Trans. Nonferrous Met. Soc. China 20(2010) s719-s722

Application of semisolid process to Zr-based metallic glass matrix composites
T. Tamura, A. Makaya, K. Miwa
Solidification Processing Group, Materials Research Institute for Sustainable Development,
National Institute of Advanced Industrial Science and Technology(AIST), Nagoya 463-8560, Japan
Received 13 May 2010;accepted 25 June 2010
Abstract:
The effect of the cooling slope on the structure of Zr-based metallic glass matrix composites was investigated by changing the cooling slope. The synthesis of bulk metallic glass composites was made by a process combining cooling slope casting and Cu mold casting for Zr66.4Nb6.4Cu10.5Ni8.7Al8 alloys. The results show that the semisolid slurry which consists of the spheroidal or rosette-type BCC crystals and the liquid phase which forms metallic glass phase can be formed by the cooling slope process in this alloy system. However, the semisolid slurry cannot reach to the mold. It is considered that higher viscosity of the liquid phase which forms metallic glass phase causes this result. Thus, parameters of the cooling slope have to be examined further.
Key words:
semisolid processing; bulk metallic glass; composite; crystal particle; cooling slope casting;
1 Introduction
Bulk metallic glasses with amorphous structure are studied owing to their attractive properties such as high strength, attractive soft magnetic properties and good corrosion resistance[1]. However, their use in practical structural applications is hindered by their limited plastic strain under deformation and the resulting brittle-like behavior. The origin of such behavior was identified as the localization of strain in a few shear bands that propagated through the material and eventually led to catastrophic failure. Thus, numerous researches for improvement of the toughness and ductility were conducted[2-7]. In these studies, it was reported that micrometer-sized ductile dendritic crystals formed in-situ during solidification could improve the ductility of Zr-based bulk metallic glasses[4-7]. The ductility improvement of such composites has been reported to arise from the pinning and multiplication of propagating shear bands by the crystalline particles, resulting in a more uniform deformation and improved global plastic strain[4]. However, it is known to be difficult that the sample, in which the primary dendritic crystal particles are dispersed into the metallic glass phase uniformly, is prepared by Cu mold casting, because the cooling rate is different between the outside and the inside of the sample. On the other hand, in Al and Mg alloys, such as Al-7%Si (mass fraction) and AZ91, semisolid processing is researched and industrially used [8-13]. The present authors reported the synthesis of bulk metallic glass composites by a process combining cooling slope casting and suction casting, for the Zr66.4Nb6.4Cu10.5Ni8.7Al8 alloy composition[14]. The obtained materials show a semisolid structure with a dispersion of spheroidal and rosette-type ductile BCC crystals in a glassy matrix. However, the microstructure modification leading to the semisolid structure seems to occur during flow in the mold. Thus, the purpose of this study is to investigate the effect of the cooling slope on the structure of Zr-based metallic glass matrix composites by changing the cooling slope to a material of higher heat conductivity.
2 Experimental
The alloy composition investigated here is Zr66.4Nb6.4Cu10.5Ni8.7Al8 which was reported by KǖHN et al[6]. A master alloy was prepared by arc melting the mixtures of pure sponge Zr, Nb powder, Cu, Ni and Al under a purified argon atmosphere.
The cooling slope casting experiments consist of casting the arc-melted buttons using the setup shown in Fig.1. The alloy with 12 g was inserted in a quartz tube with a 0.7 mm diameter hole drilled at the bottom and placed in the chamber. The chamber was then evacuated with a diffusion pump to typically 4 mPa and subsequently filled with Ar gas to about 10 kPa. The alloy was melted by induction and maintained at 1 530 K for 20 s for composition homogenization. The temperature of the melt was monitored with a calibrated pyrometer. The opening of valve A was then automatically triggered for a set injection time, releasing the Ar gas contained in the tank and leading to injection of the metal onto the cooling slope. The Ar tank was preliminarily filled to a chosen pressure and the injection pressure p1 was recorded with a gauge placed at exit of the tank. The controlled injection time was 0.7 s. The effective injection pressure, which represents the difference between the injection pressure p1 measured at the exit of the Ar tank and the pressure of about 10 kPa measured in the chamber, was set at 28 kPa. Thus, the mass of injected melt onto the slope was about 3.5 g.
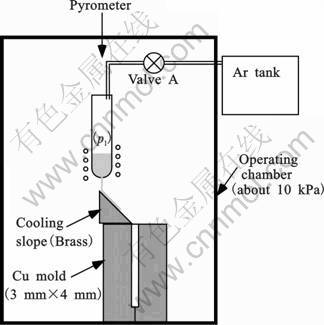
Fig.1 Schematic representation of the cooling slope casting
In the previous study, a half-pipe of boron nitride (internal diameter was 9 mm, external diameter was 15 mm, heat conductivity was 33 W/(m·K) at room temperature) was used as the cooling slope [14]. In this study, a brass block (heat conductivity was 106 W/(m·K) at 273 K) was used as the cooling slope. Fig.2 shows a shape of the cooling slope. The Cu mold, which has a square cavity with width of 3 mm and 4 mm, was placed under the cooling slope. However, in this study, the melt cannot reach the mold.
The microstructure of the samples was analyzed by optical microscopy, after polishing and etching in a solution of 0.3% HF, 4% HNO3 and 95.7% distilled water (volume fraction) for 15 s.
3 Results and discussion
Fig.3 shows a picture of a perpendicular cross section in the sample on the cooling slope. The brass block with no coating shown in Fig. 2 was used as the cooling slope. The melt was injected onto the cooling slope from the top and flowed onto the cooling slope by about 12 mm from a to c. However, after the melt reached at the c point, the melt overflowed from the cooling slope and could not reach to the mold. Fig.4 shows optical micrographs in the sample shown in Fig. 3. The micrographs shown in (a), (b) and (c) correspond to the points a, b and c in Fig. 3, respectively. The optical micrographs show the microstructures on the surface cut down by 0.5 mm from the outside which contacts the slope in parallel with the slope. The white phase is BCC phase which is the primary crystals, namely, the solid phase. And the gray phase is the featureless metallic glass phase or the crystalline phases which transforms from the metallic glass phase, namely, the liquid phase. When the cooling rate is not high, the metallic glass phase transforms to crystalline phases. These phases were confirmed by XRD and DSC in the previous study[14]. At point a to which the melt injects, very fine BCC dendritic crystals with DAS seemed sub-micrometer and BCC dendritic crystals which diameter of about 50 mm were observed. The very fine BCC dendritic crystals were considered to crystallize from the liquid phase after the cooling slope casting because quantity of the solid phase was few. Thus, it is considered that the phase which has very fine BCC dendritic crystals is liquid phase during the cooling slope casting. At point b, very fine BCC dendritic crystals disappeared and BCC dendritic crystals with diameter of about 10 mm are observed. The shape of these BCC dendritic crystals is round. At point c, micron-sized spheroidal and rosette-type BCC crystals are observed. These results indicate that the semisolid slurry can be formed by the cooling slope process in this alloy system. It is known that the melt which forms metallic glass phase has higher viscosity than that of conventional alloys. Thus, in the present study, the semisolid slurry can not reach to the mold. However, it is considered that semisolid processes can be applied to the production of Zr-based metallic glass matrix composites.
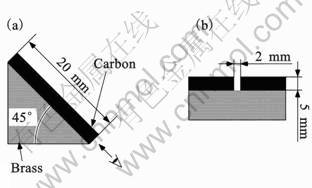
Fig.2 Schematic representation of shape of cooling slope
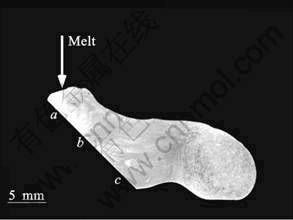
Fig.3 Picture of perpendicular cross section in sample on cooling slope (Brass block with no coating iIs used is cooling slope)
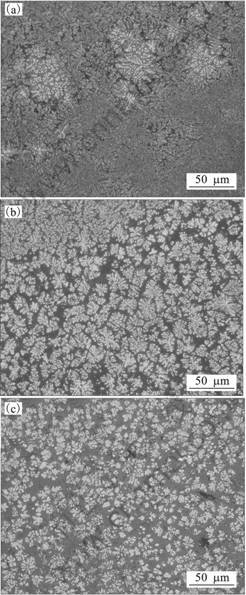
Fig.4 Optical micrographs in sample shown in Fig. 3: (a)Point a; (b) Point b; (c) Point c.
Fig.5 shows a picture of a perpendicular cross section in the sample on the cooling slope. The brass block with BN coating was used as the cooling slope. The thickness of BN coating on the brass block is about 0.05 mm. The cooling slope is the same as that shown in Fig.2 except BN coating on the brass block. The melt is injected onto the cooling slope from the top and flows onto the cooling slope by about 17 mm from a to c. However, after the melt reached at the point c, the melt overflows from the cooling slope and cannot reach to the mold. The length of the melt flow on the cooling slope with BN coating is longer than that on the cooling slope with no coating. It is considered that the difference of the heat conductivity causes this result. Fig.6 shows optical micrographs in the sample shown in Fig.5. The micrographs shown in Figs.6 (a), (b) and (c) correspond to a, b and c points in Fig.5, respectively. The optical micrographs show the microstructures on the surface cut down by 0.5 mm from the outside which contacts the slope in parallel with the slope. At point a to which the melt injects, very fine BCC dendritic crystals which DAS seems sub-micrometer and BCC dendritic crystals with diameter of about 50 mm were observed. This result is the same as that in the sample on the cooling slope with no coating shown in Fig.4. However at point b, the microstructure is similar to that at the point a. This result is different from that in the sample on the cooling slope with no coating. Moreover at point c, coarse BCC dendritic crystals were observed. This micro-structure is similar to that shown in Fig.4(b). These results are considered to indicate that the semisolid slurry was not formed yet because the cooling slope has low heat conductivity. Thus, it is considered that the longer cooling slope is needed when low heat conductivity materials are used as the cooling slope. However, the melt stopped flowing onto the cooling slope at point c although the spheroidal and rosette-type BCC crystals are not formed. It is presumed that higher viscosity of the liquid phase which forms metallic glass phase causes this result. Thus, it is considered that parameters of the cooling slope (slope material, slope angle, slope shape, slope length, flow rate of melt, etc.) have to be examined further.
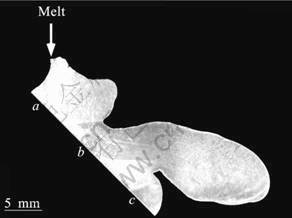
Fig.5 Picture of perpendicular cross section in sample on cooling slope(Brass block with BN coating is used as cooling slope)
4 Conclusions
1) The semisolid slurry which consists of the spheroidal or rosette-type BCC crystals and the liquid phase which forms metallic glass phase can be formed by the cooling slope process in this alloy system. When low heat conductivity materials are used as the cooling slope, the semisolid slurry has not been formed yet. Thus, it is considered that the longer cooling slope is needed. However, the semisolid slurry cannot reach to the mold.
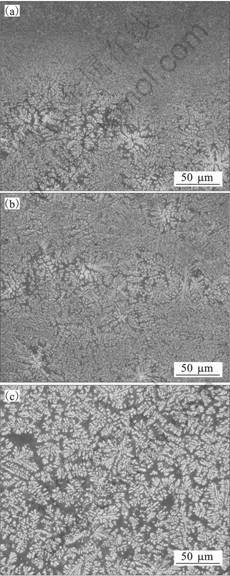
Fig.6 Optical micrographs in sample shown in Fig.5: (a) Point a; (b) Point b; (c) Point c.
2) The semisolid shlrry cannot reach to the mold. Higher viscosity of the liquid phase which forms metallic glass phase causes this result. Thus, parameters of the cooling slope (slope material, slope angle, slope shape, slope length, flow rate of melt, etc) have to be examined further.
Acknowledgments
The authors thank Mrs. T. Yamaguchi for technical assistance.
References
[1] INOUE A. Bulk amorphous alloys [M]. Switzerland: Trans Tech Publications Ltd, 1998: 2-36.
[2] TERAJIMA T, NAKATA K, KIMURA H, INOUE A. Development of W-reinforced Zr-based Metallic glass [J]. Mater Trans, 2009, 50(6): 1322-1325.
[3] HE G, ECKERT J, L?SER W, SCHULTZ L. Novel Ti-base nanostructure–dendrite composite with enhanced plasticity [J]. Nature Materials, 2003, 2(1): 33-37.
[4] HOFMANN D C, SUH J, WIEST A, DUAN G, LIND M, DEMETRIOU M D, JOHNSON W L. Designing metallic glass matrix composites with high toughness and tensile ductility [J]. Nature, 2008, 451(7182): 1085-1089.
[5] ECKERT J, K?HN U, MATTERN N, HE G, GEBERT A. Structural bulk metallic glasses with different length-scale of constituent phases [J]. Intermetallics, 2002, 10(11/12): 1183-1190.
[6] K?HN U, ECKERT J, MATTERN N, SCHULTZ L. ZrNbCuNiAl bulk metallic glass matrix composites containing dendritic bcc phase precipitates [J]. Appl Phys Lett, 2002, 80(14): 2478-2480.
[7] HAYS C C, KIM C P, JOHNSON W L. Microstructure controlled shear band pattern formation and enhanced plasticity of bulk metallic glasses containing in situ formed ductile phase dendrite dispersions [J]. Phys Rev Lett, 2000, 84(13): 2901-2904.
[8] CZERWINSKI F. Fundamentals of semisolid magnesium molding [J]. JOM, 2008, 60(11): 82-86.
[9] CZERWINSKI F. The basics of modern semi-solid metal processing [J]. JOM, 2006, 58(6): 17-20.
[10] NAFISI S, GHOMASHCHI R. The microstructural characterization of semi-solid slurries [J]. JOM, 2006, 58(6): 24-30.
[11] OMURA N, MURAKAMI Y, LI M, TAMURA T, MIWA K. Effect of volume fraction solid and injection speed on mechanical properties in new type semi-solid injection process [J]. Solid State Phenomena, 2008, 141/142/143: 761-766.
[12] RACHMAT R S, TAMURA T, MIWA K. Fluidity and Microstructures Characteristics of AZ 91D by using New Type Semi-solid Injection Process [J]. Solid State Phenomena, 2006, 116/117: 534-537.
[13] MIWA K, RACHMAT R S, TAMURA T. Effect of Solid Fraction on Microstructure and Casting Faults of AZ91D in New Type Semi-solid Injection Process [J]. Solid State Phenomena, 2006, 116/117: 441-444.
[14] MAKAYA A, TAMURA T, MIWA K. Cooling Slope Casting Process for Synthesis of Bulk Metallic Glass Based Composites with Semisolid Structure [J]. Metall Mater Trans A, 2010, 41(7): 1646-1657.
(Edited by CHEN Can-hua)
Corresponding author: T.TAMURA; Tel: +81-52-736-7277; E-mail: takuya-tamura@aist.go.jp


