKA Y. Ga2O3 thin film for oxygen sensor at high temperature [J]. Appl Surf Sci, 2001, 175/176: 721-725.
[3] SHIMIZU K, SATSUMA A, HATTORI T. Selective catalytic reduction of NO by hydro-carbons on Ga2O3/Al2O3 [J]. Applied Catalysis B: Environmental, 1998, 16: 319-326.
[4] NAKAGAWA K, KAJITA C, IDE Y, OKAMURA M, KATO S, KASUYA H, IKENAGA N, KOBAYASHI T, SUZUKI T. Promoting effect of carbon dioxide on the dehy-drogenation and aromatization of ethane over gallium-loaded catalysts [J]. Catal Lett, 2000, 64: 215-221.
[5] ZHANG Jie, LIU Zhi-guo, LIN Cui-kun, LIN Jun. A simple method to synthesize β-Ga2O3 nanorods and their photoluminescence properties [J]. Journal of Crystal Growth, 2005, 280: 99-106.
[6] ZHANG Jie, QIAO Zi-wen, LIN Cui-kun, LIN Jun. Preparation and photoluminescent properties of β-Ga2O3: Dy nanorod arrays [J]. Journal of the Chinese Rare Earth Society, 2005, 23(5): 572-575. (in Chinese)
[7] YAN Chong, CAI Ke-feng. Progress in research on one dimensional gallium oxide nanomaterials [J]. Materials Review, 2006, 20(5): 99-101. (in Chinese)
[8] KIM J S, KIM H E , PARK H L. Luminescence intensity and color purity enhancement in nanostructured β-Ga2O3?Eu3+ phosphors [J]. Solid State Communications, 2004, 132: 459-463.
[9] XIE Hong-bo, CHEN Li-miao, LIU You-nian, HUANG Ke-long. Preparation and photoluminescence properties of Eu-doped α-Ga2O3 and β-Ga2O3 phosphors [J]. Solid State Communications, 2007, 141: 12-16.
[10] GODHULI S, AMITAVA P. Generation of green, red and white light from rare-earth doped Ga2O3 nanoparticles [J]. Chemical Physics Letters, 2009, 473: 151-154.
[11] HU J Q, LI Q, MENG X M, LEE C S, LEE S T. Synthesis of β-Ga2O3 nanowires by laser ablation [J]. Journal of Physical Chemistry B, 2002, 106: 9536-9539.
[12] PARK G S, CHOI W B, KIM J M, CHOI Y C, LEE Y H, LIM C B. Structural investigation of gallium oxide(β-Ga2O3) nanowires grown by arc-discharge [J]. Journal of Crystal Growth, 2000, 220: 494-500.
[13] YANG Z X, ZHU F, WU Y J, ZHOU W M, ZHANG Y F. β-Ga2O3 nanowires and nanobelts synthesized by thermal evaporation [J]. Physical E, 2005, 27: 351-354.
[14] KIM H W, KIM N H, LEE C. Annealing effects on the structural and optical properties of gallium oxide nanowires [J]. Journal of Materials Science: Materials in Electronics, 2005, 16: 103-105.
[15] ZHAO Yan-yan, FROST R L, MARTENS W N. Synthesis and characterization of gallium oxide nanostructures via a soft-chemistry route [J]. J Phys Chem C, 2007, 111(44): 16290-16299.
[16] RAKRISHNA G, GHOSHH N. Optical and photochemical properties of sodium dode-cylbenzene sulfonate (DBS)-capped TiO2 nano-particles dispersed in nonaqueous solvents [J]. Langmuir, 2003,19: 505-508.
[17] SONG Gen-ping, BO Jie, GUO Rong. Preparation of polystyrene/Fe3O4 nanoparticles in triton X-100/sodium dodecyl benzene sulfonate mixed surfactant system [J]. Chinese Journal of Chemistry, 2005, 23: 997-1000.
[18] QIAN H S, POERNOMO G, ZHANG Y X, LIN G F, ZHENG J W, XU R. Template-free synthesis of highly uniform α-GaOOH spindles and conversion to α-Ga2O3 and β-Ga2O3 [J]. Crystal Growth & Design, 2008, 8: 1282-1287.
[19] WEI Zheng-gui, SUN Ling-dong, LIAO Chun-sheng, YAN Chun-hua, HUANG Shi-hua. Fluorescence intensity and color purity improvement in nanosized YBO3?Eu [J]. Appl Phys Lett, 2002, 80: 1447-1449.
[20] CARNELL W T. Handbook on the physics and chemistry of rare earths [M]. Amsterdam: North-Holland Publishing Company, 1979: 171.
[21] VOSEGAARD T, BYRIEL I P, BINET L, MASSIOT D, JAKOBSEN H J. Crystal structure studies by single-crystal NMR spectroscopy 71Ga and 69Ga single-crystal NMR of α-Ga2O3 twins [J]. J Am Chem Soc, 1998, 120: 8184-8188.
[22] SINHA G, PATRA A. Generation of green, red and white light from rare-earth doped Ga2O3 nanoparticles [J]. Chemical Physics Letters, 2009, 473: 151-154.
Foundation item: Project(50772133) supported by the National Natural Science Foundation of China; Project(LA 09014) supported by Innovation Projects for Graduates of Center South University, China
Corresponding author: HUANG Ke-long; Tel: +86-13974828682; E-mail: Huangkelong@163.com
DOI: 10.1016/S1003-6326(09)60321-6


