- Abstract:
- 1 Introduction ...▲
- 3 Results and discussion▲
- References
- Figure
- Fig.1 Synthetic route of extractants used in liquid-liquid extraction experiments
- Fig.2 Molecular structures and abbreviations of ester catechins monomer
- Fig.3 Effects of concentration of tert-butylcalix[8]arene on K
- Fig.4 Effects of concentration of p-tert-butykcalix[8]- arene on α
- Fig.5 Effects of different extraction temperatures on K
- Fig.6 Effects of different extraction temperatures on α
J. Cent. South Univ. Technol. (2007)06-0798-05
DOI: 10.1007/s11771-007-0152-7
![]()
Synthesis of calixarenes and their extraction performance for ester catechins
YI Jian-min(易健民)1,2, HUANG Sai-jin(黄赛金)2, TANG Ke-wen(唐课文)1, HUANG Ke-long(黄可龙)2
(1. Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology,
Yueyang 414000, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract:A series of extractants (tert-butylcalix[6]arene, tert-butylcalix[8]arene and octeacetate of tert-butylcalix[8]arene) were synthesized, and their structures were identified by IR and 1H-NMR. The distribution behavior of ester catechins monomer in the aqueous and chloroform two-phase system containing one of calixarene was studied. The influences of different extractants, concentration of tert-butylcalix[8]arene and extraction temperature on the partition coefficients and the separation factors were investigated. The experiment results show that tert-butylcalix[8]arene is the best extractant that forms a more stable supramolecular compound with gallocatechin gallate (GCG) than with epigallocathechin gallate (EGCG) or epicatechin gallate (ECG). When the concentration of p-tert-butylcalix[8]arene is 3.79 mmol/L, the extraction temperature is 4 ℃, the partition coefficients of KGCG, KECG, KEGCG are 0.987, 0.629, 0.449,the separation factors of α1 and α2 are 1.450 and 1.596, respectively. The important factors influencing the extraction properties of calixarene are discovered to be its cavity size and hydrogen bonding.
Key words:
calixarene; synthesis; ester catechin monomer; extraction;
1 Introduction
Supramolecular chemistry is one of the most attractive fields in chemistry, life science and materials science. Calixarene that is assembled by the base-catalyzed condensation of p-alkyphenols with formaldehyde, has been taken as the third generation of supramolecular host compounds after cyclodextrins and crown ethers. Calixarene has a cavity shaped architerture, which can be readily controlled by changing the number of phenol units. The scaffold of them can be modified by introducing many kinds of functional groups at the “lower” rim or the “upper” rim. The calixarene has been frequently used as extractant that takes advantage of these characteristics, being able to extract not only metal ions but also organic molecule[1-8].
Ester catechins monomers(EGCG, GCG, ECG) extracted from tea have high efficiency on cancer treatment,and their virulences are lower than any other anticancer medicine, EGCG has the highest ability to antioxidation and anticancer among them[9]. Now EGCG has been widely used in food, cosmetics and medicine because of its strong antioxidant potentials. So it is a promising work to get ester catechins monomer from tea poly-phenols(TP) solution. There are several methods to separate and purify natural ester catechins mixtures from tea, such as column chromatography[10], high-speed counter current chromatography[11], high performance liquid chromatography[12], but due to low efficiency and high cost, the prices of ester catechins monomers are very high.
In this paper, a series of extractants (tert- butylcalix[6]arene, tert-butylcalix[8]arene and octea- cetate of tert-butylcalix[8]arene) were synthesized to extract ester catechins monomer, which was expected to provide theoretical support and technical parameter for continuous separation and preparation of ester catechins monomer.
2 Experimental2.1 Apparatus and reagents
Proton NMR spectra were recorded on a Varian INOVA-300 MHz spectrometer, using Me4Si as internal standard. TLC analyses were carried out on silica gel plates. IR spectra were determined on a Nicolet AVATAR 370 spectrometer with KBr. HPLC was performed by HP1100, Hypersil ODS [4.6 mm(inner diameter)×250 mm] column was used for
the separation. All the chemical reagents used are of analytical pure grade, and p-tert-butylphenol used for extraction was recrystallized from petroleum ether.
2.2 Synthesis of extractants
Synthesis of extractants as shown in Fig.1 was referred to the method in Ref.[15].

Fig.1 Synthetic route of extractants used in liquid-liquid extraction experiments
2.2.1 Preparation of tert-butylcalix[8]arene(Ⅱ)
A slurry of 27.8 g p-tert-butylphenol, 9.0 g of paraformaldehyde, and 0.224 g of KOH in 150 mL of xylene was heated to reflux in a period of 2 h, stirring in a 500 mL flask under nitrogen. After 30 min all of the solid was put into solution, and after 1 h a white precipitate began to separate. The reaction mixture was refluxed for 4 h, then cooled and filtered. The soild product was washed, in succession, with 100 mL portions of toluene, ether, acetone and water, and then dried and recrystallized from chloroform twice to afford 17.28 g (59.3%) of the tert-butylcalix[8]arene with colorless and glistening needles. Rf=0.754 (eluting agent: Vchloroform?Vhexane=3?4).
2.2.2 Preparation of tert-butylcalix[6]arene(Ⅲ)
A slurry of 10.0 g p-tert-butylphenol, 13.9 mL (36%) of formaldehyde solution, and 1.5 g of KOH in a 250 mL flask was heated and stirred at the same time, after 15 min, connected with nitrogen. When the reaction mixture turned into gold dope, 100 mL of boiling xylene was added, then raised the reaction temperature to reflux for 3 h and then cooled and filtered. The solid product was washed with 20 mL of xylene, filtered and dried, and then the solid was suspended in 250 mL of CHCl3 and shaken with 80 mL of 1 mol/L HCl. The organic layer was separated, washed with water, dried over anhydrous MgSO4 and concentrated to 100 mL. Addition of acetone caused the precipitation of a solid, recrystallized from chloroform-methanol to give 5.07 g (47%) white solid. Rf=0.64 (eluting agent: Vchloroform?Vhexane =3?4).
2.2.3 Preparation of octeacetate of tert-butylcalix [8] arene(Ⅳ)
2.0 g of tert-butylcalix[8]arene was treated with 50 mL of acetic anhydride and 2 drops of concentrated H2SO4, and the mixture was heated under reflux for 2 h, and then cooled, filtered and recrystallized from acetic anhydride to yield 1.0 g (40%) of the octeacetate of tert-butylcalix[8]arene as glistening crystals. Rf=0.442 (eluting agent: Vchloroform?Vacetone = 20?1).
2.3 Analytical method
Chromatographic conditions were referred to Ref.[13]: mobile phases were water, methanol and acetic acid (volume ratio is 27.0?72.5?0.5). Analysis of ester catechins monomer was accomplished at a constant flow rate of 1.0 mL/min with the detection wavelength of 278 nm.
2.4 Partition experiments
The aqueous phase was ester catechins solution prepared referred to Ref.[14]. The organic phase was prepared by dissolving a certain concentration of extractants in chloroform. Equal volumes (4 mL)of the aqueous and organic solutions were mixed in a stoppered test tube to attain equilibrium at 4 ℃. After phase separation, the concentrations of ester catechins monomer were determined by HPLC. The partition coefficients can be expressed as K=(ci-caq)/caq, where is ci the initial concentration of ester catechins in aqueous phase and caq is the concentration of ester catechins in the aqueous phase at equilibrium. The separation factor α1 is the ratio of KGCG to KECG, α2 is the ratio of partition coefficients of KECG to KEGCG.
3 Results and discussion
3.1 Characterization of extractants
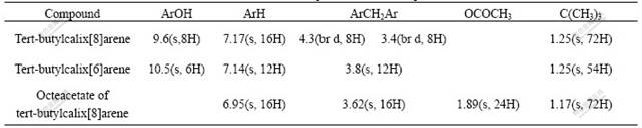
The data of IR and 1H-NMR spectra of compounds Ⅱ-Ⅳ are listed in Table 1 and Table 2, respectively.
Table 1 IR spectra results of compounds
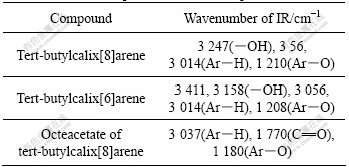
Table 2 1H-NMR spectra data for compounds
3.2 Liquid-liquid extraction experiments
3.2.1 Mechanism of molecular recognition in extraction
Calix[n]arene can recognize many organic compounds by forming supramolecular compounds with them. The driving force is hydrogen bonding, and the cavity size is the selective factors in molecular recognition process[16-17]. Molecular structures of ester catechins monomer is shown in Fig.2. GCG and EGCG are isomers, and the steric hindrance for GCG is least in ester catechins monomer. In addition, there are many hydroxy groups in ester catechins monomer, which can afford the action point for forming hydrogen bonding. benzene ring and carbonyl group in ester catechins monomer may afford the action point in the molecular recognition process. The distribution of ester catechins monomer in the aqueous and chloroform of two-phase system containing one of calixarene is the reason that calix[n]arene has a cavity shaped architecture and can form three supramolecular compounds with ester catechins monomer as follow (E represents tert-butylcalix[8]arene):
EGCG+E![]() EGCG-E
EGCG-E
ECG+E![]() ECG-E
ECG-E
GCG+E![]() GCG-E
GCG-E
but the size and the number of hydroxyl in EGCG, GCG and ECG are different (Fig.2), the steric hindrance and the cooperative non-covalent interaction such as hydrogen bonding, inclusion effect, π-π interaction and van der Waals interactions between extractant and ester catechins monomer are different. That is to say, the stability of the supramolecular compounds are different in the lipophilic organic phase, which can be represented by the free energies of partitioning, -Δ(ΔG). It can be deduced by
-Δ(ΔG)=-ΔGⅡ-(-ΔGⅠ)=RTlnKⅡ-RTlnK=RTln(KⅡ/K)=RTlnα
Only if -Δ(ΔG) is not equal to 0, that is to say, α>1, the ester catechins monomer can be separated by extraction combined with hollow fiber membrane[17-18].
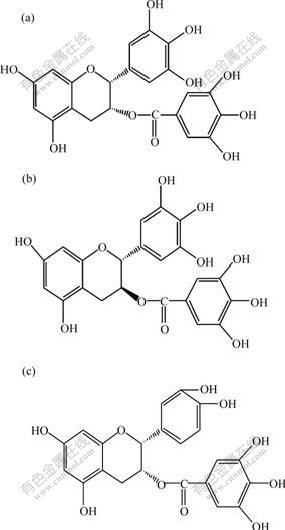
Fig.2 Molecular structures and abbreviations of ester catechins monomer
(a) EGCG (Epigallocathchin gallate); (b) GCG (Gallocatechin gallate); (c) ECG (Epicatechin gallate)
3.2.2 Effect of different extractants on K and α
The extraction performance of various extractants for ester catechins monomer is listed in Table 3. Here, the concentration of tert-butylcalix[6]arene, tert- butylcalix[8]arene and octeacetate of tert-butylcalix [8]arene were 2.4 mmol/L in the organic phase, while the concentration of tert-butylphenol was 19.2 mmol/L, in order to provide the same number of hydroxyl. The tert-butylcalix[8]arene is of the highest extractability among the host molecules tested, because the cavity size of the tert-butylcalix[8]arene fits the size of the target molecule. The cavity size of tert-butylcalix[6]arene is too small to include the guest molecule. The results indicate that the macrocyclic size of calixarenes is the one factor that results in their exceptional performance to extract compounds. The hydrogen bonding also plays an important role in the extraction performance of ester catechins. It can be seen from Table 3 that tert- butylphenol can extract ester catechins monomer, but octeacetate of tert-butylcalix[8]arene shows lower extraction abilities for ester catechins monomer because the ability of forming hydrogen bonding with ester catechins monomer declines. These results mean that hydroxyl in the lower rim is essential for creation of a high affinity to the target ester catechins monomer. So it can be concluded that the important factors influencing the extraction properties of calixarene are its cavity size and hydrogen bonding.
Table 3 Effects of different extractants on K, α and -Δ(ΔG)
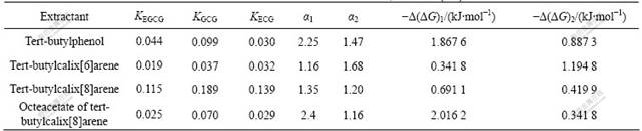
Since tert-butylcalix[8]arene can form three inclusion complexes with ester catechins monomer, it is important to investigate the influence of concentration of tert-butylcalix[8]arene on K and α (Figs.3 and 4). With the increase of the concentration of tert-butylcalix[8]- arene, the reaction equilibrium can be moved to the right, and more and more supramolecular compounds form. But the stabilities of three supramolecular compounds are different, the increases of the partition coefficient K are different. And it can be seen from Figs.3 and 4 that KGCG is always larger than KECG,KECG is always larger than KEGCG, which indicates that the cavity size of tert-butylcalix[8]arene fits the size of the target molecule GCG more, and forms a more stable complex with GCG than with EGCG or ECG.
3.2.4 Effect of extraction temperature on K and α
Tert-butylcalix[8]arene can form three supra- molecular compounds with ester catechins monomer by the cooperative non-covalent interaction such as hindrance hydrogen bonding, inclusion effect, π-π interaction, van der Waals and the steric hindrance,
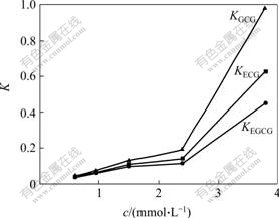
Fig.3 Effects of concentration of tert-butylcalix[8]arene on K
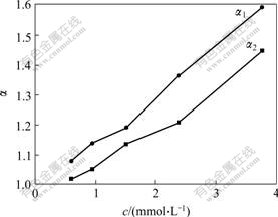
Fig.4 Effects of concentration of p-tert-butykcalix[8]- arene on α
but temperature has an important effect on hydrogen bonding, and it may have an impact on partition coefficients K and the separation factor α. So it is significant to investigate the influence of extraction temperature on K and α (Figs.5 and 6). It is found from Figs.5 and 6 that the partition coefficient K and the separation factor α decrease with the increase of extraction temperature, because it is good for the formation of hydrogen bonding when the extraction temperature is low. Molecular movement is fast when the extraction temperature increases, which is not good for the formation of hydrogen bonding.
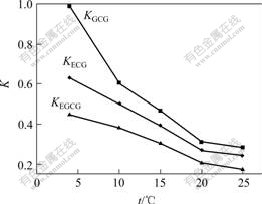
Fig.5 Effects of different extraction temperatures on K

Fig.6 Effects of different extraction temperatures on α
4 Conclusions1)A series of extractants ( tert-butylcalix[6]arene, tert-butylcalix[8]arene and octeacetate of tert- butylcalix[8]arene) are synthesized, and their structures are identified by IR and 1H-NMR.
2) The p-tert-calix[8]arene is suitable for separate ester catechins monomer, and the p-tert- butylcalix[8]arene can form a more stable supramolecular compounds with gallocatechin gallate (GCG) than with epigallocathechin gallate (EGCG) or epicatechin gallate (ECG), that is to say, it is stronger to extract GCG than EGCG or ECG. The important factors influencing the extraction properties of calixarene are its cavity size and hydrogen bonding.
3) The concentration and extraction temperature of tert-butylcalix[n]arene also play an important role on K and α, and the best extractant is p-tert-calix[8]arene in this experiment. When the concentration of p-tert-butylcalix[8]arene is 3.79 mmol/L, the extraction temperature is 4 ℃, the partition coefficients of KGCG, KECG, KEGCG are 0.987, 0.629, 0.449,the separation factors of α1 and α2 are 1.450 and 1.596, respectively. But solubility of p-tert-calix[8]arene in chloroform is not good. It is very significant for improving the solubility by some chemical modifications.
Refrences
[1] ATWOOD J L, KOUTSANTNIS G A, RASTON C L. Purification of C60 and C70 by selective complexation with calixarenes[J]. Nature, 1994, 368: 229-231.
[2] BARALAT N, BURGARD M, ASFARI Z, et al. Solevnt extraction of alkaline-earth ions by dicarboxylated calix[4]arenes[J]. Polyhedron, 1998, 17(20): 3649-3656.
[3] SHIMOJO K, OSHIMA T, GOTO M. Calix[6]arene acid extraction spicificity with respect to nucleaobases[J]. Analytica Chimica Acta, 2004, 521: 163-171.
[4] LIU Yu, YANG En-cui, CHEN Yong, et al. Molecular selective binding of pyridinium guest ions by water-soluble calix[4]arenas eur[J]. J Org Chem, 2005, 21: 4581-4588.
[5] SHIMOJO K, GOTO M. Synergistic extraction of nucleobase by the combination of calixarene and D2EHPA[J]. Separation and Purification Technology, 2005, 44: 175-180.
[6] OSHIMA T, GOTO M, FURUSAKI S. Extraction behavior of amino acids by calix[6]arene carboxylic acid derivatives[J]. J Inclusion Phenom Macrocyclic Chem, 2002, 43: 77-86.
[7] MUTIHAC L, TUDORESCU A, BUSCHMANN J H, et al. Some aspects of extract ability and transport of amino acid esters by calixarenes[J]. J Inclusion Phenom Macrocyclic Chem, 2003, 47: 123-128.
[8] OSHIMA T, GOTO M, FURUSAKI S. Complex formation of cytochrome with a calixarene carboxylic acid derivative: A novel solubilization method for biomolecules in organic media[J]. Biomacromolecules, 2002, 3:438-444.
[9] STONER G D, MUKHTAR H. Polyphenols as cancer chemopreventive agent[J]. J Cell Biochem, 1995, 22: 169-180.
[10] SAIJIO R. Isolation and chemical structure of two new catechins[J]. Nippon Kaishi Nigeikagaku, 1989, 63(4): 870-880.
[11] CAO Xue-li, TIAN YU, ZHANG Tian-you. Preparation of ester catechins monomer by high-speed counter current chromatography: CN, 99109031.4[P]. 2000-12-20. (in Chinese)
[12] ZHONG Shi-an, ZHOU Chun-shan, YANG Juan-yu. Separation and preparation of ester catechins by high performance liquid chromatography[J]. Chemical World,2003(5): 237-249. (in Chinese)
[13] YI Jian-min, LI Bao-rong, TANG Ke-wen, et al. Extraction of ester catechins with gallate ester[J]. J Cent South Univ: Science and Technology, 2006, 37(6): 1127-1131. (in Chinese)
[14] TANG Ke-wen, ZHOU Chun-shan, ZHONG Shi-an, et al. Study on adsorption of tea polyphenol and caffine with polyamide resin[J]. Chinese J Spectroscopy and Spectral Analysis, 2003, 23(1): 143-145. (in Chinese)
[15] GUTSCHE C D, DHAWAN B. Calixarene. 4. The synthesis characterization, and properties of the calixarene from p-tert-butylphenol[J]. J Am Chem Soc, 1981, 103(13): 3782-3792.
[16] IWAMOTO K, SHINKAI S. Syntheses and ion selectivity of an conformational isomers of tetrakis ((ethoxycarbonyl) methoxy) calix[4]arene[J]. J Org Chem, 1992, 57: 7066-7073.
[17] SHINKAI S. Bioorganic Chemistry Fronties 1[M]. DUGAS H. Berlin: Springer-Verlag Heidelberg, 1990.
[18] TANG Ke-wen, ZHOU Chun-shan. Enantoselective extraction of terbutaline enantiomers by lipophilic tartaricacid[J]. J Cent South Univ Technol, 2003, 10(1): 44-48.
Foundation item: Project (20576029) supported by the National Natural Science Foundation of ChinaReceived date: 2007-03-02; Accepted date: 2007-05-18
Corresponding author: TANG Ke-wen, PhD, Professor; Tel: +86-730-8646364; E-mail: tangkewen@sina.com
- Synthesis of calixarenes and their extraction performance for ester catechins


