Trans. Nonferrous Met. Soc. China 24(2014) 3922-3928
Wetting behavior of aluminium and filtration with Al2O3 and SiC ceramic foam filters
Sarina BAO1, Martin SYVERTSEN1, Anne KVITHYLD1, Thorvald ENGH2
1. SINTEF Materials and Chemistry, Trondheim 7465, Norway;
2. Department of Materials Science and Engineering, Norwegian University of Science and Technology, Trondheim 7491, Norway
Received 17 October 2013; accepted 21 November 2014
Abstract:
The wetting behavior between liquid aluminium and substrates made from industrial Al2O3 and SiC based ceramic foam filters (CFF) was investigated. The same CFF filters were also tested in plant scale filtration experiments. The wetting experiment results show that the SiC based filter material is better wetted by liquid aluminium than the Al2O3 based filter material. This indicates that the improved wetting of aluminium on a filter material is an advantage for molten metal to infiltrate the filter during priming. Also, better wetting of Al-filter might increase the removal efficiency of inclusions during filtration due to better contact between filter and metal. Non-wetted inclusions are easier to be removed.
Key words:
filtration; wettability; aluminium; Al2O3 filter; SiC filter; Al2O3 inclusion; Al4C3 inclusion;
1 Introduction
Filtration is one of the most typical refining processes to eliminate the undesired impurities from aluminium alloys. The filtration process has a complex mechanism influenced by hydrodynamic factors such as fluid flow, turbulence, surface and body forces, as well as chemical and metallurgical interactions among the inclusions [1], the filter media [2], and the liquid metal [3]. In this work, the wetting behaviour of aluminium, as a combination of surface and body forces, was investigated to improve the aluminium filtration.
There are two sequential events for inclusion removal: 1) transport of inclusion to the filter inner wall, and 2) attachment of inclusions to the wall. The wetting between aluminium and filter may change the fluid flow pattern, as well as inclusions in the fluid. Aluminium may push inclusions towards the filter wall, where inclusions can be captured. If the viscous drag on inclusion is not higher than the capture forces, the inclusions will be retained on the filter walls. Hereby, the wetting factors can be divided into two factors: the wetting of Al-inclusion and the wetting of the Al-filter.
This work focuses on the wetting of Al-inclusion (Al2O3 and Al4C3) and Al-filter (Al2O3 and SiC based filter), and its influence on the aluminium filtration behaviour.
Al2O3 and Al4C3 are two of the most typical inclusions in aluminium. In a previous work, BAO et al [4] had investigated the wetting between aluminium and various solid substrates. The contact angle of molten aluminium on alumina and graphite had been measured in high vacuum of 1.01325×10-3 Pa in the temperature range of 1000-1300 °C. Aluminium is readily oxidized (Reaction (1)) even if the oxygen partial pressure is as low as 1.01325×10-44 Pa at the filtration temperature of 700 °C [5]. Such a low oxygen partial pressure is difficult to be achieved experimentally. Nevertheless, the oxide layer on the surface of a molten aluminium drop can be removed, if the outgoing flow of gaseous Al2O, according to Reaction (2), is greater than the incoming flow of oxygen. The equilibrium partial pressure of Al2O, according to Reaction (2), is 4.357 Pa at 1000 °C. Since BAO et al [4] held the total pressure in the furnace under 1.01325×10-3 Pa, the oxide skin on the aluminium drop evaporated. This makes it possible to measure the contact angles between molten aluminium and substrates without an oxide skin on the aluminium. To describe the wetting behaviour of the Al-inclusion system at lower temperatures used in filtration and casting aluminium, a semi-empirical calculation was employed. The calculated contact angles at 700 °C are around 97° for alumina and 92° for vitreous graphite. It is also stated that the wettability of these systems with respect to time goes through steps of 1) de-oxidation of the alumina layer, 2) surface reactions: Al4C3 formation, and 3) the stable contact on the interface [6]. In conclusion, Al2O3 and Al4C3 inclusions both are non-wetted by aluminum at the casting temperature.


In the present work, the contact angle between molten aluminium and two types of ceramic foam filter materials at 1100 °C and 1200 °C was measured using the same sessile drop technique as used in an earlier study [4]. Four filtration experiments using two types of CFF filters were carried out and were presented here. The inclusion removal in the same two types of filter was discussed.
2 Experimental
The filters are shown in Fig. 1. These two types of filters were produced in the same production line by the same filter manufacturer, giving similar porosity and wall thickness. The Al2O3 based filter contains 85%- 90% of Al2O3, approximately 6% of P2O5, approximately 6% of SiO2, and approximately 1% of K2O+Na2O (mass fraction). The SiC based filter contains 58%-64% of SiC, 5%-9% of Al2O3, and 29%-33% of SiO2. The wettability of the same sintered flat materials received from the same supplier was tested in the wetting furnace. No grinding was involved. The wetting apparatus essentially consisted of a horizontal graphite tube, where aluminium on the substrate was placed, surrounded by graphite radiation shields for heating, located in a water-cooled vacuum chamber. The chamber was fitted with windows to allow a digital video camera (Sony XCD-SX910CR) to record the shape of the droplet. The contact angles and linear dimensions of the images were measured directly from the image of the drop using Video Drop Shape Analysis software. We assumed symmetry of the drop. After the experiments no asymmetry was observed.
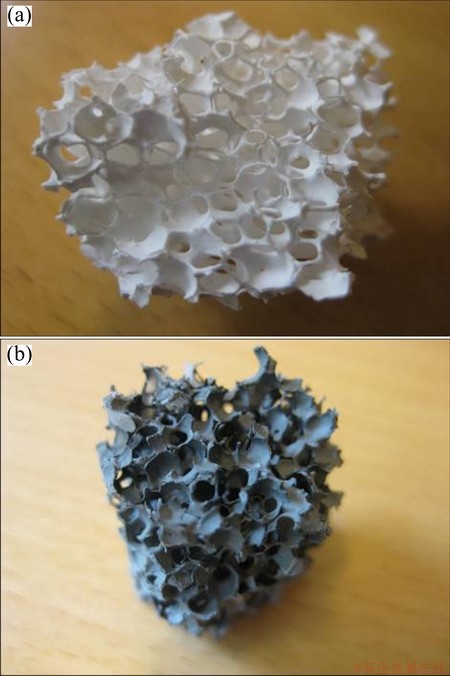
Fig. 1 Morphologies Al2O3 based filter (a) and SiC based filter (b)
The wetting experiments were carried out with the substrate of 99.999% pure aluminium (mass fraction). The aluminium rod with a diameter of 2 mm was cut into small pieces around 2 mm in length, then polished by 30 μm sandpaper and cleaned with ethanol in order to prevent further oxidation. When the wetting furnace attained a vacuum of 1.01325×10-3 Pa, the sample was quickly heated to 950 °C in about 80 s to remove the oxide layer, and then heated to 1100 and 1200 °C at a heating rate of 50 °C/min. In all the experiments, the contact angle of the droplet was recorded simultaneously during the isothermal period at 1100 and 1200 °C.
Four plant scale filtration experiments were performed with these two types of 10"×10"×2", 30ppi filters: Experiments 1 and 3 with the Al2O3 based filter and Experiments 2 and 4 with the SiC based filter. All experiments lasted for 1 h. The aluminium alloy contained approximately 1.00% of Mg, 0.14% of Fe, and 0.07% of Si (mass fraction). The contents of other elements were all less than 0.05%. Two Liquid Metal Cleanliness Analysers (LiMCA) II [7] which gave on-line information for inclusion level were positioned before and after the filter. Two lasers positioned before and after the filter bowl gave the metal height (pressure drop) in the launder. Finally, a thermocouple positioned in the launder measured the temperature after the filter. The filter in the filter bowl was preheated by a gas burner in the lid to avoid thermal shock and freezing of the metal when filtration started. When the metal primed the filter, it filled the lower space of the filter bowl, and went out into the launder again, as indicated in Fig. 2. In 1 h filtration, three groups of PoDFA samples were taken at time 0 min, 30 min, and 60 min before and after the filter. 1.25 kg of metal was pushed through the PoDFA filter disk with under pressure. The surface area of the inclusions and the types of the inclusions were examined after it solidified.
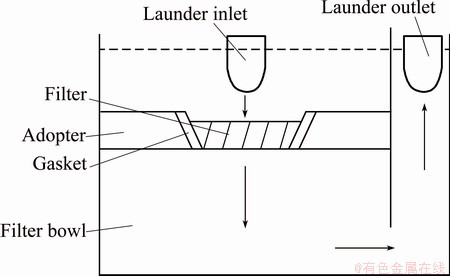
Fig. 2 Schematic cross section view of filter bowl
3 Results and discussion
3.1 Results
Contact angles measured on ceramics in temperature range of 1100-1200 °C are summarized in Figs. 3-6. The wettability improves with time and stabilizes. In Fig. 3, the contact angle decreases from 126° to approximately 91° in 64 min for Al2O3 based filter at 1100 °C. To know the trend of the wetting with time, the fitting according to Boltzmann distribution law [8], i.e., the exponential decay with equation (3), is introduced in this case. The fitted line gives the stable contact angle of 84° with R2 value of 0.928. R2 provides a measure of how well future outcomes are likely to be predicted by the model.
y=y0+A1exp(-χ/t1) (3)
With the same fitting approach, the contact angle of 44° is achieved for Al2O3 based filter at 1200 °C with R2 value of 0.992. SiC based filter gives the stable contact angles of 39° (R2=0.948) and 28° (R2=0.993) at the temperatures of 1100 °C and 1200 °C, respectively. Due to the large oxide content in the filter, 1100 °C and higher temperatures, instead of 1000 °C in previous work [4], are needed to remove the oxide skin on the aluminium droplet.
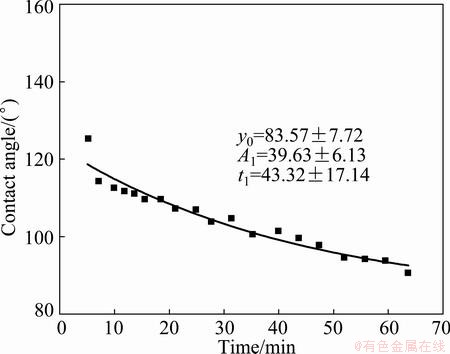
Fig. 3 Contact angle vs time for Al on Al2O3 based filter at 1100 °C
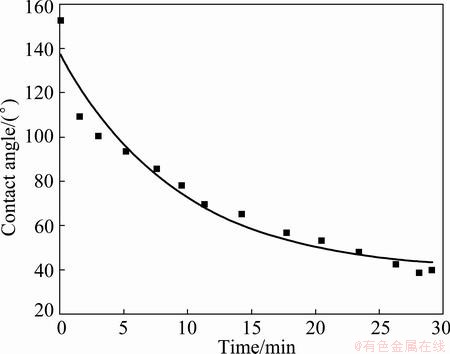
Fig. 4 Contact angle vs time for Al on SiC based filter at 1100 °C
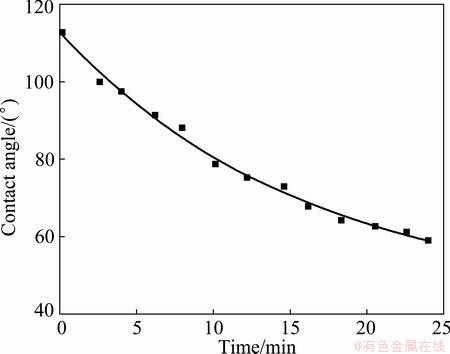
Fig. 5 Contact angle vs time for Al on Al2O3 based filter at 1200 °C
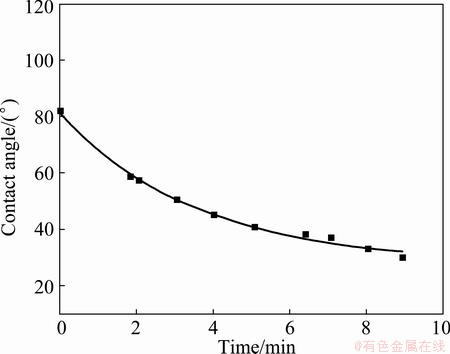
Fig. 6 Contact angle vs time for Al on SiC based filter at 1200 °C
Figure 7 shows the pressure drop and the temperature from the priming to the end of all the four filtration experiments. Note that the filtration starts from time zero. During filtration, temperatures change in the range of 718-732 °C, with a stable pressure drop at 31-33 mm in Experiment 1. There is a similar situation in Experiment 2. The temperatures change in the range of 695-737 °C, with the pressure drop at 29-31 mm. In Experiment 3, the temperature is relatively high, 730-746 °C, while the pressure drop decreases from 27 mm to 18 mm in the beginning for 16 min and stabilizes at 16-18 mm until the end of filtration. In Experiment 4, the pressure drop decreases from 26 mm to 17 mm during the first 16 min and stabilizes. The temperatures are 719-732 °C.
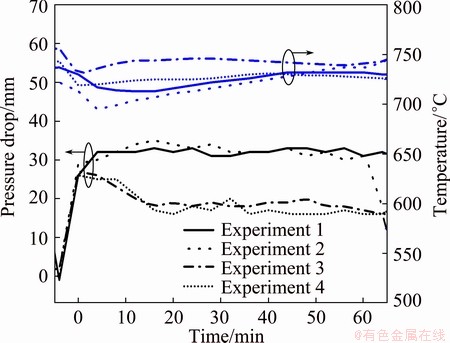
Fig. 7 Pressure drop and temperature in plant experiments
The inclusion contents before and after the filters were measured with two constantly running LiMCA units. Careful statistical treatment of the time dependent LiMCA II data was performed as described in Ref. [9], considering the settling of inclusion in the furnace and launder. While the removal efficiency range is summarized in Fig. 8. Inclusion removal efficiency is defined as the percentage of removed inclusions compared to the incoming inclusions, within ±1 standard deviation. At lower temperature, Experiment 1 gives the similar efficiency as Experiment 3 for the Al2O3 based filter. However, at lower temperature, Experiment 2 gives relatively smaller efficiency than Experiment 4 for the SiC based filter, especially for inclusions <30 μm. Uncertainty is increased for larger inclusions due to their few amounts.
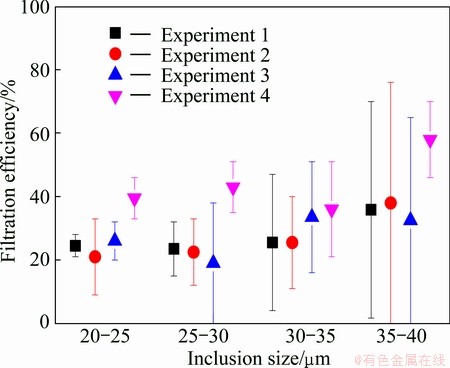
Fig. 8 Inclusion removal efficiency vs inclusion size distribution from LiMCA reading
Figure 9 summarizes the analysed PoDFA results. Considering the area of inclusions before and after the filter, oxide has been removed, except at the filtration starting for Experiment 4 where there is a small increase in oxides. Carbides have been removed slightly at 60 min for Experiments 1 and 4, and increase at 30 min for Experiments 2 and 3. In the start of the Experiment 4, most of the carbides are removed whereas for Experiment 3 there is a small increase in carbide content.
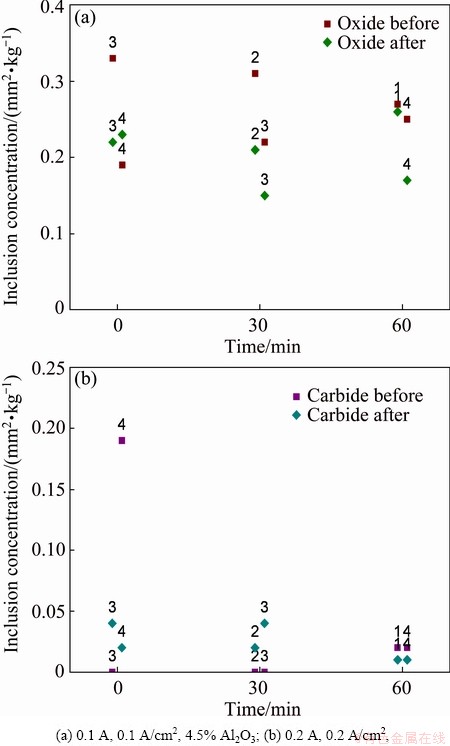
Fig. 9 Inclusion concentration by area attained by PoDFA (Labels above symbols represent different filtration experiments)
3.2 Discussion
Filtration of aluminium is carried out in air at 1.01325×105 Pa atmosphere, when the liquid aluminium is definitely covered with oxide films. However, contact angles have been measured in high vacuum for a long time to remove the oxide films. This addresses the case that aluminium is in direct contact with the filter material. Based on the vacuum studies, the behaviour of aluminium filtration will be discussed.
When replacing a filter for molten aluminium, it is necessary to pre-heat the filter and the filter bowl in which it is seated in order to prime the filter (allow metal to infiltrate the filter without freezing) and to avoid cracking the filter from thermal shock. Liquid aluminium forms an Al2O3 skin rapidly when it is exposed to the atmosphere. Increasing temperature speeds up oxidation. In a typical filter start-up, the liquid aluminium is covered by oxides created by the hot atmosphere and from the filter material in the pores of the filter. Thus, plugging of the filter and freezing of the aluminium are common problems in the industry.
When liquid aluminium penetrates the “baked” filter, oxide skin covers the aluminium and the filter. One part of the oxide skin forms Al2O3 inclusions and the rest may adhere on the filter/Al interface. For metal to manage to enter the filter, a capillary extrusion mechanism must take place, when aluminium breaks through the oxide layer. The metallostatic pressure would break the oxide layer on filter/Al interface. Metal is then in direct contact with the filter material. Then, oxidation is not a problem due to the low solubility of oxygen in aluminium, around 1.43×10-6 at 700 °C [10]. So, wettability under high vacuum should describe closely the case where metal and ceramics are in contact.
The wetting behaviors of pure Al and ceramics were studied in the wetting lab, even though the aluminium alloy with 1.00% Mg was used in the plant trails. The alloying elements should influence the wettability. CANDAN [11] concluded that Mg, Ca, and Pb would improve the wetting for the Al/SiC system. The Mg might react with Al2O3 filter to form spinel at the interface, reducing the interface tension between the aluminium and oxide. The similar mechanism was elucidated for alloying elements Ca and Li as well [12]. But wetting is not significantly affected by Si addition [13,14] for SiC ceramics.
In the one hour filtration experiments [15], the metal composition was measured every 30 min. No change was detected for all four experiments. Thus, we believe that the distribution of alloying elements into the filter interface takes time that the kinetics is very slow here. In this work, we assume the alloying elements have the same wetting influence on two types of filter.
3.2.1 Wettability of filter materials with aluminium
As shown in Figs. 3-6, the Al2O3 based filter (84°, 44°) gives higher contact angle than SiC based filter (39°, 28°) at temperatures of 1100 °C and 1200 °C. Thus, Al2O3 based filter will be non-wetted by aluminium at the filtration temperature around 700 °C, where the contact angle is larger than 90° since increasing temperature improves the wetting. SiC based filter probably gives better wetting than Al2O3 based filter at the filtration temperature, since it is influenced more by the temperature, and thus is better wetted by aluminium. Effort has been paid to extrapolate the contract angle to lower temperatures as the method described in previous work [4]. However, the missing literature data as well as the missing surface energy of the actual filter build up the barrier to have the actual contact angle calculated.
3.2.2 Pressure drop vs wettability of Al-filter
As shown in Fig. 7, Experiment 1 (Al2O3) and Experiment 2 (SiC) give similar pressure drop but with a higher temperature in Experiment 1 than in Experiment 2. The same difference is seen between Experiment 3 (Al2O3) and Experiment 4 (SiC). Thus, Al2O3 based filter requires a higher temperature to give similar pressure drop as the SiC based filter. For Al2O3 based filter, Experiment 1 gives higher pressure drop with low temperature than Experiment 3. Similarly, Experiment 2 also gives higher pressure drop than Experiment 4 for SiC based filters. Thus, the improvements of the temperature help to improve the wetting, and thus reduce the pressure drop. With very similar temperature in Experiments 1 and 4, Experiment 1 (Al2O3) gives much higher pressure drop than Experiment 4 (SiC). With improved wetting, aluminium would be more easily to penetrate the filter and less driving force, e.g., pressure, is required to push the metal through the metal. After prime, the pressure drop decreases in Experiments 3 and 4 and stabilizes, probably because the wetting improves faster at high temperatures.
3.2.3 Filtration efficiency vs wettability of Al-filter
With the limited amount of only four experiments, we may only get a clue that wettability affects the filtration efficiency. The temperature did not seem to affect the filtration efficiency of the Al2O3 based filter probably because this Al2O3 based filter does not wet aluminium at filtration temperature. However, the improvement of the filtration efficiency is concluded for the SiC filter with higher temperature in Experiment 4 than that in Experiment 2. This is probably due to the fact that this SiC based filter is wetted by aluminium and may be much more sensitive to the temperature. At similar filtration temperature, Experiments 1 and 2 do not give much difference in filtration efficiency. While at higher temperature, Experiment 4 (SiC) gives much higher filtration efficiency than Experiment 3 (Al2O3). Thus, the SiC based filter may be more efficient to remove inclusions.
3.2.4 Inclusion removal vs wettability of Al-inclusion
LiMCA counts inclusions constantly through the filtration. PoDFA is an off-line method that we take PoDFA samples at time 0, 30, and 60 min here. One advantage with PoDFA is that the analysis can distinguish between the types of inclusions. These filters tend to remove more oxide than carbides. There seems to be an increase in carbides in Experiment 3 at time zero, and in Experiments 2 and 3 at 30 min. Al2O3 inclusions (contact angle of 97°) are more non-wetted by aluminium than Al4C3 (92°) at the casting temperature. Hereby, aluminium is most likely to push away those non-wetting inclusions. This improves the chance of capturing inclusions on the filter inner wall.
3.2.5 Possible wetting theory in filtration
As indicated in Fig. 10, the metal may travel closer to the wall when the filter-Al wettability increases. The inclusions carried in the metal will get more chances to come close to the wall. Meanwhile, less wetted inclusions may be pushed away from the metal. Then they get possibility to collide with the wall and be captured by it. The liquid prefers rough surface (it gives better wetting) than a flat one. Thus, the wandering passages in the filter improve inclusion removal. From these points of view, Al2O3 inclusions are easier to be removed than Al4C3 inclusions as can be seen in these four experiments. The SiC based filter may be more efficient to remove inclusions than the Al2O3 one due to the better wetting between the filter and liquid aluminium.
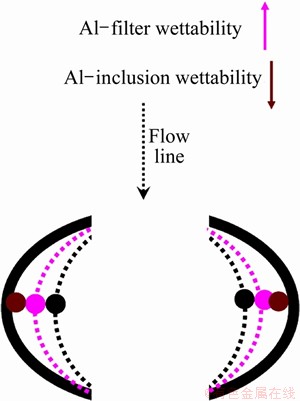
Fig. 10 Schematics of wetting in a filter cell
With the increase of the temperature, the surface tension decreases and thus the wetting improves. However, temperature is not the only way to improve wetting in filtration. Increasing pressure drop is another way generally being utilized by aluminium industries. More driving force as pressure drop is required for less wetting filter materials. This calls for further study.
4 Conclusions
1) Four plant scale filtration tests were investigated for two types of ceramic foam filters. The wettability of the aluminium and filters was tested and the wettability of Al2O3 and Al4C3 inclusions in aluminium was summarized from the earlier work.
2) The less the filter is wetted with aluminium, the more the driving force, e.g., pressure drop, is required to penetrate the filter. The Al2O3 based filter builds up more pressure drop than the SiC based filter (better wetted filter) at the similar filtration temperature.
3) Compared to the less wetted Al2O3 based filter, the SiC based filter gives more inclusion removal at high temperatures. Al2O3 (contact angle of 97°) inclusions are more easily to be removed than Al4C3 (92°) inclusions, and therefore, the bad wetted inclusions are pushed from the melt and may get more chances to collide with the good wetting filter walls.
Acknowledgement
This research was carried out as part of the Research Council of Norway (RCN) funded BIP Project No. 179947/I40 and BIA Project No. 219940/O30, which include the partners: Alcoa Norway ANS, SAPA Heat Transfer AB, Hydro Aluminium AS, SINTEF MK Trondheim, and NTNU. Funding by the industrial partners and RCN is gratefully acknowledged. Drache is acknowledged for supplying the filters.
References
[1] ZHOU Ming. Effect of filtration condition on melt filtration through ceramic particles with active coating [J]. Journal of Aeronautical Materials, 2003, 23: 43.
[2] CHEN Fu-wang, HUANG Xue-bing. Investigation on the foam filter to remove inclusions in revert superalloy [J]. Materials Letters, 1998, 34: 372-376.
[3] KACAEFE D, MURRAY-CHIASSON A, KOCAEFE Y, WAITE P. Study of inclusion re-entrainment in a filter bed [J]. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science, 2004, 35(5): 999-1009.
[4] BAO Sarina, TANG Kai, KVITHYLD A, ENGH T, TANGSTAD M. Wetting of pure aluminium on graphite, SiC and Al2O3 in aluminium filtration [J]. Transactions of Nonferrous Metals Society of China, 2012. 22, 8: 1930-1938.
[5] STULL D R, PROPHET H. JANAF Thermochemical tables [R]. 2nd edition. Washington DC: National Standard Reference Data System, 1971.
[6] EUSTATHOPOULOS N, NICHOLAS M G, DREVET B. Wettability at high temperatures [M]. London: Elsevier, 1999.
[7] LIU L, SAMUEL F H. Assessment of melt cleanliness in A356.2 aluminium casting alloy using the porous disc filtration apparatus technique: Part I. Inclusion measurements [J]. Journal of Materials Science, 1997. 32: 5907-5925.
[8] WIDOM B. Statistical mechanics: A concise introduction for chemists [M]. Cambridge: Cambridge University Press, 2002.
[9] SYVERTSEN M, BAO Sarina, ENGH T, KVITHYLD A. Performance evaluation of two different industrial foam filters with LiMCA II data [J]. Metallurgical and Materials Transactions B, 2014, 12: 1-8.
[10] TANG Kai. Wettability of Al on Al2O3 [R]. Trondheim: SINTEF, 2009: 6.
[11] CANDAN E. Effect of alloying elements to aluminium on the wettability of Al/SiC system [J]. Turkish Journal of Engineering & Environmental Sciences, 2002, 26: 1-5.
[12] LI Jian-guo. Wetting of ceramic materials by liquid silicon, aluminium and metallic melts containing titanium and other reactive elements: A review [J]. Ceramics International, 1994, 20: 391-412.
[13] LAURENT V, CHATAIN D, EUSTATHOPOULOS N. Wettability of SiC by aluminium and Al-Si alloys [J]. Journal of Materials Science, 1987, 22(1): 244-250.
[14] BAO Sarina. Filtration of aluminium-experiments, wetting and modelling [M]. Trondheim: SINTEF, 2011.
[15] BAO Sarina, SYVERTSEN M, NORDMARK A, KVITHYLD A, ENGH T, TANGSTAD M. Plant scale investigation of liquid aluminium filtration by Al2O3 and SiC ceramic foam filters [J]. Light Metals, 2013: 981-986.
铝的润湿行为以及Al2O3和SiC陶瓷过滤器的过滤行为
包萨日娜1,Martin SYVERTSEN1,Anne KVITHYLD1,Thorvald ENGH2
1. SINTEF Materials and Chemistry, Trondheim 7465, Norway;
2. Department of Materials Science and Engineering, Norwegian University of Science and Technology, Trondheim 7491, Norway
摘 要:检测了工业用Al2O3过滤器和SiC过滤器与液态铝的润湿性并在工厂使用以上2种陶瓷过滤器过滤铝液。实验结果表明:SiC过滤器比Al2O3过滤器更易于润湿液态铝。提高液态铝与过滤器的润湿性有助于铝液透过过滤器,提高夹杂物的去除率,同时,易于去除与铝不浸润的杂质。
关键词:过滤;润湿性;铝;Al2O3 过滤器;SiC 过滤器;Al2O3 夹杂;Al4C3 夹杂
(Edited by Yun-bin HE)
Corresponding author: Sarina BAO; Tel: +47-93003325; E-mail: sarina.bao@sintef.no
DOI: 10.1016/S1003-6326(14)63552-4
Abstract: The wetting behavior between liquid aluminium and substrates made from industrial Al2O3 and SiC based ceramic foam filters (CFF) was investigated. The same CFF filters were also tested in plant scale filtration experiments. The wetting experiment results show that the SiC based filter material is better wetted by liquid aluminium than the Al2O3 based filter material. This indicates that the improved wetting of aluminium on a filter material is an advantage for molten metal to infiltrate the filter during priming. Also, better wetting of Al-filter might increase the removal efficiency of inclusions during filtration due to better contact between filter and metal. Non-wetted inclusions are easier to be removed.


