
Controllable synthesis and catalytic activity of SnO2 nanostructures at room temperature
ZHAO Qing-rui(赵清锐)
Beijing Research Institute of Chemical Industry, SINOPEC, Beijing 100013, China
Received 13 March 2009; accepted 25 May 2009
Abstract:
SnO2 hollow spheres and rod bundles were prepared using SnSO4 as raw material and sodium dodecyl benzenesulfonate and poly(vinyl pyrrolidone) as templates at room temperature through oxidation-crystallization of colloidal spheres in different systems. The products were characterized with X-ray diffractometer, X-ray photoelectron spectrometer, transmission electron microscope and scanning electron microscope. Meanwhile, the catalytic performance of the SnO2 hollow spheres and rod bundles toward CO oxidation was studied. The result indicates that SnO2 hollow spheres with the uniform size exhibit a better catalytic activity toward CO oxidation, suggesting that the morphology of the materials has exerted a noticeable influence on the catalytic performance.
Key words:
SnO2; hollow spheres; rod bundles; CO oxidation;
1 Introduction
Tin oxide, SnO2, is an n-type semiconductor with a wide band gap (Eg=3.6 eV, at 300 K) and is well known for potential applications as excellent gas sensors[1], electrode materials in Li/SnO2 batteries[2], catalysts[3] and so on. The performance of these devices is influenced by the nanosized structure of SnO2 crystals, including their size, morphology and so on. Thus, much attention has been focused on the synthesis of well-defined SnO2 nanocrystals, and various SnO2 nanostructures such as nanorods[4], nanoflakes[5], and hollow spheres[6] have been successfully synthesized. For example, ZHONG et al[6] used polystyrene particles as templates to fabricate mesoscale hollow spheres of SnO2 with an average diameter of 400 nm.
Since 1989, low temperature CO oxidation has received much attention due to the important applications in indoor air cleaning and automotive exhaust treatment [7]. To our knowledge, most of the studies on noble metal based catalysts have been reported. However, high cost of noble metals and their sensitivity to sulfur poisoning have stimulated the search for substitute catalysts. Because of the price and the limited availability of previous metals, considerable attention has been paid to various metal oxides. Previously, Au-SnO2 catalysts have exhibited extraordinarily high activity for CO oxidation at 80 ℃, in which SnO2 can enhance the catalytic activity[8]. Tin oxide materials have been widely used as catalysts in chemical reactions, including synthesis of vinyl ketone[9], oxidation of methanol[10] and so on. Therefore, it is believed that SnO2 nanomaterials display potential catalytic activity for CO oxidation. In this work, the catalytic properties of the prepared SnO2 hollow nanospheres and rod bundles were examined.
Among many preparative methods for well-defined structures, template-assisted synthesis is an effective approach in which hard templates[11-12], and soft templates[13-14] have been utilized. Surfactant tends to self-assemble to form micelles with desired structures and have been widely used as soft templates[15]. In this work, SnO2 hollow spheres and rod bundles on a large scale in the air at room temperature were fabricated using surfactants as soft templates. And the products were characterized.
2 Experimental
2.1 Chemicals
Stannous sulfate (SnSO4), sodium dodecyl benzene- sulfonate(SDBS), sodium dodecyl sulfate(SDS), cetyltri- methyl ammonium bromide(CTAB) and poly-(vinyl pyrrolidone) (PVP, polymerization degree 360) were of analytical grade and were used as received.
2.2 Synthesis of SnO2 hollow spheres
SnSO4 (1 mmol) and SDBS (1.5 mmol) were dissolved in 10 mL distilled water and stirred at room temperature for 8-10 h. After the reaction was completed, the white products were collected from solution, rinsed several times with distilled water and absolute ethanol, and then dried under vacuum at 40 ℃ for 4 h.
2.3 Synthesis of SnO2 rods
SnSO4 (1 mmol), SDBS (1 mmol), PVP (0.05 g) and 10 mL distilled water were mixed, and stirred at room temperature for 8-10 h. After the reaction was completed, the white products were collected from solution, rinsed with distilled water and absolute ethanol, and then dried under vacuum at 40 ℃ for 4 h.
2.4 Characterization
The samples were characterized by X-ray powder diffraction(XRD) with a Japan Rigaku D/max rA X-ray diffractometer equipped with graphite monochromatized high-intensity Cu Kα radiation (λ=1.541 78 ?), recorded with 2θ ranging from 20? to 70?. The transmission electron microscopy(TEM) images and electron diffraction(ED) patterns were performed with a Hitachi Model H-800 instrument with a tungsten filament, using an accelerating voltage of 200 kV. The field emission scanning electron microscopy(FE-SEM) images were taken on FEI Sirion-200 SEM. XPS was performed on ESCALAB MKII with Mg Kα (hν=1 253.6 eV) as the excitation source. The binding energies obtained in the XPS spectral analysis were corrected for specimen charging by referencing C 1s to 284.6 eV.
2.5 Catalytic activity measurements
The catalytic activity of SnO2 catalysts towards CO oxidation was carried out in a continuous flow reactor. The reaction gas, a mixture composition of about 1% CO and 0.5% O2 in nitrogen, was fed to a 0.3 g catalyst at a rate of 70 mL/min. Steady-state catalytic activity was measured at each temperature with the reaction temperature rising from room temperature to 630 ℃ in step of 20 ℃. The effluent gas was analyzed on-line by an on-stream gas chromatograph (SP-6800A) equipped with a Porapak Q column (5 ? molecular sieve columns). The activity test was reproducible within the error of 5%.
3 Results and discussion
The typical X-ray powder diffraction(XRD) patterns of the as-prepared samples are shown in Fig.1. All the sharp and strong diffraction peaks in Figs.1(a) and (b) could be readily indexed to the tetragonal phase of SnO2 (JCPDS card No. 41-1445), with lattice constants of a= 4.738 ?, and c=3.187 ?, which was found to match well with the standard XRD pattern. No characteristic peaks are observed for the impurities.
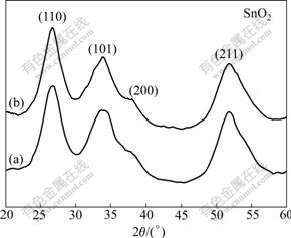
Fig.1 XRD patterns of as-prepared products: (a) SnO2 hollow spheres; (b) SnO2 rod bundles
To further characterize the product, XPS was carried out to investigate the surface compositions and chemical states of the as-prepared product obtained in different systems. Fig.2 shows typical spectra of SnO2 hollow spheres, which resemble that of SnO2 rod bundles. The binding energies obtained in the XPS analysis were corrected for specimen charging by referencing C 1s to 284.6 eV. The sample appears as a spin-orbit doublet at about 486.8 eV (3d5/2) and about 495.2 eV (3d3/2) (Fig.2(a)), which is in agreement with the reported values in Ref.[2]. The O 1s binding energy of 531.5 eV (Fig.2(b)) indicates that the oxygen atoms exist as O2- species in the compounds. Consequently, based on the results of XRD and XPS measurements, the as-synthesized products could be determined as SnO2.
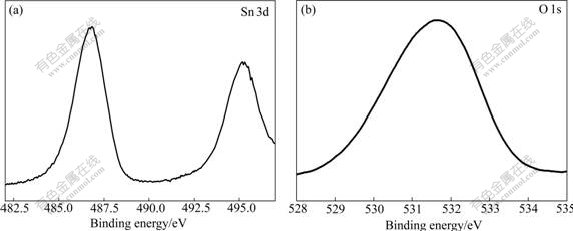
Fig.2 XPS spectra of SnO2 hollow spheres: (a) Sn region; (b) O region
The structure and morphology of the products were investigated by SEM and TEM. Morphologies of SnO2 hollow spheres are displayed in Fig.3. From the panoramic morphology shown in Fig.3(a), a bright contrast (dark/bright) between the boundary and the center of the spheres is seen, confirming their hollow structure. The external diameter of the hollow spheres is 200-250 nm and the thickness of the shell is 20-30 nm. The inset in Fig.3(a) corresponds to the SAED pattern performed on the shell, and the circular characteristic indicates the polycrystalline SnO2 shell. FESEM observations (Fig.3(b)) also reveal that the SnO2 nanospheres are hollow. Interestingly, the morphology of the product is changed from hollow spheres to rod-like bundles as the used surfactant is changed from SDBS to the polymer(PVP)-surfactant(SDBS) complexes. It could be observed from Fig.3(c) that many rods stick together in the middle forming rod bundles. A typical SEM image (Fig.3(d)) displays that the rods grown from the middle have almost uniform widths of about 200 nm and lengths up to several micrometers.
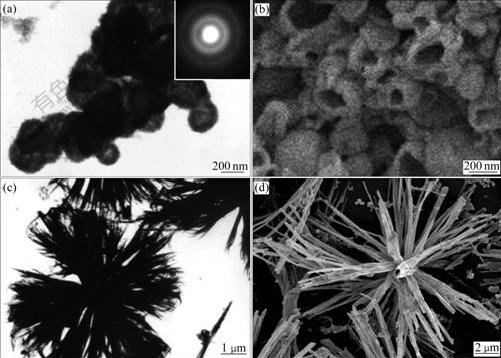
Fig.3 Electron microscopy images of SnO2 nanostructures: (a) TEM image of SnO2 hollow spheres (inset showing corresponding ED patterns); (b) SEM image of SnO2 hollow spheres; (c) TEM image of SnO2 rod bundles; (d) SEM image of SnO2 rod bundles
In the experiment, the reaction system was maintained in the air. The reaction process in the system can be expressed by the following equations:
2SnSO4+O2+2H2O→2SnO2+2H2SO4 (1)
On the basis of the above observation, the formation of SnO2 hollow spheres can be explained by surfactant-assisted oxidation-crystallization mechanism. SDBS has the tendency to form vesicles, which could act as the soft templates[16]. TEM image shown in Fig.4(a) for the diluted solutions of SDBS system indicates the well-defined micelles. As SDBS is an anionic surfactant, Sn2+ can strongly adsorb on the surface of the vesicles, which provides domains for the subsequent oxidation and crystallization in the solutions. In another case, the surfactant SDBS associating with the polymer PVP chains forms micellar aggregates, which can act as templates for the precipitation and crystallization of SnO2. There exists an interaction between PVP and SDBS micelle due to the electric attraction between the anionic SDBS micelle and the positive charged PVP, resulting in the adherence of SDBS micelles to PVP. Therefore, the surfactant SDBS associating with the polymer PVP chains form micellar aggregates as shown in Fig.4(b), which can act as template for the precipitation and crystallization of SnO2. SDBS can act as stabilizer for the crystals, preventing particles from precipitation and aggregation. PVP has been proved to be face-inhibited function surfactant favoring the 1D growth[17]. Thus, the SDBS-stabilized particles adsorbed on the polymer PVP chains are connected into rods[18-19].
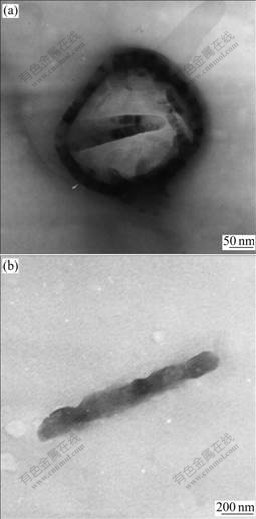
Fig.4 TEM images of micelles formed in reaction system: (a) SDBS micelle; (b) SDBS–PVP micelle
Recently, the development of carbon monoxide oxidation catalysts has been an active research area due to the many important environments and technological applications including CO gas detection sensors and air purification[20]. Commercial catalysts for CO oxidation were usually noble-metal-based materials such as platinum-tin oxide. Therefore, more economical and effective alternative catalysts are being sought[21]. Among various materials, metal oxides have been widely used due to their low cost and availability[22]. In the current work, the catalytic activities of different SnO2 samples for CO oxidation were investigated. Table 1 and Fig.5 show the relationship between CO conversion and temperature with the obtained SnO2 materials as catalysts. The CO oxidation could be described in the following equation:
2CO+O2![]() 2CO2 (2)
2CO2 (2)
Table 1 CO conversion at different temperatures
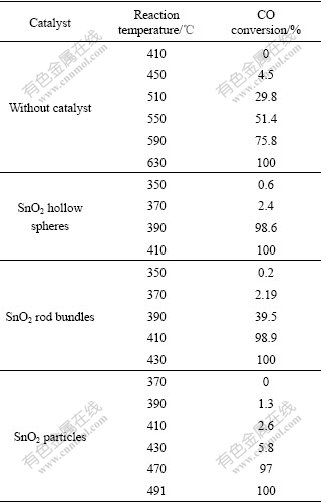
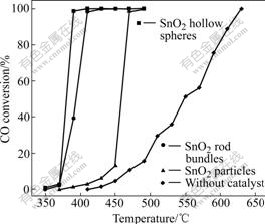
Fig.5 Effect of reaction temperature on CO conversion
The CO conversion over each sample generally increases with the increase of reaction temperature. SnO2 hollow spheres exhibit excellent catalytic activity above 350 ℃ and reach 100% conversion at 400 ℃. When SnO2 rod bundles function as catalyst, the catalytic activity decreases considerably. 100% CO conversion is achieved at 430 ℃. The activity of SnO2 chains is similar to that of SnO2 rod bundles. The curve of SnO2 particles with the diameter of 200 nm indicates that it is much more inactive than either the SnO2 hollow spheres or rod bundles. Without SnO2 samples as catalysts, CO oxidation starts at 430 ℃ and reaches 100% conversion at 630 ℃. From the current measurements, it is clear that the catalytic activity of SnO2 hollow spheres is better than that of other samples, which reduces the oxidation temperature of CO greatly. Most probably, the catalytic performance may be ascribed to the hollow spheres, in that, more chemicals (gases) could access reactive centers of the porous frameworks and have more chances to participate in reactions[23], greatly improving the catalytic efficiency of the obtained materials. Thus, SnO2 hollow spheres reveal possibilities to be applied as suitable catalyst for CO oxidation.
4 Conclusions
1) The present study describes a micelle system generated by SDBS for the preparation of SnO2 hollow spheres on a large scale. Such a system may represent a promising microreactor for the synthesis of other inorganic materials with unique properties.
2) SnO2 rod bundles are successfully fabricated by using the complex surfactant of SDBS and PVP micelles as templates. Their cooperative functions contribute to the final morphology of SnO2 rod bundles.
3) The obtained SnO2 hollow spheres and rod bundles exhibit potential catalytic activity toward CO oxidation, which may be useful in industrial applications.
References
[1] FAGLIA G, BARATTO C, SBERVEGLIERI G. Adsorption effects of NO2 at ppm level on visible photoluminescence response of SnO2 nanobelts [J]. Applied Physics Letters, 2005, 86: 11923-11926.
[2] WANG Y, LEE J Y. Molten salt synthesis of tin oxide nanorods: Morphological and electrochemical features [J]. Journal of Physics Chemistry B, 2004, 108: 17832-17837.
[3] YU K, WU Z C, ZhAO Q R, LI B X, XIE Y. High-temperature-stable Au@SnO2 core/shell supported catalyst for CO oxidation [J]. Journal of Physics Chemistry C, 2008, 112: 2244-2247.
[4] CHENG B, RUSSELL J M, SHI W S, ZHANG L, SAMULSKI E T. Large-scale, solution-phase growth of single-crystalline SnO2 nanorods [J]. Journal of American Chemistry Society, 2004, 126: 5972-5973.
[5] YANG Tian-zu, DU Zuo-juan, GU Ying-ying, QIU Xiao-yong, JIANG Ming-xi, CHU Guang. Preparation of flake AgSnO2 composite powders by hydrothermal method [J]. Trans Nonferrous Met Soc China, 2007, 17(12): 434-438.
[6] ZHONG Z Y, YIN Y D, GATES B, XIA Y N. Preparation of meso-scale hollow spheres of TiO2 and SnO2 by templating against crystalline arrays of polystyrene beads [J]. Advanced Materials, 2000, 12: 206-209.
[7] GULTEN G, THOMAS H. The oxidation of carbon monoxide on platinum-supported binary oxide catalysts [J]. Applied Catalysis A, 2000, 192(1): 51-55.
[8] CHIANG C W, WANG A Q, WAN B Z. High catalytic activity for CO oxidation of gold nanoparticles confined in acidic support Al-SBA-15 at low temperatures [J]. Journal of Physical Chemistry B, 2005, 109: 18042-18047.
[9] CARRENO N L V, FAJARDO H V, MACIEL A P, VALENTINI A, PONTES F M, PROBSST L F D, LRITE E R, LONGO E. Selective synthesis of vinyl ketone over SnO2 nanoparticle catalysts doped with rare earths [J]. Journal of Molecular Catalysis A: Chemical, 2004, 207: 91-96.
[10] JIANG L H, SUN G. Q, ZHOU Z H, SUN S G., WANG Q, YAN S Y, LI H Q, TIAN J, GUO J S, ZHOU B, XIN Q. Size-controllable synthesis of monodispersed SnO2 nanoparticles and application in electrocatalysts [J]. Journal of Physics Chemistry B, 2005, 109: 8774-8778.
[11] BREEN M L, DINSMORE A D, PINK R H, QADRI S B, RATNA B R. Sonochemically produced ZnS-coated polystyrene core-shell particles for use in phonic crystals [J]. Langmuir, 2001, 17: 903-907.
[12] YANG Z, NIU Z, LU Y, HU Z, HAN C C. Templated synthesis of inorganic hollow spheres with a tunable cavity size onto core-shell gel particles [J]. Angewandte Chemie International Edition, 2003, 42: 1943-1945.
[13] XIONG Y J, XIE Y, YANG J, ZHANG R, WU C Z, DU G A. In situ micelle-template-interface reaction route to CdS nanotubes and nanowires [J]. Journal of Materials Chemistry, 2002, 12: 3712-3716.
[14] YANG H G, ZENG H C. Creation of intestine-like interior space for metal-oxide nanostructures with a quasi-reverse emulsion [J].Angewandte Chemie International Edition, 2004, 43: 5206-5209.
[15] CHEN D, SHEN G Z, TANG K B, LIANG Z H, ZHENG H G. AOT-microemulsions-based formation and evolution of PbWO4 crystals [J]. Journal of Physical Chemistry B, 2004, 108: 11280-11284.
[16] WANG S F, GU F, LU M K. Sonochemical synthesis of hollow PbS nanospheres [J]. Langmuir, 2006, 22: 398-401.
[17] ZHANG B, DAI W, YE X C, ZUO F, XIE Y. Photothermally assisted solution-phase synthesis of microscale tubes, rods, shuttles, and an urchin-like assembly of single-crystalline trigonal selenium [J]. Angewandte Chemie International Edition, 2006, 45: 2571-2574.
[18] LEONTIDIS E, KYPRIANIDOU-LEODIDOU T, CASERI W, KYRIACOU K C. From beads-on-a-string to colloidal aggregation: Novel crystallization phenomena in the PEO-SDS system [J]. Langmuir, 1999, 15: 3381-3385.
[19] STOLL S, BUFFLE J. Computer simulation of bridging flocculation processes: The role of colloid to polymer concentration ratio on aggregation kinetics [J]. Journal of Colloid Interface Science, 1996, 180: 548-563.
[20] OH S H, HOFLUND G B. Chemical state study of palladium powder and ceria-supported palladium during low-temperature CO oxidation [J]. Journal of Physics Chemistry A, 2006, 110: 7609-7613.
[21] OKUMURA M, JUAN M, CORONADO J S, MASATAKE H, JOS? C C. EPR study of CO and O2 interaction with supported Au catalysts [J]. Journal of Catalysis, 2001, 203: 168-174.
[22] WANG C B, TANG C W, GAU S J, CHIEN S H. Effect of the surface area of cobalt oxide on carbon monoxide oxidation [J]. Catalysis Letters, 2005, 101: 59-63.
[23] CHEN J, XU L N, LI W Y, GOU X H. α-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications [J]. Advanced Materials, 2005, 17: 582-586.
Corresponding author: ZHAO Qing-rui; Tel: +86-10-59202717; E-mail: zhaoqingrui@brici.ac.cn
DOI: 10.1016/S1003-6326(08)60433-1


