- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Result and discuss...▲
- 4 Conclusions▲
- References
- Figure
- Figure 1 XRD patterns of CaZnAl-CO3-LDHs with different Ca/Zn/Al molar ratios:
- Figure 2 FT-IR spectra of CaZnAl-CO3-LDHs with different Ca/Zn/Al molar ratios:
- Figure 3 Typical SEM images of CaZnAl-CO3-LDHs with different Ca/Zn/Al molar ratios:
- Figure 4 EDX analysis of CaZnAl-CO3-LDHs with different Ca/Zn/Al molar ratios:
- Figure 5 Particle size distribution of CaZnAl-CO3-LDHs with n(Ca):n(Zn):n(Al)=3.6:0.4:2
- Figure 6 Thermal stability results of PVC samples with different CaZnAl-CO3-LDHs at 180°C
- Figure 7 UV-VIS spectra of pure PVC and PVC stabilized with different CaZnAl-CO3-LDHs heated at 180 °C for 0 min (a) and 60 min (b)
- Figure 8 Effect of different contents of Ca3.6Zn0.4Al2- CO3-LDHs on thermal stability of PVC resin at 180 °C
- Figure 9 Thermal stability time of PVC samples stabilized by Ca3.6Zn0.4Al2-CO3-LDHs at different contents detected with Congo red test
- Figure 10 Thermal stability of PVC samples with Ca3.6Zn0.4Al2-CO3-LDHs (6 phr) incorporated with different amounts of CaSt2:
- Figure 11 UV-VIS spectrum of PVC samples with Ca3.6Zn0.4Al2-CO3-LDHs (6 phr) incorporated with different amounts of CaSt2 heated at 180 °C for (a) 0 min and (b) 60 min

J. Cent. South Univ. (2020) 27: 797-810
DOI: https://doi.org/10.1007/s11771-020-4332-z
Synthesis of CaZnAl-CO3 ternary layered double hydroxides and its application on thermal stability of poly (vinyl chloride) resin
ZENG Xiao(曾笑)1, 2, YANG Zhan-hong(杨占红)1
1. College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Kunming Training Crops, Fire and Rescue Bureau, Ministry of Emergency Management,Kunming 650208, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
In this work, hydrothermal method was used to prepare the CaZnAl-CO3 ternary layered double hydroxides (CaZnAl-CO3-LDHs) with various Ca/Zn/Al molar ratios, which were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscope (FT-IR) and scanning electron microscopy (SEM) techniques. The obtained results demonstrate that the samples were well-crystallized and flake-structured. The CaZnAl-CO3-LDHs used alone for thermal stability of poly (vinyl chloride) (PVC) resin, with different Ca/Zn/Al molar ratios and varying additive amounts, were investigated through the tests such as static thermal aging, mass loss and congo red, respectively. The optimum Ca/Zn/Al molar ratio and additive amount were 3.6:0.4:2 and 5 phr (parts per hundred PVC resin), respectively. In addition, the synergistic effects of Ca3.6Zn0.4Al2-CO3-LDHs and CaSt2 were discussed in detail, showing better thermal stability compared with Ca3.6Zn0.4Al2-CO3-LDHs used alone, and the optimum additive amounts of Ca3.6Zn0.4Al2-LDHs and CaSt2 were 6 and 1.0 phr, respectively.
Key words:
CaZnAl-CO3-LDHs; synergistic effects; thermal stability; poly vinyl chloride;
Cite this article as: ZENG Xiao, YANG Zhan-hong. Synthesis of CaZnAl-CO3 ternary layered double hydroxides and
its application on thermal stability of poly vinyl chloride resin [J]. Journal of Central South University, 2020, 27(3): 797-810. DOI: https://doi.org/10.1007/s11771-020-4332-z.
1 Introduction
Poly vinyl chloride (PVC) as a kind of thermoplastic exhibits remarkable performance such as high corrosion resistance, high mechanical strength and relatively low cost [1-3]. Therefore, PVC has been widespreadly used in commodity including food packing, toys, water pipes, wire cables and medical devices [4-6]. However, one of the main drawbacks of PVC is that it is easy to decompose at temperatures below the processing temperature, resulting in serious discoloration and damage of some performance [7]. As pyrolysis process of PVC takes place when being heated over 110 °C, hydrogen chloride (HCl) will escape from PVC and accelerate the pyrolysis process of PVC in return [8-10]. To improve the thermal stability and insure the essential properties of PVC, thermal stabilizer needs to be added into the PVC matrix [11]. According to the structure characteristics, heat stabilizer can be divided into lead salt, organotin, organoantimony, rare earth, metal soap, hydrotalcite and so on [12-14]. In recent years, a great deal of attention is drawn to exploit that the thermal stabilizers are non-toxic, environmentally friendly, economically interesting and high efficient due to the increasing awareness of environmental protection [15].
Layered double hydroxides (LDHs), also called hydrotalcite-like compounds, are known as synthetic anionic clays with a brucite-like layered structure [16]. Its general molecular formula is expressed as [M(II)1-xM(III)x(OH)2]x+(An-)x/n·mH2O, in which An- represents an interlayer anion while M(II) and M(III) are the divalent and trivalent metal ions, respectively [17]. Recently, LDHs have been widely employed as catalysts [18, 19], drug delivery materials [20, 21], flame retardant [22], battery active materials [23–26], rubber filler [27], hydrogel [28], chemically tailored functional material [29] and so on, which is mainly attributed to their special lamellar structure. In particular, LDHs are extensively used as a new type of inorganic thermal stabilizer due to their low price, non-poisonous and environmentally friendly properties [30]. During the thermal degradation process of PVC, the LDHs with alkalescency and layered structure can efficiently react with and absorb the HCl gas to improve the thermal stability of PVC resin. Since Kyowa Chemical Industries of Japan [31] first demonstrated that MgAl-CO3-LDHs can improve the heat stability of PVC and VAN DER VEN et al [32] further investigated the stabilization mechanism, extensive works have been performed to study the MgAl-CO3-LDHs and their derivatives as thermal stabilizer for PVC resin. LIN et al [33] found that the MgAl-CO3-LDH with the molar ratio of n(Mg)/n(Al)=2 containing the highest quantity of interlayer CO32- ions exhibits the best effect to enhance the thermal stability of PVC compared with that of other Mg/Al molar ratios, revealing that the reaction of CO32- ions and HCl gas is very important to stabilize the thermal degradation of PVC. LIU et al [34] reported that the nanoscale LDHs dispersed in PVC matrix can more easily react with HCl gas and thus reduce its autocatalytic effection to delay the thermal degradation process of PVC. WANG et al [35] found that the introduction of Zn element into MgAl-CO3-LDHs can facilitate eliminating HCl gas generated during the pyrolysis process of PVC and enhance the heat stability of PVC [35]. ZHANG et al [36] synthesized the MgAl-BP-LDHs compound where the BP is 2-hydroxy-4- methoxybenzophenone-5-sulfonic acid anion by an ion-exchange method, which can effectively absorb the released HCl gas to increase the thermal stability time of PVC resin. LABUSCHAGHE et al [37] discussed the effect of MgAl-CO3-LDH and its derivatives such as MgFeAl-CO3-LDH, MgCuAl- CO3-LDH, MgZnAl-CO3-LDH on the thermal stability of PVC respectively, showing that the conventional MgAl-CO3-LDH achieved the best dynamic heat stability and the MgCuAl-CO3-LDH dramatically restrained the release of volatile HCl gas, while the MgZnAl-CO3-LDH exhibited better color retention. LIU et al [38] prepared a novel MgAl-SbS3-LDH nanocomposite applied as thermal stabilizers for PVC, showing some prominent superiority in enhancing transparency, high temperature resistance, and long-term stability. ZHANG et al [39] reported that PVC/MgAl- CO(NH2)2-LDH composites have improved the thermal stability of PVC obviously due to a fact that it can effectively increase the temperatures for the initiate decomposition and at the maximum degradation rate, as well as the residue yields during the thermal degradation in nitrogen. In addition, our group had revealed that the CaAl-CO3-LDHs can promote the thermal stability of PVC markedly. WEN et al [40] successfully synthesized the CaAl-CO3-LDHs by a green synthesis route with zero discharge, which exhibits favorable long-term stabilization effect for PVC. YAN et al [41] reported that the CaAl-HPO3-LDH can improve the thermal stability of PVC resin in the field of both short-term and long-term stability compared with CaAl-CO3-LDHs, which was ascribed to a fact that these allyl chloride and conjugated double bonds in PVC can be eliminated by the interlayer phosphite in LDHs. YANG et al [42] demonstrated that the reformative CaAl-CO3- LDHs with sodium stearate had been endowed with a good compatibility with PVC and exhibited better thermal stability compared with that of unmodified CaAl-CO3-LDHs.
In our previous work, it was reported that the heat stability of PVC with CaAl-CO3-LDHs is superior to that with MgAl-CO3-LDHs, which is attributed that the Ca2+ is more easily combined with Cl- than the Mg2+ [40]. However, the CaAl-CO3-LDHs used as thermal stabilizer alone for PVC would lead to the initial coloring and the growth of ‘polyolefin structure’ taking place due to a fact that it can not react with the labile chlorine atoms (such as allyl chloride) in the PVC. On the contrary, the ZnAl-CO3-LDH can react with allyl chloride to suppress the initial coloring and the growth of polyolefin structure [43]. Therefore, the zinc element (Zn) was doped into the CaAl-CO3- LDH to synthesize CaZnAl-CO3-LDH served as thermal stabilizer, which can meet both initial thermal stability and long-term stability effect of PVC composites. Meanwhile, the thermal stabilizer containing excessive zinc element will result in the phenomenon of zinc burning, resulting in PVC samples turn black quickly [44, 45]. In previous report, calcium stearate (CaSt2), also known as a metal soap, is generally employed as an auxiliary thermal stabilizer in conjunction with other thermal stabilizers due to the poor thermal stability when metal soaps are used alone [46, 47]. It was reported that CaSt2 can inhibit zinc burning that was caused by zinc stearate (ZnSt2), indicating that CaSt2 and ZnSt2 show a good synergistic effect [48]. So, we think that CaZnAl-CO3-LDH incorporated with CaSt2 would generate synergistic effects on the heat stability of PVC resin.
In this work, CaZnAl-CO3-LDHs with various Ca/Zn/Al molar ratios were synthesized using hydrothermal method, and their microstructures were analyzed by X-ray diffraction (XRD), FT-IR spectrometer and scanning electron microscope (SEM). According to the results of static thermal aging test, mass loss test and Congo red test, the optimum Ca/Zn/Al molar ratio and additive amount of CaZnAl-CO3-LDHs employed as the thermal stability for PVC resin were obtained. After that, the synergistic effects of CaZnAl-CO3-LDHs and CaSt2 on the thermal stability of PVC were discussed in detail.
2 Experimental
2.1 Materials
The PVC resin (SG-5 type, suspension grade) was supplied by the chemical plant of Zhuzhou, China. The calcium nitrate (Ca(NO3)2), zinc nitrate hexahydrate (Zn(NO3)2·6H2O), aluminum nitrate nonahydrate (Al(NO3)3·9H2O), sodium hydroxide (NaOH) and sodium carbonate (Na2CO3) were all of analytical reagent (A.R.) grade and were purchased from Guang Fu Fine Chemical Institute, Tianjin, China. The dioctyl phthalate (DOP) was of chemical pure (C.P.) grade and was purchased from Sinopharm Chemical Reagent Co. Ltd., Shanghai, China. The calcium stearate was of industrial grade and was kindly supplied by Jiangxi Hongyuan Chemical Co. Ltd., Yichun, China. The carbon dioxide (CO2) in the de-ionized water was eliminated by distilling twice.
2.2 Synthesis of CaZnAl-CO3-LDHs
A salt solution containing zinc nitrate, calcium nitrate, and aluminum nitrate (AR, Tianjin City Guang Fu Fine Chemical Institute) and another alkaline solution containing NaOH (2 mol/L) and Na2CO3 (0.5 mol/L) (AR, Tianjin City Guang Fu Fine Chemical Institute) were simultaneously dripped into a beaker with a small amount of deionized water at a rate of one drop per second. The total metal ion concentration of the added salt solution maintained at 1.0 mol/L and the various Ca/Zn/Al molar ratios were 4:0:2, 3.9:0.1:2, 3.6:0.4:2, 3.4:0.6:2 and 3.2:0.8:2, respectively. Meantime, the obtained mixed solution was under vigorous stirring at 65°C for 30 min and the reaction pH was controlled at a constant value of 10. Afterward, the resultant mixture was transferred into a Teflon-lined autoclave and heated at 120°C for 24 h. After being cooled to room temperature, the gained product was filtered, washed for three times with deionized water and ethyl alcohol, dried at 60 °C for 24 h followed by grinding to fine powder.
2.3 Preparation of PVC-LDHs composites
Firstly, a certain amount of PVC, dioctyl phthalate (DOP), CaZnAl-CO3-LDHs and CaSt2 with different ratios were thoroughly mixed in a blender. Then the mixture was milled in a double-roller machine at a temperature of 180 °C for 5 min. At last, the obtained thin slice of PVC-LDHs composites was cut up into 2 cm×2 cm strips with a thickness of about 1.0 mm [49].
2.4 Characterizations of CaZnAl-CO3-LDHs
The structure of as-prepared CaZnAl-CO3- LDHs was analyzed by XRD (Siemens D-500) using Cu Kα radiation with a scanning rate of 2θ= 8°/min at 36 kV and 30 mA. The FT-IR spectra (KBr pellets) were performed on a Nicolet Nexus 670 FT-IR spectrometer over a wave-number region from 4000 to 400 cm-1. A scanning electron microscope (JSM-6360LV) was employed to obtain the SEM images of the as-prepared samples. A laser particle size analyzer (Malvern 2000) was used to study the particle size distribution of the samples.
2.5 Procedure for thermal stability testing
2.5.1 Static thermal aging test
In a typical procedure of the static thermal aging test, the sample strips (2 cm×2 cm×1.0 mm) of PVC-LDHs composites obtained by a double-roller mixer were placed in a thermal aging test box at 180 °C. Afterward, a digital camera was used to record the color changes of these strips being taken out of the box every ten min. The thermal stability of PVC resin can be evaluated by the color changes of the sample strips.
2.5.2 Congo red test
The Congo red test was carried out according to ISO standard 182/1-1990 (E) [40]. A test tube immersed into an oil bath was loaded with some fragments of as-prepared PVC composites and the Congo red test paper was located at 2 cm above the sample. Then, the tube was heated at 195°C to record the color change of Congo red test paper. The thermal stability time was obtained when the color of Congo red test paper changed from red to blue. In order to ensure the accuracy of test results, each sample was tested three times and the average time was gained.
2.5.3 UV-VIS spectroscopy test
The UV-VIS spectra were collected on a UV-2600PC UV-VIS spectrometer (Shimadzu Scientific Instruments, Kyoto, Japan) with the wavelength from 200 to 400 nm, and the slit width was set as 2 nm. The PVC samples were first dissolved by tetrahydrofuran (THF), and the supernatant was detected using UV-VIS spectrometer [50].
3 Result and discussion
3.1 Characterization of CaZnAl-CO3-LDHs
3.1.1 X-ray diffraction analysis of CaZnAl-CO3- LDHs
Figure 1 shows the XRD patterns of CaZnAl- CO3-LDHs with various molar ratios of Ca/Zn/Al. As shown in Figure 1, the XRD baselines of all specimens are relatively stable and the diffraction peaks are narrow, sharp and symmetrical, indicating that the specimens are well-crystallized. The strong and sharp diffraction peaks of CaZnAl-CO3-LDHs at 2θ=11.67°, 23.391°, 29.96°, 36.12°, 38.3° are corresponding to the (001), (002), (110), (003) and (113) planes in the standard XRD pattern (JCPDS No. 41-0219) of hydrocalumite with hydrotalcite- like layered structure and rhombohedral symmetry (space group R3m). The highest diffraction peak is present at 2θ=11.67°, indicating that the CaZnAl- CO3-LDHs with a typical hexagonal crystal structure are highly crystallized. These peaks intensities of CaZnAl-CO3-LDHs are close to those of CaAl-CO3-LDHs, implying that calcium atoms in lattice can be superseded by zinc atoms availably and these bonds are not destroyed. As observed in Figure 1, the emergence of some additional weak peaks of samples d and e compared with CaAl-CO3-LDHs (sample a) manifests that the excessive zinc additive would result in the disproportion of CaAl-CO3-LDHs lattice. The diffraction peak of sample c is sharper than that of sample b, demonstrating that CaZnAl-CO3-LDHs with Ca/Zn/Al=3.6:0.4:2 can optimally maintain CaAl-CO3-LDHs structure due to appropriate amount of calcium atoms replaced effectively.
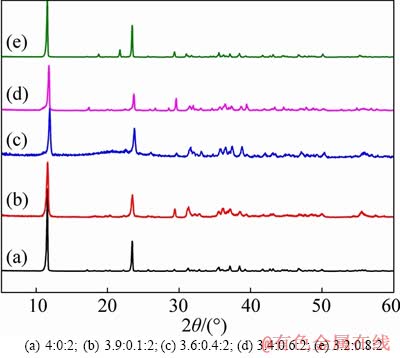
Figure 1 XRD patterns of CaZnAl-CO3-LDHs with different Ca/Zn/Al molar ratios:
3.1.2 Fourier transform infrared spectroscopy analysis of CaZnAl-CO3-LDHs
The typical FT-IR spectra of CaZnAl-CO3- LDHs with four different molar ratios of Ca/Zn/Al and CaAl-CO3-LDHs are depicted in Figure 2. As shown in Figure 2(a), the FT-IR spectra of four samples corresponding different ratios of Ca/Zn/Al are similar. A broad absorption peak round 3546 cm-1 can be assigned to the stretching frequency of OH clusters attached to metal ions in the layers of LDHs, which moved in a lower direction mildly in comparison with the free OH group at about 3640 cm-1, implying that the OH clusters of LDHs are in an associating state. The obvious absorption peaks around 1425 cm-1 belong to the antisymmetric stretching vibrations modes of interlayer anion CO32-. As observed carefully from the Figure 2(a), it is found a smaller absorption peak at about 1500 cm-1 on account of the asymmetric vibration of O—C—O in carbonate. Several absorption peaks below 800 cm-1 should belong to the stretching vibration patterns between the metal cation and oxygen atom such as Ca—O, Zn—O, Al—O. Furthermore, the FT-IR spectra of CaZnAl-CO3-LDHs (Figure 2(a)) are basically the same as that of CaAl-CO3-LDHs (Figure 2(b)), demonstrating that Zn element had been introduced into the lattice of hydrocalumite and the Ca-Zn-Al ternary hydrotalcite has been synthesized successfully, which is in agreement with XRD pattern.
3.1.3 Morphology and EDS analysis of CaZnAl- CO3-LDHs
The morphology and composition of the synthesized CaZnAl-CO3-LDHs were studied by SEM and EDS. As shown in Figure 3, all of the CaZnAl-CO3-LDHs with various molar ratios of Ca/Zn/Al show the lamellar shape, and some of them present a regular hexagonal shape, which is consist with the morphology of hydrotalcite-like materials reported previously. The result indicates that suitable addition of Zn element should not break the basic construction of LDHs, which is in accordance with the XRD spectra. These samples are approximately 80 nm thick with an average size of 500-600 nm, indicating that the obtained CaZnAl-CO3-LDHs are of nano-scale material.
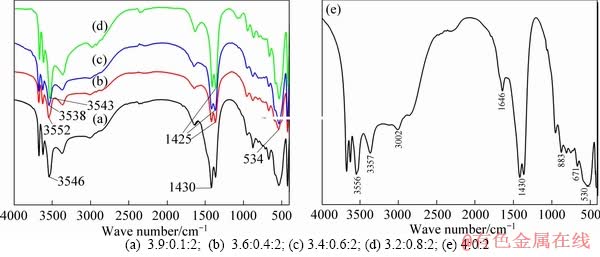
Figure 2 FT-IR spectra of CaZnAl-CO3-LDHs with different Ca/Zn/Al molar ratios:

Figure 3 Typical SEM images of CaZnAl-CO3-LDHs with different Ca/Zn/Al molar ratios:
Figure 4 displays the EDS spectra of CaZnAl-CO3-LDHs synthesized with various molar ratios of Ca/Zn/Al. As seen in Figure 4, the proportions of Ca, Zn and Al in the four samples prepared are close to 3.9:0.1:2, 3.6:0.4:2, 3.4:0.6:2 and 3.2:0.8:2, respectively. The results further prove that the Ca-Zn-Al ternary hydrotalcite has been synthesized successfully.
According to previous reports, the possible growth mechanism of the CaZnAl-CO3-LDHs films is proposed. The chemical reaction equations (e.g. Ca/Zn/Al=3.6:0.4:2) can be expressed as follows [51]:
3.6Ca2++0.4Zn2++2Al3++12OH-+CO32-+mH2O Ca3.6Zn0.4Al2(OH)12CO3·mH2O (1)
Ca3.6Zn0.4Al2(OH)12CO3·mH2O (1)
Firstly, under alkaline condition, metal cations (M2+ and M3+) and OH- complexes form M(OH)6 with octahedral structure, which constitute two- dimensional sheets by sharing edge and can be stacked together by hydrogen bonds between the hydroxyl groups of adjacent sheets. Then, the two- dimensional sheets and anions CO32- complexes form CaZnAl-CO3-LDHs crystal nuclei. Finally, according to the evolution selection growth mechanism, the nucleus grows into hexagonal crystal structure along plane (001) that is the preferential growth direction due to its own crystal structure features. As a result, the hexagon layer structure of the LDH is obtained on the basis of the brucite composed of edge sharing M(OH)6 octahedra. Furthermore, the intercalated anions CO32- is to balance the positive charge, which is ascribed to that partial M2+ cations are replaced by M3+ anions on the host layers. Therefore, the sheets can firmly attach to the anions and hydrone located at the interlayer via the electrostatic forces,hydrogen bonds and van der Waals forces.
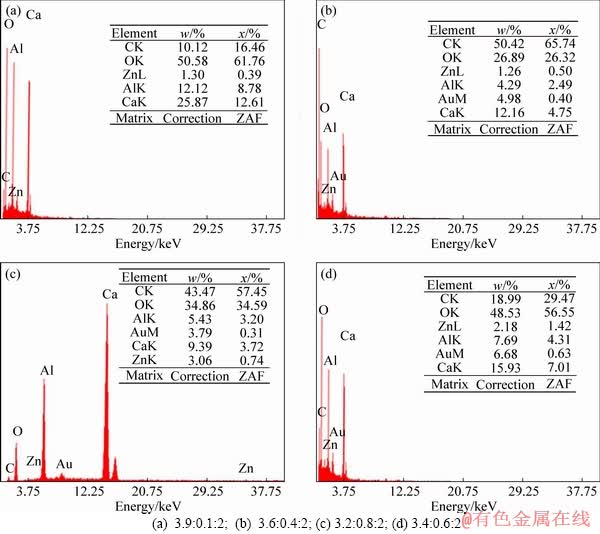
Figure 4 EDX analysis of CaZnAl-CO3-LDHs with different Ca/Zn/Al molar ratios:
3.1.4 Particle granularity analysis of CaZnAl-CO3- LDHs
The particle size distribution curve of CaZnAl- CO3-LDHs (Ca/Zn/Al as 3.6:0.4:2) is shown in Figure 5. It can be seen that there is only one relatively large peak with a normal distribution and the average particle size is 560 nm, which demonstrates that particle size distribution of the as-synthesized sample is very uniform and in good agreement with the result of SEM discussed above.
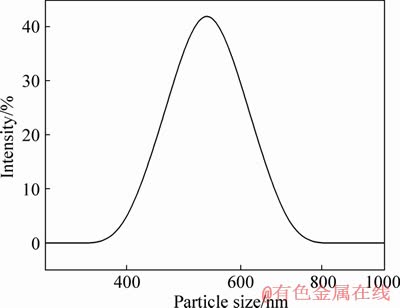
Figure 5 Particle size distribution of CaZnAl-CO3-LDHs with n(Ca):n(Zn):n(Al)=3.6:0.4:2
3.2 Effect of CaZnAl-CO3-LDHs with different Ca/Zn/Al molar ratio on PVC stability
The mixtures including 100 phr PVC, 70 phr DOP, and 3 phr CaZnAl-CO3-LDHs with different molar ratios of Ca/Zn/Al were served to investigate the PVC stability through the static thermal aging test. The thermal stability of the thermal stabilizers on PVC can be reflected by the color changes of PVC samples. As seen in Figure 6, the PVC samples without addition of any stabilizer turned black after 30 min under 180 °C. As is well known, the PVC undergo the destructive reactions such as autocatalytic dehydrochlorination and the HCl- catalytic under the impact of temperatures and energy radiation, producing multiple sequences of conjugated double bonds. Moreover, the discoloration of PVC will take place when the conjugated polyene sequences contain more than 6 double bonds due to it is a chromophore. These two processes of PVC degradation can be described as follows:
1) PVC autocatalytic dehydrochlorination reaction:
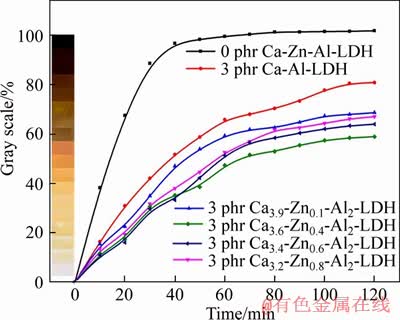
Figure 6 Thermal stability results of PVC samples with different CaZnAl-CO3-LDHs at 180°C
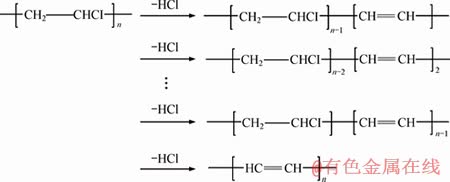 (2)
(2)
2) HCl catalytic reaction:
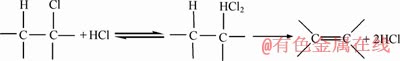 (3)
(3)
The main reason for hydrotalcite used as PVC thermal stabilizer is that hydrotalcite can effectively react with hydrogen chloride gas in the process of PVC thermal dehydrochlorination and inhibit the autocatalytic degradation of PVC. The overall reaction of hydrotalcite and hydrogen chloride gas can be expressed below:
Ca3.6Zn0.4Al2(OH)12CO3·5H2O+14HCl→3.6CaCl2+0.4ZnCl2+2AlCl3+CO2+18H2O (4)
As shown in Figure 6, the PVC samples with stabilizers don’t turn black in 120 min at 180 °C. Furthermore, the heat stability of the four samples with CaZnAl-CO3-LDHs is superior to that of the sample with CaAl-CO3-LDHs. It is attributed to the introduction of zinc element which can react with allyl chloride to suppress the early coloring and hamper the increase of polyolefin structure. In CaZnAl-CO3-LDHs, the presence of Zn can improve the early-stability of PVC samples, while the existence of Ca can enhance the long-term heat stability. Thus, the Ca and Zn have a synergistic effect on improving the both initial and long-term heat stability of PVC. The CaZnAl-CO3-LDHs synthesized by combining calcium and zinc will show better effect on the heat stability of PVC resin. From Figure 6, as the zinc content increases, the thermal stability of the corresponding PVC samples also gradually increases in the first 50 min of the test. However, as the test time increases, the heat stability of PVC samples with the highest zinc content of the hydrotalcite (Ca3.2Zn0.8Al2-LDHs) has actually declined in contrast with that of PVC samples with Ca3.6Zn0.4Al2-LDHs. This could be explained that the ZnCl2 as a strong Lewis acid derived from the reaction between CaZnAl-CO3- LDHs and HCl is deemed as a highly efficient catalyst for the dehydrochlorination reaction of PVC, while exceeding ZnCl2 in system will result in the pernicious degradation of PVC and becoming black suddenly as named “zinc burning”. It is obtained that the molar ratios of Ca/Zn/Al on the heat stability of PVC should have a suitable range, because the low amount of zinc in Ca3.9Zn0.1Al2- CO3-LDHs isn’t enough to increase the initial- stability of PVC, while the high amount of zinc in Ca3.2Zn0.8Al2-CO3-LDHs will accelerate the PVC degradation. According to the test results, PVC sample with Ca3.6Zn0.4Al2-CO3-LDHs has excellent thermal stability among the all test samples. For synthesizing CaZnAl-CO3-LDHs on the PVC stability, the optimum molar ratios of Ca/Zn/Al should be 3.6:0.4:2.
The UV-VIS spectroscopy test was carried out to investigate the dehydrochlorination reaction of PVC samples, which would form conjugated double bonds (Cdb). The UV-VIS absorption wavelength corresponds to the conjugated chain length of PVC degradation products, and the height of absorption peak reflects the concentration of Cdb. Figure 7 shows the UV-VIS spectra of pure PVC and PVC stabilized by 3 phr of CaZnAl-CO3-LDHs with different molar ratios of Ca/Zn/Al, which were heated at 180 °C for 0 min and 60 min, respectively. As seen in Figure 7(a), the main absorption wavelengths of all of the five samples are around 240 nm, which corresponds to the Cdb with chain length of 3. Pure PVC presents the highest concentration of Cdb, indicating that CaZnAl-CO3-LDHs are effective to improve the thermal stability of PVC samples. In addition, as the increases of zinc content in the molar ratios of Ca/Zn/Al, the peak height of corresponding PVC sample decreases, showing the lowest Cdb generated in the PVC sample with Ca3.2Zn0.8Al2-LDHs. It can be ascribed to a fact that the zinc element is effective in improving the initial color of PVC sample. After having been heated for 60 min, the Cdb of all PVC samples in Figure 7(b) increases at different degrees. In particular, the increment of Cdb in pure PVC sample is the largest, indicating that pure PVC without stabilizer suffered from a severe thermal degradation. However, the PVC sample with Ca3.6Zn0.4Al2-LDHs has the lowest Cdb among PVC samples. It indicates that the degree of thermal degradation of PVC sample with excessive zinc contents increases greatly, due to a fact that the ZnCl2 produced from the reaction between zinc ions and HCl would catalyze the PVC degradation and generate more Cdb. The results are in accord with the results of the static thermal aging tests in Figure 6.
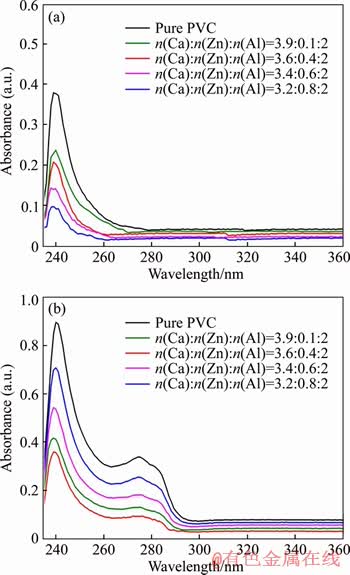
Figure 7 UV-VIS spectra of pure PVC and PVC stabilized with different CaZnAl-CO3-LDHs heated at 180 °C for 0 min (a) and 60 min (b)
3.3 Effect of different contents of CaZnAl-CO3- LDHs on PVC stability
For getting the optimum amount of CaZnAl- CO3-LDHs added to PVC samples, the effects of different contents of CaZnAl-CO3-LDHs on the heat stability of PVC were investigated. 100 phr PVC, 70 phr DOP and various amounts of CaZnAl-CO3-LDHs (2, 3, 4, 5, 6 and 7 phr) with the optimum molar ratio of n(Ca):n(Zn):n(Al)= 3.6:0.4:2 were mixed, respectively. The heat stability of these obtained PVC samples is shown in Figure 8. In the first 40 min of the test, it is found from Figure 8 that the curve of one PVC sample with higher content stabilizer will have more gentle slope, indicating the initial-stability of the corresponding PVC samples gradually increased as the increase of CaZnAl-CO3-LDHs content. Nevertheless, the PVC samples with 6 and 7 phr CaZnAl-CO3-LDHs have suffered from an acute coloring between 40 and 70 min in the test, blacking obviously at 110 min. On the contrary, the PVC samples with lower content stabilizer from 2 to 5 phr don’t show this phenomenon above, which named the “zinc burning” as adding excess CaZnAl-CO3-LDHs, resulting in the excessive generation of ZnCl2 which can accelerate the dehydrochlorination and weaken the long-term heat stability of PVC samples. From Figure 8, the thermal stability of PVC samples is gradually improved in the entire test process with the contents of CaZnAl-CO3-LDHs increased from 2 to 5 phr. The PVC sample especially with 5 phr CaZnAl- CO3-LDHs exhibits the best thermal stability, retaining slight yellow after 150 min. Therefore, the optimum content of CaZnAl-CO3- LDHs on the heat stability of PVC is determined to be 5 phr.
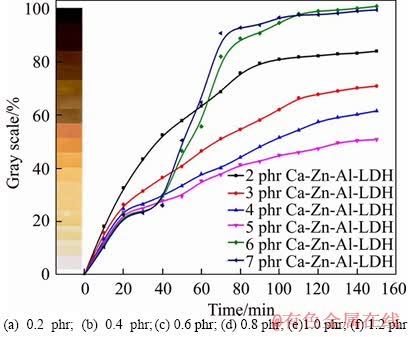
Figure 8 Effect of different contents of Ca3.6Zn0.4Al2- CO3-LDHs on thermal stability of PVC resin at 180 °C
The Congo red test was carried out to further investigate the thermal stability of PVC samples. As seen in Figure 9, the thermal stabilization time is extended as the amounts of CaZnAl-CO3-LDHs increase from 2 to 5 phr, indicating that the heat stability of PVC samples is enhanced gradually. However, the thermal stabilization time of PVC samples with 6 and 7 phr CaZnAl-CO3-LDHs is only 83 and 76 min, respectively. Obviously, the heat stabilization time of PVC samples with 6 and 7 phr CaZnAl-CO3-LDHs is much shorter than that of PVC samples with 2 to 5 phr CaZnAl-CO3-LDHs, revealing that further increment of CaZnAl-CO3- LDHs in PVC samples weakens the heat stability instead. This is because the Congo red test reflects the capacity of thermal stabilizers to eliminate HCl, which is released from the degradation of the PVC samples. The faster the Congo red test strip discolors, the shorter the thermal stabilization time of the corresponding PVC samples is. The results above are attributed to exceeding Zn contents in corresponding PVC samples, resulting in the acceleration of the PVC thermal degradation and more released HCl, which is consistent with the experimental results of static thermal aging test.
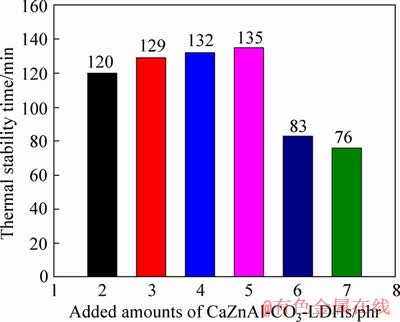
Figure 9 Thermal stability time of PVC samples stabilized by Ca3.6Zn0.4Al2-CO3-LDHs at different contents detected with Congo red test
3.4 Synergetic thermal stabilizing effect of CaZnAl-CO3-LDHs with calcium stearate
On the basis of the above test, the appropriate Zn contents in the CaZnAl-CO3-LDHs can significantly improve the thermal stability of PVC samples compared with CaAl-CO3-LDHs. However, the excessive Zn contents in thermal stabilizers will cause the phenomenon of “zinc burning” for PVC samples. From Figure 8, the additive amount of CaZnAl-CO3-LDHs reaching 6 phr has improved significantly the initial-stability, but also the phenomenon of zinc burning takes place. In order to overcome this problem, CaSt2 was incorporated with CaZnAl-CO3-LDHs to inhibit the zinc burning and enhance the long-term stability of PVC samples. The synergetic effects between CaSt2 and CaZnAl-CO3-LDHs on the heat stability of PVC samples were investigated to gain the optimum additive amounts of CaZnAl-CO3-LDHs and CaSt2. 100 phr PVC, 70 phr DOP, 6 phr CaZnAl-CO3- LDHs (molar ratio of n(Ca):n(Zn):n(Al)=3.6:0.4:2) and various amounts of CaSt2 (0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 phr) were mixed, respectively. The heat stability of these corresponding PVC samples is illustrated in Figure 10, which displays that PVC samples containing CaZnAl-CO3-LDHs (6 phr) and CaSt2 did not show signs of zinc burning during the test process, indicating that CaSt2 combined with CaZnAl-CO3-LDHs is beneficial to inhibiting the degradation of PVC samples. It is ascribed to the significantly synergetic effects between CaSt2 and CaZnAl-CO3-LDHs on the heat stability of PVC samples. During the heat degradation process of PVC samples, ZnCl2 as a strong Lewis acid is generated by Eq. (6). Then, the reaction equation of ZnCl2 with CaSt2 can be represented as following:
ZnCl2+Ca(C17H35COO)2→CaCl2+Zn(C17H35COO)2(5)
According to Eq. (7), ZnCl2 is eliminated to prevent the phenomenon of zinc burning from happening, while CaCl2 production doesn’t promote sudden dehydrochlorination and ZnSt2 production is a excellent thermal stabilizer for PVC samples. From Figure 10, as the increase of CaSt2 content in the system from 0.2 to 1.0 phr, the color of the corresponding sample is lighter at the same test time. However, as the content of CaSt2 reached 1.2 phr, the color of the corresponding PVC sample is darker than that of PVC sample with 1.0 phr CaSt2 at the same test time, indicating that the heat stability of PVC sample with 1.0 phr CaSt2 is superior to that of PVC sample with 1.2 phr CaSt2. This can be explained that the content of CaZnAl- CO3-LDHs added in these samples is constant, resulting in the fact that the content of ZnCl2 generated by the reaction between CaZnAl-CO3- LDHs and HCl is also certain. Furthermore, CaSt2 doesn’t play a strong role in absorbing hydrogen chloride gas derived from the pyrolysis of PVC samples when used alone, while combined with zinc-containing thermal stabilizer will improve significantly thermal stability for PVC samples. Therefore, excessive CaSt2 incorporated with a certain amount of CaZnAl-CO3-LDHs can not further increase the heat stability of PVC samples. Furthermore, the phenomenon of segregation and scaling will appear on PVC samples due to the adding of excessive CaSt2. Moreover, compared Figure 10 with Figure 8, the color of the PVC sample with CaZnAl-CO3-LDHs (6 phr) incorporated with CaSt2 (1.0 phr) is obviously lighter than that of PVC sample with CaZnAl- CO3-LDHs (5 phr) at the same test time, indicating that CaZnAl-CO3-LDHs incorporated with CaSt2 show better thermal stability than CaZnAl-CO3- LDHs employed alone.
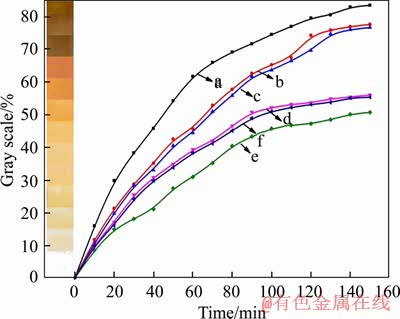
Figure 10 Thermal stability of PVC samples with Ca3.6Zn0.4Al2-CO3-LDHs (6 phr) incorporated with different amounts of CaSt2:
Figure 11 shows UV-VIS spectra of PVC samples with Ca3.6Zn0.4Al2-CO3-LDHs (6 phr) incorporated with different amounts of CaSt2, which were heated at 180 °C for 0 min and 60 min, respectively. As seen in Figure 11(a), the main absorption wavelengths of all of the six samples are around 240 nm, which corresponds to the Cdb with chain length of 3. It is noted that the Cdb of PVC samples decreases with the CaSt2 content in the system increasing from 0.2 to 1.0 phr, demonstrating that Ca3.6Zn0.4Al2-CO3-LDHs and CaSt2 have a good synergetic effect in enhancing the thermal stability of PVC samples. However, when the dosage of CaSt2 is increased to 1.2 phr, the Cdb of corresponding PVC sample increases greatly. Therefore, the lowest Cdb in PVC sample with 6 phr Ca3.6Zn0.4Al2-CO3-LDHs and 1.0 phr CaSt2 indicates that this component had the best synergistic effect on PVC thermal stability. After being heated for 60 min, the Cdb of all PVC samples in Figure 11(b) increases at different degrees. In particular, the increment of Cdb in PVC sample with 6 phr Ca3.6Zn0.4Al2-CO3-LDHs and 0.2 phr CaSt2 is the largest, indicating that it suffered from a severe thermal degradation. It results from the excessive zinc content in the stabilizer, which would generate more ZnCl2 to accelerate the dehydrochlorination of PVC sample. The Cdb of the PVC sample stabilized with 6 phr Ca3.6Zn0.4Al2-CO3-LDHs and 1.0 phr CaSt2 is the lowest one of the six samples, which is in agreement with the results of the static thermal aging tests in Figure 10. It can be concluded that Ca3.6Zn0.4Al2-CO3-LDHs combined with CaSt2 exhibit significantly synergetic effects in enhancing the thermal stability of PVC samples, and the optimum additive amounts of Ca3.6Zn0.4Al2-CO3- LDHs and CaSt2 are 6 and 1.0 phr respectively.
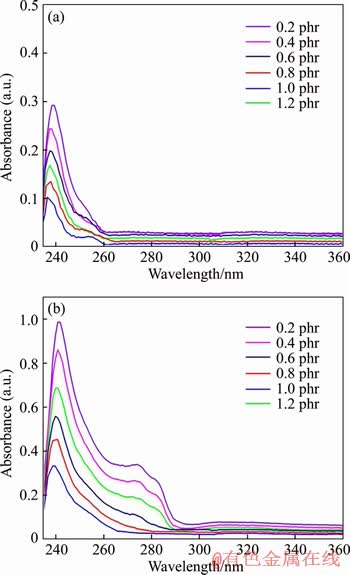
Figure 11 UV-VIS spectrum of PVC samples with Ca3.6Zn0.4Al2-CO3-LDHs (6 phr) incorporated with different amounts of CaSt2 heated at 180 °C for (a) 0 min and (b) 60 min
4 Conclusions
In this work, CaZnAl-CO3-LDHs were successfully synthesized and served as thermal stabilizers for PVC resin. The CaZnAl-CO3-LDHs showed better thermal stability compared with CaAl-CO3-LDHs, due to a fact that the calcium and zinc have synergistic effects on improving the heat stability of PVC in both initial and long term. The synthesized CaZnAl-CO3-LDHs with the Ca/Zn/Al molar ratio of 3.6:0.4:2 show the optimum thermal stabilization for PVC resin. When Ca3.6Zn0.4Al2- CO3-LDHs used alone, the initial- stability of the corresponding PVC samples gradually increased as the increase of additive amounts. However, the additive amount of Ca3.6Zn0.4Al2-CO3-LDHs exceeding 6 phr will result in zinc burning for PVC resin. So, the optimum additive amount is 5 phr when Ca3.6Zn0.4Al2-CO3- LDHs is used alone. As Ca3.6Zn0.4Al2-CO3-LDHs incorporated with CaSt2, it can effectively inhibit the phenomenon of zinc burning and exhibit significantly synergetic effects in improving the heat stability of PVC samples, showing better thermal stability compared with Ca3.6Zn0.4Al2-CO3- LDHs used alone. The testing results display that the optimum additive amounts of Ca3.6Zn0.4Al2- CO3-LDHs and CaSt2 are 6 phr and 1.0 phr respectively.
References
[1] MORENO M, LLIGADAS G, RONDA J C, GALIA M, CADIZ V. Flame retardant high oleic sunflower oil-based thermosetting resins through aza- and phospha-michael additions [J]. Journal of Polymer Science Part A: Polymer Chemistry, 2013, 51(8): 1808-1815.
[2] CHEN Guang-ming. Preparation of a poly (vinyl chloride)/ layered double hydroxide nanocomposite with a reduced heavy-metal thermal stabilizer [J]. Journal of Applied Polymer Science, 2007, 106(2): 817-820.
[3] LIU Jun, CHEN Guang-ming, YANG Ji-ping. Preparation and characterization of poly (vinyl chloride)/layered double hydroxide nanocomposites with enhanced thermal stability [J]. Polymer, 2008, 49(18): 3923-3927.
[4] ESPINOSA L M D, GEVERS A, WOLDT B, GRAβ M, MEIER M A R. Sulfur-containing fatty acid-based plasticizers via thiolene addition and oxidation: Synthesis and evaluation in PVC formulations [J]. Green Chemistry, 2014, 16(4): 1883-1896.
[5] SILVA M D, VIEIRA M G A, MACUMOTO A C G. Polyvinylchloride (PVC) and natural rubber films plasticized with a natural polymeric plasticizer obtained through polyesterification of rice fatty acid [J]. Polymer Testing, 2011, 30(5): 478-484.
[6] SAEKI Y, EMURA T. Technical progress for PVC production[J]. Progress in Polymer Science, 2002, 27(10): 2055-2131.
[7] MOHAMED N A, YASSIN A A, KHALIL K D, SABAA M W. Organic thermal stabilizers for rigid poly (vinyl chloride) I. Barbituric and thiobarbituric acids [J]. Polymer Degradation and Stability, 2000, 70(1): 5-10.
[8] STATHEROPOULOS M, KAKALI G. A parallel study of PVC degradation and stabilization by Ba/Cd stearates using differential scanning calorimetry and mass spectrometry [J]. European Polymer Journal, 1989, 25(4): 405-409.
[9] BUDDHIRANON S, CHANG Teng, TANG Kang-li, KYU T. Stabilization of epoxidized soybean oil-plasticized poly (vinyl chloride) blends via thermal curing with genistein [J]. Journal of Applied Polymer Science, 2018, 135(31): 46472.
[10] WANG Chao, LI Na, HUO Li, GAO Jun-gang. Effect of carbon nanotube on the mechanical, plasticizing behavior and thermal stability of PVC/poly (acrylonitrile-styrene- acrylate) nanocomposites [J]. Polymer Bulletin, 2015, 72(8): 1849-1861.
[11] MURPHY J. Heat stabilizers [J]. Plastics Additives and Compounding, 1999, 1(4): 24-29.
[12] WANG Ming, XU Jia-you, WU Hong, GUO Shao-yun. Effect of pentaerythritol and organic tin with calcium/zinc stearates on the stabilization of poly(vinyl chloride) [J]. Polymer Degradation and Stability, 2006, 91(9): 2101-2109.
[13] WEN Rui-juan, YANG Zhan-hong, CHEN Hong-yan, HU You-wang, DUAN Ji-an. Zn-Al-La hydrotalcite-like compounds as heating stabilizer in PVC resin [J]. Journal of Rare Earths, 2012, 30(9): 895-902.
[14] YI Shi, YANG Zhan-hong, WANG Sheng-wei, LIU Dong-ren, WANG Su-qin, LIU Qing-yan, CHI Wei-wei. Effects of MgAlCe-CO3 layered double hydroxides on the thermal stability of PVC resin [J]. Journal of Applied Polymer Science, 2011, 119(5): 2620-2626.
[15] LIN Yan-jun, LI Dian-qing, EVANS D G, DUAN Xue. Modulating effect of Mg-Al-CO3 layered double hydroxides on the thermal stability of PVC resin [J]. Polymer Degradation and Stability, 2005, 88(2): 286-293.
[16] MARTIN K J, PINNAVAIA T J. Layered double hydroxides as supported anionic reagents. Halide-ion reactivity in zinc chromium hexahydroxide halide hydrates [Zn2Cr(OH)6X· nH2O](X=Cl, I) [J]. Journal of American Chemical Society, 1986, 108(3): 541-542.
[17] LIU Zhao-ping, MA Ren-zhi, OSADA M, IYI N, EBINA Y, TAKADA K, SASAKI T. Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies [J]. Journal of American Chemical Society, 2006, 128(14): 4872-4880.
[18] IQBAL K, IQBAL A, KIRILLOV A M, WANG Bing-kai, LIU Wei-sheng, TANG Yu. A new Ce-doped MgAl- LDH@Au nanocatalyst for highly efficient reductive degradation of organic contaminants [J]. Journal of Materials Chemistry A, 2017, 5(14): 6716-6724.
[19] WANG Yan-na, DOU Li-guang, ZHANG Hui. Nanosheet array-like palladium-catalysts Pdx/rGO@CoAl-LDH via lattice atomic-confined in situ reduction for highly efficient heck coupling reaction [J]. ACS Applied Materials & Interfaces, 2017, 9(44): 38784-38795.
[20] GAO Yan-shan, TEOH T W, WANG Qiang, WILLIAMS G R. Electrospun organic-inorganic nanohybrids as sustained release drug delivery systems [J]. Journal of Materials Chemistry B, 2017, 5(46): 9165-9174.
[21] YAN Li, ZHOU Meng-jiao, ZHANG Xiu-juan, HUANG Long-biao, CHEN Wei, ROY V A L, ZHANG Wen-jun, CHEN X. A novel type of aqueous dispersible ultrathin- layered double hydroxide nanosheets for in vivo bioimaging and drug delivery [J]. ACS Applied Materials & Interfaces, 2017, 9(39): 34185-34193.
[22] YE Lei, DING Peng, ZHANG Ming, DU Bao-jun. Synergistic effects of exfoliated LDH with some halogen- free flame retardants in LDPE/EVA/HFMH/LDH nanocomposites [J]. Journal of Applied Polymer Science, 2017, 107(6): 3694-3701.
[23] ZENG Xiao, YANG Zhan-hong, LIU Feng-liang, LONG Jun, FENG Zhao-bin, FAN Mao-kui. An in situ recovery method to prepare carboncoated Zn-Al-hydrotalcite as the anode material for nickel-zinc secondary batteries [J]. RSC Advances, 2017, 7(70): 44514-44522.
[24] LONG Jun, YANG Zhan-hong, HUANG Jian-hang, ZENG Xiao. Self-assembly of exfoliated layered double hydroxide and grapheme nanosheets for electrochemical energy storage in zinc/nickel secondary batteries [J]. Journal of Power Sources, 2017, 359: 111-118.
[25] WEN Xing, YANG Zhan-hong, XIAO Xiang, YANG Huan, XIE Xiao-e, HUANG Jian-hang. The impact of hydrocalumites additives on the electrochemical performance of zinc-nickel secondary cells [J]. Electrochimica Acta, 2016, 187: 65-72.
[26] LONG Jun, YANG Zhan-hong, ZENG Xiao, HUANG Jian-hang. A new class of nanocomposites of Zn-Al-Bi layered double oxides: Large reversible capacity and better cycle performance for alkaline secondary batteries [J]. RSC Advances, 2016, 6(95): 92896-92904.
[27] XIAO Suo, FENG Jian-xiang, ZHU Jin, WANG Xi, YI Chun-wang, SU Sheng-pei. Preparation and characterization of lignin-layered double hydroxide/styrene-butadiene rubber composites [J]. Journal of Applied Polymer Science, 2013, 130(2): 1308-1312.
[28] HU Zi-qiao, CHEN Guang-ming. Novel nanocomposite hydrogels consisting of layered double hydroxide with ultrahigh tensibility and hierarchical porous structure at low inorganic content [J]. Advanced Materials, 2014, 26(34): 5950-5956.
[29] XUE Sheng-guo, WU Yu-jun, LI Yi-wei, KONG Xiang-feng, ZHU Feng, WILLIAM Hartley, LI Xiao-fei, YE Yu-zhen. Industrial wastes applications for alkalinity regulation in bauxite residue: A comprehensive review [J]. Journal of Central South University, 2019, 26(2): 268–288. DOI: https://doi.org/10.1007/s11771-019-4000-3.
[30] BAO Yong-zhong, HUANG Zhi-ming, LI Shen-xing, WENG Zhi-xue. Thermal stability, smoke emission and mechanical properties of poly(vinyl chloride)/hydrotalcite nanocomposites [J]. Polymer Degradation and Stability, 2008, 93(2): 448-455.
[31] MIYATA S, KURODA M. NO.4299759, method for inhibiting the thermal or ultraviolet degradation of thermoplastic resin and thermoplastic resin compositions having stability to thermal or ultraviolet degradation: US Patent[P]. 1981.
[32] van DER VEN L, van GEMENT M L M, BATENBURG L F, KEERN J J, GIELGENS L H, KOSTER T P M, FISCHER H R. On the action of hydrotalcitelike clay materials as stabilizers in polyvinylchloride [J]. Applied Clay Science, 2000, 17: 25-34.
[33] LIN Yan-jun, LI Dian-qing, EVANS D G, DUAN Xue. Modulating effect of Mg-Al-CO3 layered double hydroxides on the thermal stability of PVC resin [J]. Polymer Degradation and Stability, 2005, 88: 286-293.
[34] LIU Jun, CHEN Guang-ming, YANG Ji-ping, DING Li-ping. Thermal stability of poly (vinyl chloride)/layered double hydroxide nanocomposites [J]. Journal of Applied Polymer Science, 2010, 116(4): 2058-2064.
[35] WANG Gong-ling, YANG Mei, LI Zhi-wen, LIN Kai-feng, JIN Quan, XING Chao-jian, HU Zhu-dong, WANG Dan. Synthesis and characterization of Zn-doped MgAl-layered double hydroxide nanoparticles as PVC heat stabilizer [J]. Journal of Nanoparticle Research, 2013, 15: 1882.
[36] ZHANG Xiao-fei, ZHOU Liang-chun, PI Hong, GUO Shao-yun, FU Jing-wei. Performance of layered double hydroxides intercalated by a UV stabilizer in accelerated weathering and thermal stabilization of PVC [J]. Polymer Degradation and Stability, 2014, 102: 204-211.
[37] LABUSCHAGNE F J W J, MOLEFE D M, FOCKE W W, WESTHUIZEN I V D, WRIGHT H C, ROYEPPEN M D. Heat stabilising flexible PVC with layered double hydroxide derivatives [J]. Polymer Degradation and Stability, 2015, 113: 46-54.
[38] LIU Shu-ting, ZHANG Ping-ping, YAN Kang-kang, ZHANG Yuan-hu, YE Ying, CHEN Xue-gang. Sb-intercalated layered double hydroxides-poly (vinyl chloride) nanocomposites: Preparation, characterization, and thermal stability [J]. Journal of Applied Polymer Science, 2015, 132(39): 42524. DOI: 10.1002/APP.42524.
[39] ZHANG Hong-mei, ZHANG Shu-hua, STEWART P, ZHU Chen-hui, LIU Wei-jun, HEXEMER A, SCHAIBLE E, WANG Cheng. Thermal stability and thermal aging of poly (vinyl chloride)/MgAl layered double hydroxides composites [J]. Chinese Journal of Polymer Science, 2016, 34(5): 542-551.
[40] WEN Xing, YANG Zhan-hong, YAN Jie, XIE Xiao-e. Green preparation and characterization of a novel heat stabilizer for poly(vinyl chloride)-hydrocalumites [J]. RSC Advance, 2015, 5: 32020-32026.
[41] YAN Jie, YANG Zhan-hong. Intercalated hydrotalcite-like materials and their application as thermal stabilizers in poly (vinyl chloride) [J]. Journal of Applied Polymer Science, 2017, 134(22): 44896.
[42] YANG Huan, YANG Zhan-hong. The effect of sodium stearate-modified hydrocalumite on the thermal stability of poly(vinyl chloride) [J]. Journal of Applied Polymer Science, 2018, 135(4): 45758.
[43] TONG Meng-liang, CHEN Hong-yan, YANG Zhan-hong, WEN Rui-juan. The effect of Zn-Al-hydrotalcites composited with calcium stearate and β-diketone on the thermal stability of PVC [J]. International Journal of Molecular Sciences, 2011, 12(3): 1756-1766.
[44] IIDA T, GOTO K. Stabilization of poly (vinyl chloride).V. Synergism between metal soaps and polyols upon stabilization of poly (vinyl chloride) [J]. Journal of Applied Polymer Science, 1980, 25(5): 887-900.
[45] IIDA T, KAWATO J, TANIE S, GOTO K. Stabilization of poly (vinyl chloride). X. Synergisms between epoxidized polybutadienes and metal soaps on the stabilization of poly (vinyl chloride) [J]. Journal of Applied Polymer Science, 1989, 37(6): 1685-1698.
[46] BENAVIDES R, EDGE M, ALLEN N S, SHAH M. The mode of action of metal stearate stabilisers in poly(vinyl chloride)—IV. The application of fluorescence spectroscopy to characterise chromophoric species [J]. Polymer Degradation and Stability, 1997, 57(1): 25-30.
[47] LIU Yan-bin, LIU Wei-qu, HOU Meng-hua. Metal dicarboxylates as thermal stabilizers for PVC [J]. Polymer Degradation and Stability, 2007, 92(8): 1565-1571.
[48] BENAVIDES R, EDGE M, ALLEN N S. The mode of action of metal stearate stabilisers in poly (vinyl chloride). I. Influence of pre-heating on melt complexation [J]. Polymer Degradation and Stability, 1994, 44(3): 375-378.
[49] LI Yue-peng, LI De-gang, HAN Wen-yuan, ZHANG Man-qi, AI Bing, ZHANG Li-peng, SUN Hong-qi, CUI Zhen. Facile synthesis of di-mannitol adipate ester-based zinc metal alkoxide as a bi-functional additive for poly (vinyl chloride) [J]. Polymers, 2019, 11: 813. DOI: 10.3390/ polym11050813.
[50] HAN Wen-yuan, ZHANG Man-qi, LI De-gang, DONG Tian-bao, AI Bing, DOU Jian-ping, SUN Hong-qi. Design and synthesis of a new mannitol stearate ester-based aluminum alkoxide as a novel tri-functional additive for poly (vinyl chloride) and its synergistic effect with zinc stearate [J]. Polymers, 2019, 11: 1031. DOI: 10.3390/ polym11061031.
[51] BOCLAIR J W, BRATERMAN P S. Layered double hydroxide stability. 1. Relative stabilities of layered double hydroxides and their simple counterparts [J]. Chemistry of Materials, 1999, 11(2): 298-302.
(Edited by ZHENG Yu-tong)
中文导读
CaZnAl-CO3三元层状双氢氧化物的合成及其在聚氯乙烯树脂热稳定性中的应用
摘要:采用水热法制备了含不同Ca/Zn/Al摩尔比的CaZnAl-CO3三元层状双氢氧化物(CaZnAl-CO3-LDHs),并用X射线衍射(XRD)、傅立叶变换红外光谱(FT-IR)和扫描电镜(SEM)对其进行表征分析,其结果表明,合成的样品均呈现水滑石的片状结构且晶形良好。静态热老化实验、刚果红法和紫外可见光谱测试等分析结果表明,当将CaZnAl-CO3-LDHs单独用于聚氯乙烯(PVC)热稳定剂时,最佳Ca/Zn/Al摩尔比为3.6:0.4:2,最佳添加量为5份。在此基础上进一步研究了Ca3.6Zn0.4Al2-CO3-LDHs和硬脂酸锌(CaSt2)复配对PVC热稳定性能的协同效应,结果表明,复配体系的热稳定性能优于单独使用Ca3.6Zn0.4Al2-CO3-LDHs,Ca3.6Zn0.4Al2-LDHs和CaSt2的最佳添加量分别为6份和1份。
关键词:CaZnAl-CO3-LDHs;协同效应;热稳定性;聚氯乙烯
Foundation item: Project(21371180) supported by the National Natural Science Foundation of China; Project (K1303015-11) supported by the Science and Technology Project of Changsha City, China; Project(20130162110018) supported by the Specialized Research Fund for the Doctoral Program of Higher Education, China
Received date: 2018-08-28; Accepted date: 2019-11-07
Corresponding author: YANG Zhan-hong, PhD, Professor; Tel: +86-731-88879616; E-mail: zhanhongyang611@163.com; ORCID: 0000-0002-5595-2469
Abstract: In this work, hydrothermal method was used to prepare the CaZnAl-CO3 ternary layered double hydroxides (CaZnAl-CO3-LDHs) with various Ca/Zn/Al molar ratios, which were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscope (FT-IR) and scanning electron microscopy (SEM) techniques. The obtained results demonstrate that the samples were well-crystallized and flake-structured. The CaZnAl-CO3-LDHs used alone for thermal stability of poly (vinyl chloride) (PVC) resin, with different Ca/Zn/Al molar ratios and varying additive amounts, were investigated through the tests such as static thermal aging, mass loss and congo red, respectively. The optimum Ca/Zn/Al molar ratio and additive amount were 3.6:0.4:2 and 5 phr (parts per hundred PVC resin), respectively. In addition, the synergistic effects of Ca3.6Zn0.4Al2-CO3-LDHs and CaSt2 were discussed in detail, showing better thermal stability compared with Ca3.6Zn0.4Al2-CO3-LDHs used alone, and the optimum additive amounts of Ca3.6Zn0.4Al2-LDHs and CaSt2 were 6 and 1.0 phr, respectively.
- Synthesis of CaZnAl-CO3 ternary layered double hydroxides and its application on thermal stability of poly (vinyl chloride) resin


