
Synthesis and photocatalytic properties of InVO4 sol containing nanocrystals by mild hydrothermal processing
FANG Hai-bo(方海波), XU Ming-xia(徐明霞), GE Lei(戈 磊), HE Zhi-yuan(何智远)
School of Materials Science and Engineering, Key Laboratory for Advanced Ceramics and
Machining Technology of Ministry of Education, Tianjin University, Tianjin 300072, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
The precursor precipitation of InVO4 was synthesized by co-precipitation using indium trichloride (InCl3), ammonium metavanadate (NH4VO3) and ammonia (NH3?H2O) as raw materials. The InVO4 sols with orthorhombic phase were obtained by hydrothermal treatment (the precursor precipitation solution at 423 K, for 4 h). The precursor and sol of InVO4 were characterized by X-ray diffraction (XRD), Fourier Transform Infra-red spectra (FT-IR), scanning electron microscopy (SEM) measurements. The XRD patterns indicate that the InVO4 precursor is amorphous phase, InVO4 sol contains orthorhombic InVO4 nanocrystals. The results also reveal that the pH value of the reaction mixture and reaction temperature play important roles to the target phase. InVO4-TiO2 thin films on glass slides were prepared by the dip-coating method from the composite sol. The photocatalytic properties of the InVO4-TiO2 thin films were investigated by the photocatalytic degradation of methyl orange solution. The results indicate that it has better photocatalytic activities than pure TiO2 thin films or pure InVO4 thin films with UV light.
Key words:
InVO4 nanocrystals; mild hydrothermal processing; co-precipitation; photocatalytic properties;
1 Introduction
As a well-known photocatalyst, TiO2 is one of the most popular and promising materials because it is stable in various solvents under photoirradiation, available commercially, and easy to prepare in the laboratory [1, 2]. However, the band gap of TiO2 is so wide that it only responds to the ultra violet (UV) light, which occupies only about 4% of the solar spectrum. Therefore, there is an urgent need to develop new photocatalysts with higher photocatalytic activities. In recent years, some papers were published on novel photocatalysts active under visible light irradiation, including ABO4(A=Bi, In, B=V, Nb, Ta)[3-6],Bi2MNbO7[7], CaIn2O4[8], etc. The new type of semiconductor InVO4 has been developed by YE et al [4], which was used as water splitting photocatalysis under visible irradiation. Several methods have been developed to synthesize InVO4, including the solid-state reaction between In2O3 and V2O5 [4], ‘chimie douce’method[9], sol-gel approach[10,11] and precipitation of the aqueous solution of InCl3 and NH4VO3[12], etc. Nevertheless, in orden to obtain crystalline InVO4, all of these ways needed heat treatment process with high temperature, which consume more energy and meet the difficulty in controlling the component, homogeneity and crystal size of the products [13]. XIAO et al [13] and CHEN et al [14] have synthesized nano-crystalline InVO4 powders by hydrothermal approach. However, there were two disadvantages: firstly, the agglomeration of InVO4 nanoparticles was obvious; secondly, the organic additives in the experiment were expensive and not friendly to environment.
In this study, the InVO4 sol was prepared from a mild hydrothermal method without organic additives at low temperature of 423 K, which was much lower than the solid-state reaction. The photocatalytic activity of the composite thin films was evaluated by the photocatalytic decoloration of methyl orange (MO) aqueous solution.
2 Experimental
The starting materials for the synthesis of InVO4 sol were indium trichloride (InCl3, AR), ammonium metavanadate (NH4VO3, AR) and ammonia (NH3?H2O, 3 mol/L). In a typical procedure, InCl3 was first dissolved with deionized water, then, precipitated by adding NH4VO3 solution resulting in the formation of yellow deposit with continual magnetic stirring until the stoichiometric proportion was 1. Under the vigorous stirring, the pH value of the mixture was adjusted to about 7 with ammonia solution. After centrifuging the precipitate, appropriate distilled water was added to disperse the precipitate homogeneously. InVO4 sol containing nanocrystals was obtained by reacting in a Teflon-lined autoclave, which was used to prepare composite sol (InVO4-TiO2). The TiO2 sol had been prepared by GE et al [15]. Glass slides were used as substrates. Before the deposition, substrates were ultrasonically cleaned in acetone and absolute ethanol for 10 min, respectively. Finally, they were thoroughly rinsed with distilled water. InVO4-TiO2 thin films were deposited on substrates by a dip-coating process at room temperature. The layers could be thickened by means of consecutive dip-coating processes. Afterward, the films were calcined to 773 K at 6 K/min, and then calcined for 30 min in an air flow oven.
The precursor and sol of InVO4 were characterized by FT-IR (BIO-RAD FTS-3000), XRD (Rigaka D/max 2 500 v/pc, Cu Kα, 40 kV and 40 mA) and SEM (JEOL JSF-6700F, FE-SEM, 10kV) measurements, respectively. The photocatalytic activities of the composite films were evaluated from an analysis of the degradation of MO solution under UV illumination. Two pieces of 25 mm×75 mm glass slides coated with composite films were settled in a watch glass containing 50 mL MO solution (10 mg/L) and exposed to UV-irradiation. Two UV-light lamps were used to provide the UV light-source. The distance between the films and the light source was 15 cm. UV absorption spectra of samples were measured every 20 min with a UV-vis spectrophotometer (7 230 G, Shanghai, China). The changes in concentrations of MO were estimated from the changes in absorbance of the absorption maximum at 500 nm.
3 Results and discussionFig.1 shows the FT-IR spectra of the dried InVO4 sol. The absorption bands of the dried powders at 406.7 cm-1 and 434.1 cm-1 are ascribed to V—O—V deformations mode. The 462.4 cm-1 band is ascribed to the V—O—V scissoring vibration. The bands of the samples at 748.7, 775.4 and 909.1 cm-1 originate from the V—O—In stretching vibration mode. The band of 951.0 cm-1 is assigned to the terminal V—O stretching.
The influence of the pH value and reaction temperature on the formation of InVO4 crystalline phase has been investigated by XRD in Fig.2 and Fig.3, respectively. The XRD patterns of the InVO4 samples
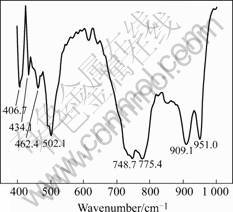
Fig.1 FT-IR spectra of dried InVO4 sol (pH=7, T=423 K, 4 h)
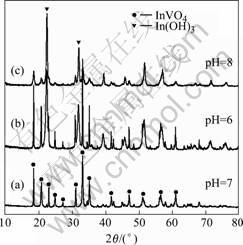
Fig.2 XRD patterns of samples (T=423 K, 4 h): (a) pH=7; (b) pH=6; (c) pH=8
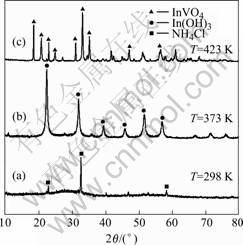
Fig.3 XRD patterns of samples (pH=7, 4 h): (a) T=298 K; (b) T=373 K; (c) T=423 K
synthesized at different pH values are shown in Fig.2. All the reflection peaks in Fig.2(a) could be indexed to the orthorhombic phase of InVO4 (JCPDF card 48-0898), no other impurities, such as In(OH)3, is detected in the samples. It indicates that the InVO4 samples prepared from hydrothermal method are absolutely orthorhombic phase. When pH value is less than or more than 7, In(OH)3 gradually becomes the main crystalline phase in the sol. In Fig.2(b), the main phase is orthorhombic InVO4, together with a spot of In(OH)3 detected as parent peaks at around 2θ=22.36? and 31.89?. When the pH value reaches to 8 (Fig.2(c)), the intensity of the peaks indexed as InVO4 decreases. The majority of crystalline phase is In(OH)3. From Fig.3(a), it can be seen that the precursor precipitation of InVO4 is amorphous. Upon increasing the synthetic temperature, no peaks of orthorhombic InVO4 appear in the XRD pattern, the crystalline phase is In(OH)3 (Fig.3(b)). We obtain the X-ray signature of the orthorhombic InVO4 phase at 423 K (Fig.3(c)). From Ref. [11], the InVO4 with the pure orthorhombic phase was obtained above 873 K. In the present work, it gives a new method to prepare the orthorhombic phase at a low temperature.
The morphologies of the InVO4 sol and as-prepared InVO4-TiO2 composite films are shown in Fig.4. All the InVO4 sols are obtained at pH=7. From Fig.4(a), we can clearly see the round, homogenous and fairly small crystals in the sols. The average grain size is 100 nm. The size of sphere particles grows with increasing reaction temperature (Fig.4(b)). Fig.4(b) also shows that the grain size distribution is wider with higher temperature, such as the larger particles reach to 200 nm in diameters. When extending the reaction time, it is found that the sols contain regular polygonal crystals of about 400-500 nm in size as shown in Fig.4(c). Fig.4(d) shows that the InVO4-TiO2 composite thin films are compact, uniform and no cracks, and abnormal large particles are observed. The composite films consist of rice-bodies crystals, which are 100 nm in length and 50 nm in width.
Photocatalytic performance of the InVO4-TiO2 composite thin films was assessed by investigating photodegradation effect of methyl orange(MO)under UV illumination, as shown in Fig.5. For comparison, the decoloration of pure TiO2 and InVO4 films are given as well. Temporal changes in the concentration of MO are monitored by examining the variations in maximal absorption in UV-vis spectra at 500 nm. From Fig.5, it can be seen that the decoloration of MO increases with extending of the irradiation time. And the results show that the InVO4-TiO2 composite thin films exhibit an extremely high activity (Fig.5(c)); almost 100% of methyl orange is degraded in 80 min. This is attributed to the efficient separation of e--h+ pairs in the excited state, where the InVO4 and TiO2 are coupled; and electrons, which are promoted from conduction band of the InVO4 under UV light, are easily injected into the conduction band of TiO2; whereas holes in the valence band of TiO2 are transferred into the InVO4, which could product —OH radical on the surface of TiO2, leading to the higher activity[16,17]. At the same time, the photocatalytic activity of the TiO2 (Fig.6(a)) and InVO4 (Fig.6(b)) shows that the catalytic activities of them are low, because the photo-generated electron–holes are de-coupled easily on the surface of InVO4 and the electrons are not easily excited in pure TiO2 under these conditions.
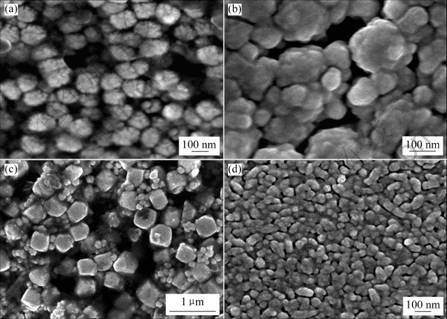
Fig.4 FE-SEM micrographs of as-prepared samples (pH=7): (a) InVO4 sol, 423 K, 4 h; (b) InVO4 sol, 453 K, 4 h; (c) InVO4 sol, 423 K, 6h; (d) InVO4-TiO2 composite thin films, using (a) InVO4 sol
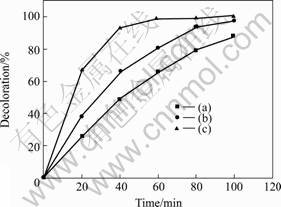
Fig.5 Decoloration rate curves of methyl orange under UV light irradiation: (a) TiO2 thin films; (b) InVO4 thin films; (c) InVO4-TiO2 composite thin films
4 Conclusions
InVO4 sol containing pure orthorhombic phase was synthesized by hydrothermal method at low temperature. The optimal conditions for the synthesis are: pH=7, T=423 K, reaction time: 3-6 h. It is found that the sols consist of the round, homogenous nanocrystals. The average grain size is 100 nm. The photocatalytic exper-
iment exhibits that the InVO4-TiO2 composite films have the highest photocatalytic activity among the three thin films with UV light.
References
[1] BUNSHO O, YOSHIMASA O, SEIICHI N. Photocatalytic activity of amorphous-anatase mixture of titanium (IV) oxide particles suspended in aqueous solutions [J]. J Phys Chem B, 1997, 101: 3746-3752.
[2] HIROMICHI I, HIROAKI K. Photocatalytic activities of coating films prepared from peroxotiatanic acid solution-derived anatase sols [J]. J Ceramic Society of Japan 1998, 106(3): 344-347.
[3] AKIHIKO K, HIDEKI K, ISSEI T. Strategies for the development of visible-light-driven photocatalysts for water splitting [J]. Chem Lett, 2004, 12(33): 1534-1539.
[4] YE J H, ZOU Z, OSHIKIRI M, MATSUSHITA A, SHIMODA M, IMAI M, SHISHIDO T. A novel hydrogen-evolving photocatalyst InVO4 active under visible light irradiation [J]. Chem Phys Lett, 2002, 356: 221-226.
[5] ZOU Z G, YE J H, Arakawa H. Structural properties of InNbO4 and InTaO4: correlation with photocatalytic and photophysical properties [J]. Chem Phys Lett, 2000, 332(12): 271-277.
[6] ZOU Z G, YE J H, SAYAMA K. Photocatalytic hydrogen and oxygen formation under visible light irradiation with M-doped InTaO4 (M=Mn, Fe, Co, Ni and Cu) photocatalysts [J]. J Photochem Photobio A, 2002, 148: 65-69.
[7] ZOU Z G, ARAKAWA H. Direct water splitting into H2 and O2 under visible light irradiation with a new series of mixed oxide semiconductor photocatalysts [J]. J Photochem Photobio A, 2003, 158: 145-162.
[8] TANG J W, ZOU Z G, YIN J, YE J H. Photocatalytic degradation of methylene blue on CaIn2O4 under visible light irradiation [J]. Chem Phys Lett, 2003, 382: 175-179.
[9] DENIS S, BAUDRIN E, ORSINI F, OUVRARD G, TOUBOUL M, TARSCOM J M. Synthesis and electrochemical properties of numerous classes of vanadates [J]. J Power Sources, 1999, 81-82: 79-84.
[10] OREL B, SURCA V A, OPARA K U. Electrochromic and structural investigation of InVO4 and some other vanadia-based oxide films [J]. Electrochimica Acta, 2001, 46(13-14): 2059-2068.
[11] TOUBOUL M, MELGHIT K. Crystal Structure of a metastable form of indium orthovanadate, InVO4-I [J]. J Solid State Chem, 1995, 118: 93-98.
[12] RONCAGLIA D I, BOTTO I L, BARAN E J. Characterization of a low-temperature form of InVO4 [J]. J Solid State Chem, 1986, 62: 11-15.
[13] XIAO G C, LI D Z, FU X Z, WANG X X, LIU P. Synthesis for single dispersing nano-crystalline InVO4(orthorhombic) at low temperature [J]. Chinese J Inorg Chem, 2004, 20 (2): 195-198.
[14] CHEN L M, LIU Y N, LU Z G, ZENG D M. Shape-controlled synthesis and characterization of InVO4 particles [J]. J Colloid and Inter Scie, 2006, 295: 440-444.
[15] GE L, XU M X, SUN M. Synthesis and characterization of TiO2 photocatalytic thin films prepared from refluxed PTA sols [J]. Materials Letters 2006, 60(2): 287-290.
[16] IDRISS B, PRASHANT V K. Capped semiconductor colloids. synthesis and photoelectrochemical behavior of tio2-capped sno2 nanocrystallites [J]. J Phsy Chem, 1995, 99: 9182-9188.
[17] KOMORNICHI S, RADECHA M, SOBAS P. Structural, electrical and optical properties of TiO2-WO3 polycrystalline ceramics [J]. Mater Rese Bull. 2004, 39: 2007-2017.
Foundation item: Project (20030056001) supported by the Doctor Foundation of Ministry of Education of China
Corresponding author: XU Ming-xia; Tel.: +86-22-27890489; Fax: +86-22-27404724; E-mail: xumingxia@tju.edu.cn


