Trans. Nonferrous Met. Soc. China 23(2013) 524-529
Removal of chromium(III) from aqueous waste solution by predispersed solvent extraction
Jian-hong LUO, Jun LI, Zhao-peng YANG, Xue-feng LIU
College of Chemical Engineering, Sichuan University, Chengdu 610065, China
Received 13 September 2011; accepted 12 November 2012
Abstract:
The extraction experiments of chromium(Ш) from aqueous waste solution by predispersed solvent extraction (PDSE) process with extractant (HEHPEHE) and its mixture, including acidic extractant (D2EHPA), alkaline extractant (TOA) and neutral extractant (TBP) were carried out respectively. It is found that the extractant HEHPEHE exhibited high extraction selectivity to chromium(III) from aqueous waste solution. The colloidal liquid aphrons (CLAs) were successfully generated using kerosene as a solvent, HEHPEHE as an extractant, sodium dodecyl benzene sulphate (SDBS) as a surfactant in aqueous phase and polyoxyethylene sorbitol anhydride monolaurate (Tween-20) in oil phase. To study the extraction efficiency and advantages of the PDSE process in the removal of chromium(Ш), the effects of major factors, such as initial chromium(Ш) concentration, volume of colloidal gas aphrons (CGAs), HEHPEHE volume fraction, phase ratio (solvent phase to water phase), mass fraction of dodecyl trimethylammonium bromide (HTAB), mass fraction of SDBS, mass fraction of Tween-20 and initial pH of aqueous waste solution were also investigated and the appropriate process conditions were obtained. Under the appropriate conditions, the extraction efficiency of chromium(III) above 99.9 % can be achieved and the treated aqueous waste solution can be discharged directly without polluting the environment.
Key words:
predispersed solvent extraction (PDSE) process; chromium(Ш); extraction mechanism; extractant;
1 Introduction
The extensive use of chromium in leather tanning, metallurgy, electroplating and other industries has resulted in the release of aqueous chromium to the subsurface at numerous sites [1]. The most common oxidation states for chromium are +3 and +6. Chromium(Ш) cannot be absorbed, and can form complex with protein in the external layer of skin and accumulate in the lung causing lung cancer. Thus, scholars pay more and more attention to removal and recovery of chromium(III) because of the growing importance to environmental protection problems [2-5]. Consequently, the predispersed solvent extraction (PDSE) is employed to extract chromium(III) from aqueous waste solution, which has a great significance for wastewater treatment.
The PDSE was first proposed by SEBBA [6], which is applicable to treat the dilute solution with a low solvent/water ratio [7,8]. Organic solvents including extractant are predispersed to micron-sized globules, which forms an enormous interfacial area where a transfer of solute from one phase to another can occur very rapidly with a minimum energy requirement. Although the solvent is usually lighter than water and would be expected to rise naturally, it is very time-consuming because of the small size of colloidal liquid aphron (CLA). The used solvent is usually recovered by flotation using CGAs. When CGAs are added to an aqueous solution, the CGAs rise due to the natural buoyancy force, and CLAs rising velocity is further enhanced after CGAs combining with CLAs. Since CGAs have better mechanical strength and larger surface area than gas bubbles, the CGAs are effective for stripping of solvents after extraction [9]. So, the PDSE process as a new separation method presents a huge potential for the extraction from dilute solution [10-12]. There have been a number of reported applications [13,14] for PDSE process. Recently, KIM and HONG [15,16] have used CLAs to extract succinic acid, and studied the effect of salts and pH on the extraction characteristics of succinic acid by PDSE process.
In the present work, the removal of chromium(Ш) from aqueous waste solution with PDSE is investigated. The aim is to experimentally study the effects of various factors on the extraction efficiency in the PDSE process.
2 Experimental
2.1 Reagents
HEHPEHE and D2EHPA (AR grade) were produced by Luoyang Zhongda Chemical Company, China. TOA and TBP (AR grade) were purchased from Kelong Chemical Company, China. The surfactants including SDBS, HTAB and Tween-20 were all of AR grade and purchased from Kelong Chemical Company, China. The solvent kerosene (AR grade) was from Luoyang Zhongda Chemical Company, China. Chromic nitrate (Cr(NO)3) (AR grade) was purchased from Kelong Chemical Company, China. Deionized water was produced by Aquapro making-water machine (ABZ1- 1001-P) in our laboratory.
2.2 Preparation of polyaphrons and CGAs
The oil phase (500 mL) containing non-ionic surfactant was gradually added (1.0-1.5 mL/min) into a foaming aqueous ionic surfactant solution under suitable mixing conditions (600 r/min) using a mechanical stirrer. A white creamy dispersion system of CLAs was obtained as shown in Fig. 1. The phase volume ratio (PVR) was defined as the volume ratio of the oil phase to the aqueous phase. CGAs were prepared in an apparatus similar to that of SEBBA [17]. The generator is composed of a one-liter beaker containing surfactant aqueous solution and a central stirrer. A high-speed motor drived a shaft at 6000 r/min. The beaker was initially filled with 175 mL surfactant aqueous solution and was stirred at a high speed until a constant volume of creamy CGAs was generated as shown in Fig. 2. In the PDSE experiment, CGAs were generated on demand.

Fig. 1 Sample of diluted CLAs generated from SDBS in water and Tween-20 in kerosene examined by microscopic camera
Fig. 2 Sample of CGAs generated from HTAB in water examined by microscopic camera
2.3 Extraction conditions
Extraction experiment conditions were as follows: solvent of kerosene, phase volume ratio (PVR is defined as the volume ratio of the dispersed oil phase to the continuous phase) of 9:1, stirring speed of 600 r/min, solvent addition rate of 1-1.5 mL/min, oil phase surfactant (mass/volume) of 2% Tween-20, aqueous phase surfactant of 1 g/L SDSB, phase ratio(solvent phase/water phase) of 1:2, CLA of 30% HEHPEHE, CLA flow rate of 10.0 mL/min (40 mL diluted to 60 mL), CGA of 2.857 g/L HTAB in aqueous solution, CGA flow rate of 20.0 mL/min, initial chromium(Ш) concentration of 600 mg/L, initial pH of aqueous waste solution of 3, settle time of 15 min, reaction temperature of 30 °C.
2.4 Analysis
The concentration of chromium(Ш) was determined by atomic absorption spectrophotometry (GF3000).
3 Results and discussion
3.1 Influence of extractant
In the present work, studies on the extraction of chromium(Ш) from aqueous waste solution with extractant (HEHPEHE), and its mixture including acidic extractant (D2EHPA), alkaline extractant (TOA) and neutral extractant (TBP) respectively were carried out, to maintaining HEHPEHE and other extractant molar ratio 1:1, and the molar volume a constant. It is found that the extraction selectivity of chromium(Ш) with HEHPEHE from aqueous waste solution is outstanding. Therefore only the HEHPEHE is employed as an extractant.
3.2 Effect of volume of CGA
The results of the experiments are presented in Fig. 3. The figure indicates that the extraction efficiency of chromium(Ш) is improved with the increase of volume of CGA, LEE and HONG [9] pointed out that the surface areas provided by CGAs play an important role in the surface charges of both CGA and CLA. With increasing the amount of CGA dispersion, the surface area which CLAs could attach to also increases, so that the extraction efficiency increases. However, when the volume of CGAs increases to a certain value, CGAs absorb most parts of CLAs, and the extraction efficiency (E) remains almost unchanged because the extraction reaction reaches equilibrium.
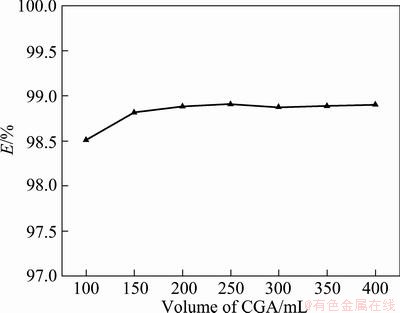
Fig. 3 Extraction efficiency (E) vs volume of CGA
3.3 Effect of initial chromium(Ш) concentration in aqueous solution
Figure 4 shows that the extraction efficiency (E) decreases with the increase of initial chromium(Ш) concentration in aqueous solution. WANG et al [18] reported that the extraction by CLA is more effective for dilute solution. Since the a mount of extractant is given, so the number of free extractant molecules taking part in the extraction reaction is also fixed.

Fig. 4 Extraction efficiency (E) vs initial chromium(Ш) concentration in aqueous solution
3.4 Effect of HEHPEHE volume fraction
Increasing HEHPEHE concentration in CLAs will increase the amount of extractant, so the amount of free extractant taking part in the extraction reaction will also increase. WANG et al [18] reported the same trend on the removal of phenol from dilute solution. Therefore, the extraction efficiency (E) increases as shown in Fig. 5.

Fig. 5 Extraction efficiency (E) vs HEHPEHE volume fraction
3.5 Effect of phase ratio (solvent phase/water phase)
Figure 6 shows the effect of phase ratio. With the increasing phase ratio, the extraction efficiency (E) increases gradually. The reason is that increasing phase ratio in CLAs will increase the amount of extractant [18,19].
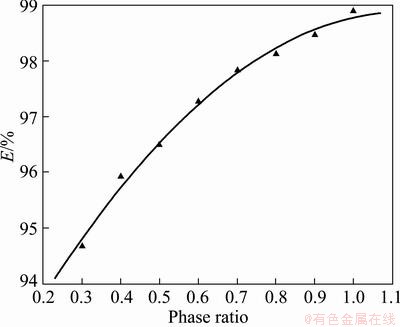
Fig. 6 Extraction efficiency (E) vs phase ratio (solvent phase/water phase)
3.6 Effect of initial pH of aqueous waste solution
HEHPEHE (HA) contains dissociable H+, so the mechanism of extracting chromium(Ш) with HA perhaps accords with the cation exchange. In general, the extraction reaction can be described as follows:
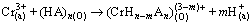 (1)
(1)
 (2)
(2)
where n is the aggregation number of HEHPEHE.
And the distribution ratio (D) of Cr3+ can be expressed as
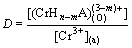 (3)
(3)
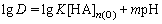 (4)
(4)
The plot of lgD—pH as shown in Fig. 7 is a straight line with a slope of approximately 0.1841, suggesting m≈0.2, which indicates the chelate complex of (CrA3·2HA) can be obtained. So the mechanism of the extraction of chromium(Ш) with HA accords with the cation exchange [20] and chelation. Therefore, the extraction distribution ratio (D) of chromium(Ш) increases rapidly as the initial pH of aqueous waste solution rises in the extraction system.
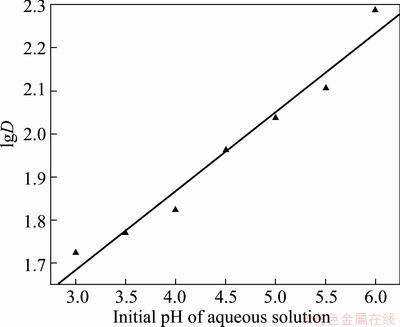
Fig. 7 Extraction efficiency (E) vs initial pH of aqueous waste solution
3.7 Effect of mass fractions of HTAB
The experimental results show that the mass fraction of HTAB does not affect the extraction efficiency (E), which can be shown from Fig. 8. The reason is that increasing the HTAB concentration does not affect the mean diameter of CGA when the HTAB concentration is much higher than the critical micelle concentration (CMC) of HTAB.
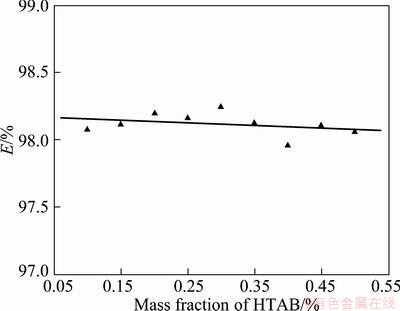
Fig. 8 Extraction efficiency (E) vs mass fraction of HTAB
3.8 Effect of mass fractions of SDBS
As can be shown from Fig. 9, with increasing the mass fractions of SDBS, the extraction efficiency increases rapidly firstly, and then remains nearly invariable. WANG et al [21] pointed out that when the concentration of SDBS is lower than its CMC, with increasing the concentration of SDBS, the mean bubble size of CLAs decreases to the minimum size near to CMC. Then with rising the concentration of SDSB, the average bubble size of CLAs tends to be unchanged. This is in agreement with the fact that there is an inverse relationship between the surface area and the radius of the aphrons [22].
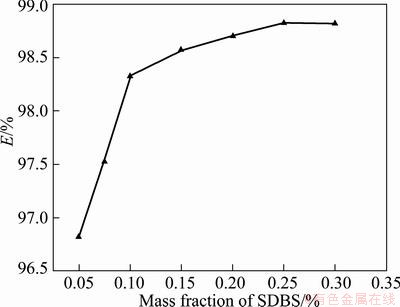
Fig. 9 Extraction efficiency (E) vs mass fractions of SDBS
3.9 Effect of mass fractions of Tween-20
From Fig. 10, with increasing the mass fraction of Tween-20, the extraction efficiency (E) increases firstly, and then remains nearly invariable. The reason is that the mean bubble size of CLAs decreases with an increase in the concentration of Tween-20 up to 2.5 g/L, and then remains approximately unchanged. MATSUSHITA et al [23] reported the same trend on the removal of dilute products with PDSE.

Fig. 10 Extraction efficiency (E) vs mass fraction of Tween-20
3.10 Examination
A kind of practical waste solution containing 350 mg/L chromium(Ш) was neutralized to pH=4.5 with acetic acid and sodium acetate. The neutralized solution was then extracted under the above mentioned optimal technology conditions. The results show that chromium(Ш) in waste solution can be effectively removed by extraction process. An extraction efficiency of more than 99.9% is attained under optimum conditions and the aqueous waste solution containing chromium(Ш) concentration is lower than 0.5 mg/L. So it can be discharged directly, as it meets the Chinese National Emission Standards by two levels of extraction.
4 Conclusions
1) PDSE treatment can be an effective method for the removal of chromium(Ш) from aqueous waste solution.
2) The optimized parameters affecting the process are as follows: the volume of CGA 200 mL, the volume fraction of D2EHPA 30%, the phase volume ratio 1:2, the mass fraction of HTAB 0.3%, the mass fraction of SDBS 0.15%, the mass fraction of Tween-20 1.0% and the initial pH of aqueous waste solution 3.5.
3) The mechanism of the extraction of chromium(Ш) with HA accords with the cation exchange and chelation.
4) An extraction efficiency more than 99.5% is attained with the optimized parameters and the aqueous waste solution can be discharged directly.
References
[1] DJANE N K, NDUNGU K, JOHNSSON C, SARTZ H, TORNSTROM T, MATHIASSON L. Chromium speciation waters using serially connected supported liquid membranes [J]. Talanta, 1999, 48: 1121-1132.
[2] ZHAO Li-feng, FEI De-jun, DANG Ya-gu, ZHOU Xiao-long, XIAO Jia-li. Studies on the extraction of chromium(III) by emulsion liquid membrane [J]. Journal of Hazardous Materials, 2010, 178: 130-135.
[3] YU K, PETER S, XUE L Z. Extraction of zinc and chromium(Ш) and its application to treatment of alloy electroplating wastewater [J]. Separation Science & Technology, 2003, 38(2): 405-425.
[4] PANDEY B D, COTE D, BAUER D. Extraction of chromium(Ш) from spent tanning baths [J]. Hydrometallurgy, 1996, 40(3): 343-357.
[5] FEI De-jun, LUO Jian-hong, DANG Ya-gu. Extraction of Cr3+ with p-tert-butylca lix[4] arene acetate from artificial tannery waste water [J]. Chinese Journal of Applied Chemistry, 2008, 25(2): 157-161. (in Chinese)
[6] SEBBA F. Foams and biliquid foams: Aphrons [M]. Wiley: New York, 1987: 5-38.
[7] MICHELSEN D L, RUETTIMANN K W, HUNTER K R, SEBBA F. Feasibility study on use of predispersed solvent extraction/flotation technique for removal of organics from wastewaters [J]. Chemical Engineering Communications, 1986, 48: 155-163.
[8] ZHANG C, VALSARAJ K T, CONTANT W D, ROY D. Studies in solvent extraction using polyaphrons. II. Semibatch and continuous countercurrent extraction/flotation of a hydrophobic organic dye from water [J]. Separation Science & Technology, 1996, 31: 1463-1465.
[9] LEE D W, HONG W H. Removal of an organic dye from water using predispersed solvent extraction [J]. Separation Science & Technology, 2000, 35: 1951-1962.
[10] WANG Yun-dong, CHENG Min, XU LI-liang, DAI You-yuan. Recent advances in predispersed solvent extraction [J]. Chemical Industry and Engineering Progress, 1997(6): 1-4. (in Chinese)
[11] ZHANG Xin, ZHANG Ping, CAI Shui-hong, LIU Hong-lai. Predispered solvent extraction technology and its application in biological separation [J]. Journal of Jishou University, 2011, 22(2): 64-66. (in Chinese)
[12] WANG Yun-dong, CHEN Min, DAI You-yuan. Mass transfer characteristics of predispersed solvent extraction process [J]. Journal of Chemical Industry and Engineering, 2004, 55(5): 737-739. (in Chinese)
[13] LYE G J, STUCKEY D C. Extraction of erythromycin—A using colloidal liquid aphrons: Part II. Mass transfer kinetics [C]//ISEC'96. Melbourne, 1996: 1399-1400.
[14] ZHANG Xin, ZHANG Ping, CAI Shui-hong, LIU Hong-lai. Predispersed solvent extraction of lincomycin from fermentation broth containing mycelium [J]. Journal of East China University of Science and Technology, 2003, 29(5): 496-499. (in Chinese)
[15] KIM B S, HONG Y K. Effect of salts on the extraction characteristics of succinic acid by predispersed solvent extraction [J]. Biotechnology and Bioprocess Engineering, 2004, 9(3): 207-211.
[16] KIM B S, HONG Y K. Effect of pH on the extraction characteristics of succinic acid and the stability of colloidal liquid aphrons [J]. Korean Journal of Chemical Engineering, 2002, 19(4): 669-672.
[17] SEBBA F. An improved generator for micron-sized bubbles [J]. Chemistry and Industry, 1985, 9: 91-92.
[18] WANG Yun-dong, CHENG Min, XU LI-liang, DAI You-yuan. Removal of phenol from dilute solutions by predispersed solvent extraction [J]. Chinese Journal of Chemical Engineering, 2000, 8(2): 103-107.
[19] LUO Jian-hong, LI Jun, JIN Yang, ZHANG Yi, ZHENG Dong-sheng. Study on Mg2+ removal from ammonium dihydrogen phosphate solution by predispersed solvent extraction [J]. Industrial & Engineering Chemistry Research, 2009, 48: 2056-2060.
[20] VAN D D, PINOY L, COURTIJN E, VERPOORT F. Influence of acetate ions and the role of the diluents on the extraction of copper (II), nickel (II), cobalt (II), magnesium(II) and iron (II, III) with different types of extractants [J]. Hydrometallurgy, 2005, 78: 92-106.
[21] WANG Yun-dong, CHENG Min, XU LI-liang, DAI You-yuan. Preparation and characteristics of colloidal liquid aphrons and colloidal gas aphrons [J]. Journal of Tsinghua University, 1998, 38(6): 42-45. (in Chinese)
[22] AMIRI M C, SOMUNK J. Effect of gas transfer on separation of whey protein with aphron flotation [J]. Separation Science & Technology, 2004, 35: 161-167.
[23] MATSUSHITA K, MOLLAH A H, STUCKEY D C. Predispersed solvent extraction of dilute products using colloidal gas aphrons and colloidal liquid aphrons: aphron preparation, dtability and size [J]. Colloids and Surfaces, 1992, 69: 65-72.
预分散溶剂萃取法处理Cr(III)废水
罗建洪,李 军,杨兆鹏,刘雪峰
四川大学 化工学院,成都 610065
摘 要:以2-乙基己基膦酸单2-乙基己基酯(HEHPEHE)为萃取剂,以及与其它萃取剂形成酸加酸、酸加碱、酸加中性混合萃取剂,通过考察萃取剂对废液中的Cr3+萃取分配比的影响,筛选出萃取Cr3+的适宜萃取剂 HEHPEHE;采用预分散溶剂萃取技术对含Cr3+废液进行分离研究,以煤油为稀释剂,HEHPEHE为萃取剂,十二烷基苯磺酸钠(SDBS)为水性表面活性剂,吐温20(Tween-20)为油性表面活性剂,制备胶质液体泡沫CLAs。同时,为了研究预分散溶剂萃取技术萃取Cr3+废液的效果,考察了废液中的Cr3+浓度、胶质气体泡沫CGAs体积、萃取剂浓度、相比、阳离子表面活性剂十六烷基三甲基溴化铵(HTAB)浓度、阴离子表面活性剂SDBS浓度、表面活性剂Tween20浓度以及Cr3+废液的pH值等影响因素对Cr3+的萃取率影响,得到了萃取Cr3+的适宜条件;并用实际的含Cr3+废液进行检验,结果表明,在获得的适宜工艺条件下,经过二级萃取后,废水可以直接排放,Cr3+的萃取率超过99.9%。
关键词:预分散溶剂萃取法;铬(III);萃取机理;萃取剂
(Edited by Hua YANG)
Foundation item: Project (NCET-07-0577) supported by New Century Excellent Talents of Ministry of Education, China
Corresponding author: Jun LI; Tel/Fax: +86-28-85460936; E-mail: lijun@scu.edu.cn
DOI: 10.1016/S1003-6326(13)62494-2
Abstract: The extraction experiments of chromium(Ш) from aqueous waste solution by predispersed solvent extraction (PDSE) process with extractant (HEHPEHE) and its mixture, including acidic extractant (D2EHPA), alkaline extractant (TOA) and neutral extractant (TBP) were carried out respectively. It is found that the extractant HEHPEHE exhibited high extraction selectivity to chromium(III) from aqueous waste solution. The colloidal liquid aphrons (CLAs) were successfully generated using kerosene as a solvent, HEHPEHE as an extractant, sodium dodecyl benzene sulphate (SDBS) as a surfactant in aqueous phase and polyoxyethylene sorbitol anhydride monolaurate (Tween-20) in oil phase. To study the extraction efficiency and advantages of the PDSE process in the removal of chromium(Ш), the effects of major factors, such as initial chromium(Ш) concentration, volume of colloidal gas aphrons (CGAs), HEHPEHE volume fraction, phase ratio (solvent phase to water phase), mass fraction of dodecyl trimethylammonium bromide (HTAB), mass fraction of SDBS, mass fraction of Tween-20 and initial pH of aqueous waste solution were also investigated and the appropriate process conditions were obtained. Under the appropriate conditions, the extraction efficiency of chromium(III) above 99.9 % can be achieved and the treated aqueous waste solution can be discharged directly without polluting the environment.


