DOI: 10.11817/j.issn.1672-7207.2016.01.004
铝酸钠溶液碳分母液深度碳分制备片钠铝石
李小斌,刘楠,周秋生,齐天贵,刘桂华,彭志宏
(中南大学 冶金与环境学院,湖南 长沙,410083)
摘 要:
酸化分解制备冶金级氢氧化铝后所得碳分母液进行深度碳分制备片钠铝石纤维,研究深度碳分过程中铝和硅的析出行为;通过FTIR,XRD和SEM等分析手段,研究深度碳分所得最终产物物相及其形貌的主要影响因素。研究结果表明:在深度碳分过程中,溶液中铝的析出存在一临界pH,即随着CO2气体的通入,当溶液pH降低到11.8以下时,溶液中铝迅速析出;溶液中硅的析出趋势与铝的析出趋势基本相同;控制深度碳分终点pH为10.5左右,所得最终产物均为片钠铝石;随着深度碳分温度升高,最终产物由颗粒状向纤维状转变;随着初始Al2O3质量浓度的增加,所得片钠铝石纤维的直径变小,长径比增大,且其均匀性提高。
关键词:
中图分类号:TF802 文献标志码:A 文章编号:1672-7207(2016)01-0020-06
Dawsonite preparation by deep carbonation decomposition of spent liquor from carbonation of sodium aluminate solutions
LI Xiaobin, LIU Nan, ZHOU Qiusheng, QI Tiangui, LIU Guihua, PENG Zhihong
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: Fibrous dawsonite was prepared by deep carbonation decomposition of spent liquor from carbonation of sodium aluminate solutions for producing metallurgical aluminum trioxide. The precipitation behavior of aluminum and silicon was studied in the deep carbonation decomposition process, and the main factors influencing the morphology of final dawsonite was determined by FTIR, XRD and SEM analyses. The results show that there is a critical pH for the aluminum precipitation from the spent liquor. Dawsonite precipitates rapidly when pH is lower than 11.8 with the continuous ventilation of CO2. The precipitation behavior of silicon species is similar to that of aluminum species. The final product of deep carbonation decomposition is fibrous dawsonite when the final pH of spent liquor is 10.5. The morphology of final product changes from particles to fibers with the increase of carbonation temperature, and the fiber diameter becomes small with the increase of initial alumina concentration for deep carbonation decomposition.
Key words: sodium aluminate solution; deep carbonation decomposition; dawsonite
铝酸钠溶液碳酸化分解(简称“碳分”)过程是烧结法生产氧化铝的关键工序之一,碳分条件直接影响分解产物氢氧化铝的结晶形貌、粒度及杂质含量等重要指标。在现行烧结法生产氧化铝工业中,为了获得物理指标和化学纯度均符合要求的氢氧化铝产品,碳分分解率常控制在90%左右,即碳分母液中仍残留约10%的氧化铝。这部分残留的氧化铝必须返回烧结法配料,这不仅增加了氧化铝生产能耗,而且降低了设备产能。而另一方面,曾有研究报道,在铝酸钠溶液碳分末期,分解体系中存在的NaHCO3可与NaAl(OH)4溶液或Al2O3·nH2O发生反应,生成片钠铝石(NaAlCO3(OH)2)[1]。片钠铝石是一种斜方晶系的钠和铝的碳酸盐矿物,晶体结构为Na和Al呈Na-O4(OH)2和Al-O2(OH)4的畸变八面体。自然界中的此类化合物是在水热环境中形成的,呈现棱形、针状、纤维状、球形和玫瑰形等不同晶体习性[2]。片钠(钾、铵等)铝石是一类具有广阔潜在应用前景的材料。基于其热分解产生无毒的CO2和水,且略呈碱性,有望在阻燃[3]、抗酸、防锈和防腐[4]等方面得到应用。基于其热分解产物具有高活性,可作为制备高效Al,Cr和Fe氧化物催化剂[5]和高性能H3O+-β-Al2O3快离子导体材料的前驱物[6];基于其热分解产物的结构记忆性,在智能材料和CO2储存[7-11]等方面具有潜在应用前景。目前,此类化合物的制备方法主要有水热法[3, 12-13]、线状分散-沉淀法[14]、共沉积法[15-16]、盐溶液慢速蒸发结晶法[17]等。由于这些方法是以Na2CO3,NaHCO3,Al2(SO4)3,C9H21O3Al和AlOOH等纯化合物为初始反应物,因而均存在制备成本较高的问题。若能以烧结法生产氧化铝工业中铝酸钠溶液碳分末期得到的碳分母液为原料,采用深度碳分的方法制备片钠铝石,则不仅能解决烧结法生产氧化铝工业上母液中氧化铝返回配料所造成的氧化铝生产能耗升高和产出率降低的问题,而且有望大幅度降低片钠铝石的制备成本。为此,本文作者对烧结法碳分母液深度碳分制备片钠铝石进行实验研究。
1 实验原料与方法
1.1 实验原料
铝酸钠溶液,为工业NaOH和工业Al(OH)3在实验室配制而成的合成溶液;碳酸钠溶液,由分析纯Na2CO3溶解于水中制备;二氧化碳,为工业纯气体。
1.2 实验方法
自制碳分实验装置如图1所示。实验前将一定体积的铝酸钠溶液和碳酸钠溶液加入碳分反应器,混匀后取样,采用容量法测定其中Al2O3,Na2Ok,Na2Oc和SiO2质量浓度[18]。然后将碳分反应器置于预先升温至预定温度的数显恒温水浴槽中,并预热一段时间,使反应器内溶液温度恒定;二氧化碳气体和压缩空气在配气罐中混合后得到空气-二氧化碳混合气体,通过调节转子流量计(LZB-6型)调整混合气体中二氧化碳体积分数(采用O2/CO2气体浓度测定仪)到实验所需值。然后,将混合气体通入置于反应器底部的自制通气装置,使气体均匀分散于铝酸钠溶液中,并开始计时;在碳分过程中采用PHS-25C酸度计测定不同时间的溶液pH,并同时取样,试样经真空抽滤,所得滤液用于溶液组成分析;滤饼经去离子水洗涤3次后置于恒温干燥箱中于60 ℃干燥12 h,然后采用扫描电镜(JSM-6360LV)分析其形貌,采用X线衍射仪(D8 Advance)和红外光谱仪(Nicolet 6700)分析其物相组成和纯度。
由于在整个碳酸化分解过程中,溶液中全碱(即溶液中的碳酸钠和苛性钠质量浓度之和)的绝对量基本不变,因此,铝酸钠溶液碳分过程中Al2O3分解率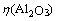 可采用如下公式进行计算:
可采用如下公式进行计算:
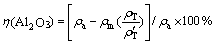
式中: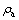 和
和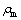 分别为碳分原液和母液中Al2O3质量浓度(g/L);
分别为碳分原液和母液中Al2O3质量浓度(g/L);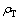 和
和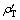 分别为碳分原液和母液中总碱质量浓度(g/L)。
分别为碳分原液和母液中总碱质量浓度(g/L)。
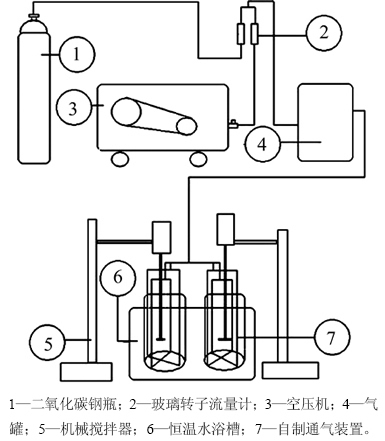
图1 碳分实验设备连接示意图
Fig. 1 Schematic diagram of deep carbonation decomposition equipment
2 结果与讨论
2.1 铝酸钠溶液深度碳分过程中铝和硅的析出行为
2.1.1 铝的析出行为
铝酸钠溶液深度碳分过程中,随着CO2气体的通入,溶液中苛性碱逐渐被CO2中和而生成碳酸钠,但氢氧化铝或片钠铝石并不会随着溶液苛性比的降低而迅速析出,存在1个诱导期。图2所示为铝酸钠溶液在55 ℃时深度碳分过程中溶液的Al2O3质量浓度随pH的变化曲线。从图2可知:在铝酸钠溶液深度碳分过程中,溶液中氧化铝质量浓度随pH的升高依次经历缓慢下降、骤降和基本不变3个阶段;当pH从12.7降到约11.7时,母液中氧化铝质量浓度略下降,铝的析出不明显,此时主要发生CO2与溶液中苛性碱的中和反应,导致溶液过饱和度快速上升;随着CO2继续通入,溶液中氧化铝质量浓度超过其最大容许介稳质量浓度,此时含铝物相从溶液中迅速析出;当溶液中铝大量析出后,溶液中氧化铝的过饱和度又迅速减小,含铝物相的析出速度再次变慢;当pH小于10.8时,溶液中的铝几乎全部析出。
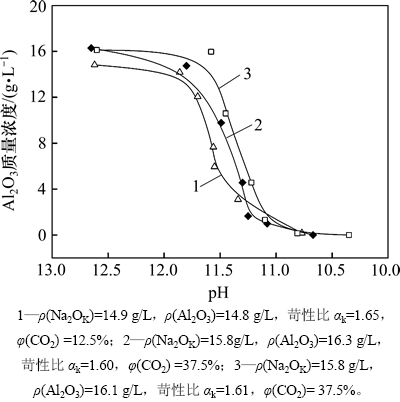
图2 55 ℃下深度碳分时母液中Al2O3质量浓度随溶液pH的变化
Fig. 2 Relationship between Al2O3 mass concentration of spent liquor and pH in deep carbonation decomposition process at 55℃
反应时间是影响碳分深度的重要因素。控制其他条件相同,研究反应时间对铝酸钠溶液深度碳分过程的影响,结果如图3所示。由图3可知:碳分分解率随着反应时间的增加而增加;同时,对比通入不同CO2体积分数φ(CO2)反应结果可知:在反应前10 min内,CO2体积分数为12.5%的溶液分解率较体积分数为28.5%和37.5%时的低。这是由于提高CO2体积分数缩短了溶液中氢氧化铝或片钠铝石析出的诱导期,从而促进了反应的进行;当反应50 min时,溶液分解率出现明显区别,φ(CO2)为12.5%时溶液分解率约为30%,而φ(CO2)为37.5%的溶液分解率达100%。
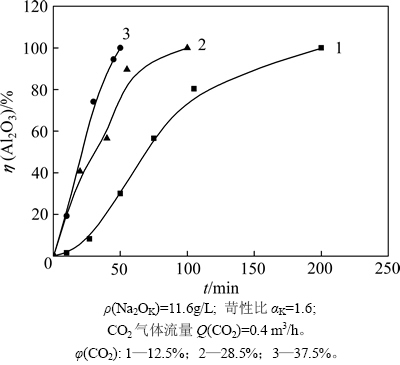
图3 不同CO2体积分数下深度碳分分解率随时间的变化
Fig. 3 Relationship between carbonation ratio and time during deep carbonation process with different carbon dioxide mass concentrations
2.1.2 硅的析出行为
随着深度碳分的进行,溶液中苛性碱逐渐被CO2所中和,使得溶液中二氧化硅的溶解度变小,导致硅酸根离子从溶液中析出,作为杂质进入含铝析出产物中。图4所示为不同条件下深度碳分过程中母液的ρ(Al2O3)及ρ(SiO2)随溶液pH的变化曲线。从图4可看出:在深度碳分过程中,溶液中ρ(SiO2)的变化趋势始终与溶液中氧化铝质量浓度的变化趋势保持一致,当溶液中铝大量析出时,二氧化硅质量浓度亦出现大幅度下降,这与氧化铝工业上铝酸钠溶液碳分生产氢氧化铝过程中铝硅的析出规律基本一致[19-20]。所不同的是:在深度碳分过程中,铝最终以片钠铝石形式从溶液中析出,而在氧化铝生产工业碳分过程中铝以氢氧化铝的形式从溶液中结晶析出;在铝酸钠溶液深度碳分过程中,溶液中的SiO2以水合铝硅酸钠的形式析出。由于铝硅酸钠在碳酸钠溶液中溶解度极小,当溶液中的Na2O全部变成Na2OC以及Al2O3完全析出时,SiO2也会基本析出。
2.2 深度碳分产物的物相表征
在碳分母液深度碳分初期,当溶液中还存在一定量苛性碱时,在通入CO2气泡与铝酸钠溶液界面上生成的片钠铝石将与母液中苛性碱反应,变成碳酸钠和铝酸钠;但随着深度碳分的进行,溶液中苛性碱含量逐渐减少直至全部被中和,此时,生成的片钠铝石将在母液中稳定存在。因此,铝酸钠溶液深度碳分的最终产物应为片钠铝石。图5所示为不同深度碳分条件下所得最终产物的红外光谱图。从图5可看出:不同深度碳分条件下所得最终产物的红外谱图变化不大,其中3 290.96 cm-1和1 098.35 cm-1处的振动分别对应O—H的伸缩振动和面内变形振动;1 391.96 cm-1和1 569.37 cm-1处的振动分别对应—O伸缩振动;指纹区中500~800 cm-1处的振动对应Al—O以及Na—O的特征振动。这与片钠铝石的红外光谱图基本一致[21],说明所得产物为片(丝)钠铝石。图6所示为深度碳分最终产物的X线衍射图谱。对比标准卡片可知,所得物相为单一的片钠铝石(NaAl(OH)2CO3),与图5所示结果一致。
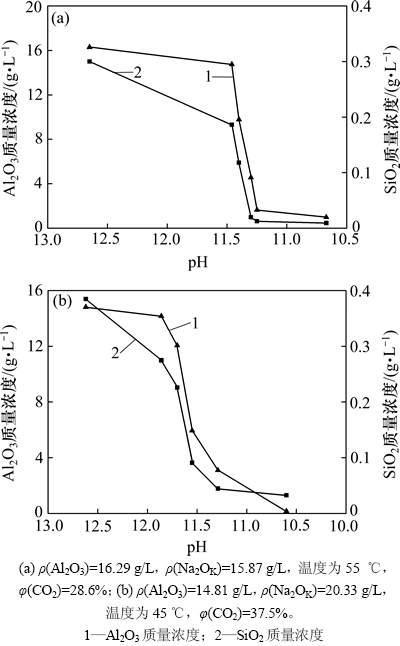
图4 深度碳分母液中的Al2O3和SiO2质量浓度随pH的变化
Fig. 4 Relationship between mass concentration of alumina and silica species in spent liquor and pH in deep carbonation decomposition process
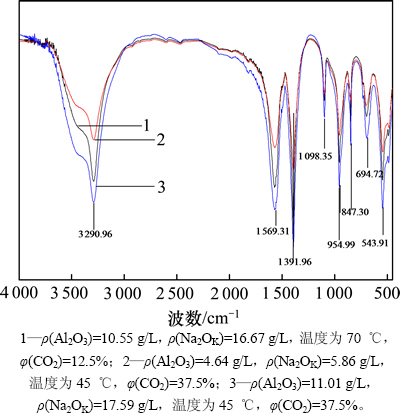
图5 铝酸钠溶液深度碳分产物的红外光谱图
Fig. 5 FTIR spectra of deep carbonation decomposition products of sodium aluminate solution
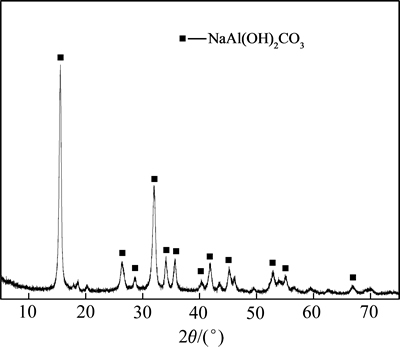
图6 深度碳分产物的X线衍射图谱
Fig. 6 X-ray diffraction pattern of deep carbonation decomposition product from sodium aluminate solution
2.3 片钠铝石产物形貌的影响因素
2.3.1 深度碳分温度的影响
碳分温度是影响铝酸钠溶液深度碳分过程的重要因素,直接影响CO2气体的溶解及其相关反应的速率以及碳分产物的结晶过程,从而最终影响碳分产物的形貌。为了确定温度对碳分产物形貌的影响,控制碳分终点pH为10.5,在其他条件相同的情况下,分别考察45,55和70 ℃这3种温度下所得最终深度碳分产物的形貌特征,结果如图7所示。从图7可看出:45 ℃时所得片钠铝石呈颗粒状,粒径为1~2 μm,颗粒间团聚较明显;当碳分温度升高到55 ℃时,颗粒明显变粗,团聚体表面开始呈现纤维状;当碳分温度进一步提高到70 ℃时,片钠铝石完全纤维化,且长径比大、分布较均匀。
2.3.2 初始Al2O3质量浓度的影响
碳分母液中Al2O3的质量浓度直接关系到反应速率。为了考察铝酸钠溶液中初始ρ(Al2O3)对片钠铝石形貌的影响,实验控制碳分温度为70 ℃,反应终点pH控制在10.5,研究初始Al2O3质量浓度对分解产物形貌的影响,结果如图8所示。从图8可看出:在铝酸钠溶液深度碳分过程中,溶液中初始Al2O3质量浓度对反应最终产物的形貌亦有较大影响。虽然在实验条件下,当初始Al2O3质量浓度不同时所得产物均为片钠铝石纤维,但随着初始Al2O3质量浓度从4.39 g/L增加到15.35 g/L,总体上纤维直径由0.2~0.3 μm减小到约0.1 μm,且长径比呈增大趋势,纤维逐渐均匀。
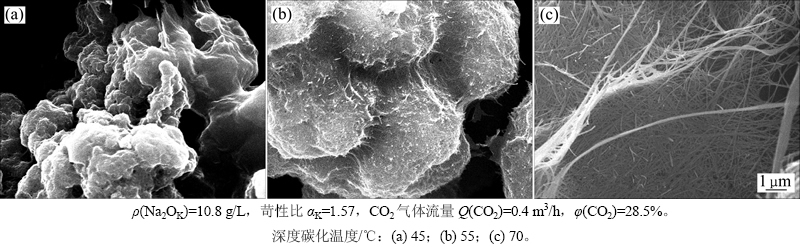
图7 不同温度下深度碳分产物的扫描电镜照片
Fig. 7 SEM photographs of deep carbonation decomposition products obtained at different carbonation temperatures
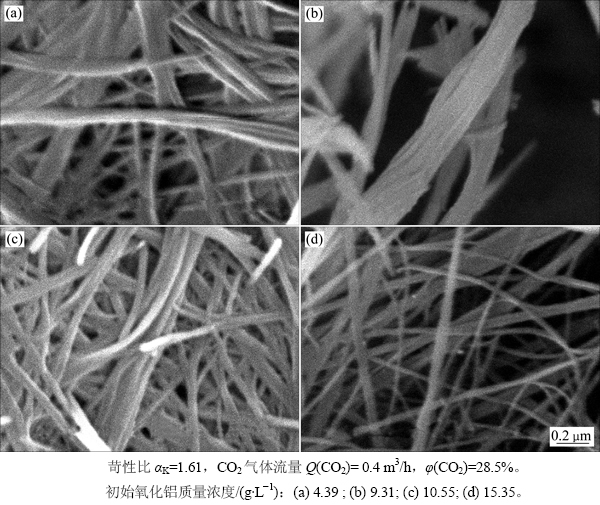
图8 铝酸钠溶液中初始氧化铝浓度对深度碳分产物形貌的影响
Fig. 8 Influence of initial alumina concentration of sodium aluminate solutions on morphology of deep carbonation decomposition products
3 结论
1) 在铝酸钠溶液深度碳分过程中,铝的析出存在一临界析出pH。随着CO2气体的通入,当溶液pH降低到11.8以下时,溶液中的铝迅速析出;溶液中硅的析出趋势与铝的析出趋势基本相同。
2) 控制碳分母液深度碳分的终点pH为10.5左右,所得最终深度碳分产物均为片钠铝石。
3) 深度碳分温度和初始Al2O3质量浓度影响最终深度碳分产物片钠铝石的形貌。随着深度碳分温度升高,产物由颗粒状向纤维状转变;随着初始Al2O3质量浓度增大,所得片钠铝石纤维直径变小,长径比增大,均匀性提高。
参考文献:
[1] 杨重愚. 氧化铝生产工艺学[M]. 北京: 冶金工业出版社, 1993: 78-95.
YANG Zhongyu. Process technology of alumina[M]. Beijing: Metallurgical Industry Press, 1993: 78-95.
[2]  P, PALMER D A, ANOVITZ L M, et al. Dawsonite synthesis and reevaluation of its thermodynamic properties from solubility measurements: Implications for mineral trapping of CO2[J]. Geochimica et Cosmochimica Acta, 2007, 71(18): 4438-4455.
P, PALMER D A, ANOVITZ L M, et al. Dawsonite synthesis and reevaluation of its thermodynamic properties from solubility measurements: Implications for mineral trapping of CO2[J]. Geochimica et Cosmochimica Acta, 2007, 71(18): 4438-4455.
[3] 范蕾蕾, 叶俊伟, 李鑫, 等. NaAl(OH)2CO3阻燃晶须的水热合成及其阻燃性能[J]. 功能材料, 2009, 40(9): 1580-1583.
FAN Leilei, YE Junwei, LI Xin, et al. Hydrothermal synthesis and flam-retardant properties of NaAl(OH)2CO3 whiskers[J]. Journal of Functional Meterials, 2009, 40(9): 1580-1583.
[4] CHEN Jun, SONG Yinwei, SHAN Dayong, et al. Properties of dawsonite conversion film on AZ31 magnesium alloy[J]. Transactions of the Nonferrous Metals Society of China, 2011, 21(4): 936-942.
[5] STOICA G, ABELL S,
S,  J. Na-dawsonite derived aluminates for DMC production by transesterification of ethylene carbonate[J]. Applied Catalysis A: General, 2009, 365(2): 252-260.
J. Na-dawsonite derived aluminates for DMC production by transesterification of ethylene carbonate[J]. Applied Catalysis A: General, 2009, 365(2): 252-260.
[6] AHMAD A, WHEAT T A, CANADAY J D, et al. Processing and characterization of Na and (Na-K) beta-beta alumina ceramics[J]. Solid State Ionics, 1994, 68(3): 233-241.
[7] OKUYAMA Y. Dawsonite-bearing carbonate veins in the Cretaceous Izumi Group, SW Japan: a possible natural analogue of fracture formation and self-sealing in CO2 geological storage[J]. Energy Procedia, 2014, 63: 5530-5537.
[8] 姜伟, 曲希玉, 肖洪, 等. 钾长石-CO2相互作用水热实验研究[J]. 世界地质, 2014(4): 974-980.
JIANG Wei, QU Xiyu, XIAO Hong, et al. Research on interactively hydrothermal experiment between potassium feldspar and CO2[J]. Global Geology, 2014(4): 974-980.
[9] 郭晓静, 曲希玉, 程慧慧, 等. 温室气体CO2与钠长石相互作用研究[J]. 岩石矿物学杂志, 2014(5): 980-988.
GUO Xiaojing, QU Xiyu, CHENG Huihui, et al. Experimental research on the possibility of geological storage of greenhouse gas-CO2 in albite[J]. Acta Petrologica et Mineralogica, 2014(5): 980-988.
[10] MARTIN O, HAMMES M, MITCHELL S, et al. Design of hydrothermally-stable dawsonite-based sorbents in technical from for CO2 capture[J]. Energy & Environmental Science, 2014, 7: 3640-3650.
[11] ZHOU Bing, LIU Li, ZHAO Shuang, et al. Dawsonite formation in the Beier Sag, Hailar Basin, NE Chian tuff: a natural analog for mineral carbon storage[J]. Applied Geochemistry, 2014, 48: 155-167.
[12] ALI A A, HASAN M A, ZAKI M I. Dawsonite-type precursors for catalytic Al, Cr and Fe oxides: synthesis and characterization[J]. Chemistry of Materials, 2005, 17(26): 6797-6804.
[13] KANAZIREV V I. Process for conversion of aluminum oxide hydroxide: 7947250B2[P]. 2011-05-24.
[14] SANTIAGO M, YALFANI M S,  J. In-line dispersion-precipitation method for the synthesis of metal-substituted dawsonites: genesis of oxide materials with superior properties[J]. Journal of Materials Chemistry, 2006, 16(28): 2886-2889.
J. In-line dispersion-precipitation method for the synthesis of metal-substituted dawsonites: genesis of oxide materials with superior properties[J]. Journal of Materials Chemistry, 2006, 16(28): 2886-2889.
[15] DUBERT D C,  J, GARCIA-VALLS R. Continuous synthesis of porous ammonium dawsonite within a new microstructrured system[J]. Chemical Engineering Transactions, 2011, 25: 231-236.
J, GARCIA-VALLS R. Continuous synthesis of porous ammonium dawsonite within a new microstructrured system[J]. Chemical Engineering Transactions, 2011, 25: 231-236.
[16] LACH R,  M M, HABERKO K, et al. Dynamic study of ammonium dawsonite doped with yttrium transformation at elevated temperatures[J]. Journal of Thermal Analysis and Calorimetry, 2013, 112(2): 727-730.
M M, HABERKO K, et al. Dynamic study of ammonium dawsonite doped with yttrium transformation at elevated temperatures[J]. Journal of Thermal Analysis and Calorimetry, 2013, 112(2): 727-730.
[17] CONTRERAS C A,  J I, RAMOS E. A new preparation method of sodium aluminum hydroxy carbonate and potassium aluminum hydroxy carbonate from basic aluminum sulfate[C]// MRS. MRS Proceedings. Cambridge: Cambridge University Press, 2010: 3-11.
J I, RAMOS E. A new preparation method of sodium aluminum hydroxy carbonate and potassium aluminum hydroxy carbonate from basic aluminum sulfate[C]// MRS. MRS Proceedings. Cambridge: Cambridge University Press, 2010: 3-11.
[18] 赵国永, 杨清廉, 单仁斌, 等. 联合法生产氧化铝控制分析[M]. 北京: 冶金工业出版社, 1977: 88-110.
ZHAO Guoyong, YANG Qinlian, SHAN Renbin, et al. Control analysis of alumina production in combined process[M]. Beijing: Metallurgical Industry Press, 1977: 88-110.
[19] 韩中岭, 娄世彬, 杨桂丽. 铝酸钠溶液碳分过程机理及影响因素探讨[J]. 中国稀土学报, 2006, 24: 177-185.
HAN Zhongling, LOU Shibin, YANG Guili. Approach to decomposition mechanism and influencing factors of carbonization process of sodium aluminate solution[J]. Journal of the Chinese Rare Earth Society, 2006, 24: 177-185.
[20] 王志, 毕诗文, 杨毅宏, 等. 碳酸化分解的机理研究与进展[J]. 轻金属, 2001(12): 13-15.
WANG Zhi, BI Shiwen, YANG Yihong. Research progress in decomposition mechanism of carbonization process of sodium aluminate solution[J]. Light Metals, 2001(12): 13-15.
[21] FROST R L, L PEZ A, SCHOLZ R, et al. SEM, EDS and vibrational spectroscopic study of dawsonite NaAl(CO3)(OH)2[J]. Spectrochimica Acta Part A: Molecular & Biomolecular Spectroscopy, 2015, 136: 918-923.
PEZ A, SCHOLZ R, et al. SEM, EDS and vibrational spectroscopic study of dawsonite NaAl(CO3)(OH)2[J]. Spectrochimica Acta Part A: Molecular & Biomolecular Spectroscopy, 2015, 136: 918-923.
(编辑 陈灿华)
收稿日期:2015-01-10;修回日期:2015-03-08
基金项目(Foundation item):国家自然科学基金资助项目(51274243) (Project(51274243) supported by the National Natural Science Foundation of China)
通信作者:周秋生,教授,从事碱法冶金过程研究;E-mail: qszhou@csu.edu.cn
摘要:对铝酸钠溶液碳酸化分解制备冶金级氢氧化铝后所得碳分母液进行深度碳分制备片钠铝石纤维,研究深度碳分过程中铝和硅的析出行为;通过FTIR,XRD和SEM等分析手段,研究深度碳分所得最终产物物相及其形貌的主要影响因素。研究结果表明:在深度碳分过程中,溶液中铝的析出存在一临界pH,即随着CO2气体的通入,当溶液pH降低到11.8以下时,溶液中铝迅速析出;溶液中硅的析出趋势与铝的析出趋势基本相同;控制深度碳分终点pH为10.5左右,所得最终产物均为片钠铝石;随着深度碳分温度升高,最终产物由颗粒状向纤维状转变;随着初始Al2O3质量浓度的增加,所得片钠铝石纤维的直径变小,长径比增大,且其均匀性提高。
[1] 杨重愚. 氧化铝生产工艺学[M]. 北京: 冶金工业出版社, 1993: 78-95.
[3] 范蕾蕾, 叶俊伟, 李鑫, 等. NaAl(OH)2CO3阻燃晶须的水热合成及其阻燃性能[J]. 功能材料, 2009, 40(9): 1580-1583.
[8] 姜伟, 曲希玉, 肖洪, 等. 钾长石-CO2相互作用水热实验研究[J]. 世界地质, 2014(4): 974-980.
[9] 郭晓静, 曲希玉, 程慧慧, 等. 温室气体CO2与钠长石相互作用研究[J]. 岩石矿物学杂志, 2014(5): 980-988.
[13] KANAZIREV V I. Process for conversion of aluminum oxide hydroxide: 7947250B2[P]. 2011-05-24.
[18] 赵国永, 杨清廉, 单仁斌, 等. 联合法生产氧化铝控制分析[M]. 北京: 冶金工业出版社, 1977: 88-110.
[19] 韩中岭, 娄世彬, 杨桂丽. 铝酸钠溶液碳分过程机理及影响因素探讨[J]. 中国稀土学报, 2006, 24: 177-185.
[20] 王志, 毕诗文, 杨毅宏, 等. 碳酸化分解的机理研究与进展[J]. 轻金属, 2001(12): 13-15.


