
J. Cent. South Univ. (2018) 25: 2373-2379
DOI: https://doi.org/10.1007/s11771-018-3921-6
Effect of content of Al2O3 and MgO on crystallization of blast furnace slag during fiber formation
LI Zhi-hui(李智慧)1, 2, ZHANG Yong-jie(张永杰)1, 3, ZHANG Yu-zhu(张玉柱)1, 2, DU Pei-pei(杜培培)2, REN Qian-qian(任倩倩)2
1. School of Metallurgy, Northeastern University, Shenyang 110819, China;
2. College of Metallurgy and Energy, North China University of Science and Technology,Tangshan 063009, China;
3. Baosteel Research Institute, Shanghai 201900, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
The simulation of blast furnace slag was prepared by pure chemical reagents. Test methods like DSC, XRD and SEM were used to study the effect of Al2O3 and MgO content on crystallization of blast furnace slag during fiber formation. The results show that as Al2O3 and MgO contents in the sample changed, blast furnace slag was crystallized at the average temperature below 1232 K. When the ratio of Mg/Al in the samples is 0.6 calculated by Kissinger equation, crystallization activation energy is at the maximum value and the system is in the most stable condition. The sample crystallization phases are mainly calcium akermanite (2CaO·MgO·2SiO2) and gehlenite (2CaO·Al2O3·SiO2). Secondary crystallization phases are anorthite (CaAl2Si2O8), wollastonite minerals (WOLLA) and pyroxene minerals (cPyrA). Meanwhile, the principal crystallization phases of the samples are different types and have different contents, and the microstructures of the sample sections are different due to the difference between MgO/Al2O3 ratio.
Key words:
blast furnace slag; MgO/Al2O3 ratio; fiber formation; crystallization;
Cite this article as:
LI Zhi-hui, ZHANG Yong-jie, ZHANG Yu-zhu, DU Pei-pei, REN Qian-qian. Effect of content of Al2O3 and MgO on crystallization of blast furnace slag during fiber formation [J]. Journal of Central South University, 2018, 25(10): 2373–2379.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3921-61 Introduction
Mineral wool, as a part of silicate system, can be used for mineral wool boards, pipes and felts due to its light weight, good heat preservation, heat insulation and sound insulation performance. It is an excellent thermal insulation, sound absorption and fireproof material [1–4]. Mineral wool consists of rock wool, slag and wool etc., of which the slag wool has a wide range of chemical composition and has brought great convenience on raw material formulation during process. The composition range of slag wool is generally 36%–42% SiO2, 8%–47% CaO2, 9%–17% Al2O3 and 3%–12% MgO3. In addition, it also contains a small amount of iron and alkali metal oxides, such as Na2O, K2O [5, 6].
The composition of raw slag wool has a significant impact on the production process and fiber properties. Especially, the crystallization process of raw materials has an even more serious effect on the fiber performance in recent years. Many scholars have conducted a great deal of researches [7–12] on the effect of the composition of raw material itself on mineral wool performance. Based on the sintering method, CHENG et al [13] investigated the effect of MgO on the crystallization of CaO-Al2O3-SiO2 system. It was found that increasing the content of MgO favors decreasing the viscosity of the system and thus lowering the crystallization temperature of the system. The influence of CaO/MgO mass ratio changes on the crystallization of CaO-MgO-Al2O3-SiO2 system was studied by PENG et al [14] and YANG et al [15]. It was found that the decrease of CaO/MgO mass ratio enhanced the crystallization temperature of the system; reduced the crystallization performance; changed the crystal phase ratio, shape; reduced the grain size; increased the diopside crystal content; and improved the product abrasion resistance. In addition, since the blast furnace slag belongs to the CaO–MgO–Al2O3–SiO2 system [16], CaO and SiO2 are the obvious network modifier and network former and Al2O3 network intermediate and MgO with high field strength Mg2+ are also in this system [7, 11]. Therefore, studying the effect of MgO and Al2O3 content on the crystallization of blast furnace slag during fiber making process can provide a theoretical basis for the comprehensive utilization of secondary resources and environmental pollution control, which not only can gain good economic benefits, but also produce significant social benefits.
2 Experiments
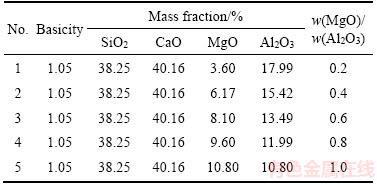
In this work, the experiments only focused on the effect of Al2O3 and MgO contents on the crystallization of blast furnace slag during fiber making process. The raw materials were prepared by simulating the composition of the blast furnace slag with selected pure chemical reagents. During the raw materials making process, the effect of other insignificant compositions was not considered, in which 38.25% of SiO2 content and 40.16% of CaO content remained consistent but only changed the Al2O3 and MgO content. For the convenience of the research, the mass ratio of MgO and Al2O3 was determined as w(MgO)/w(Al2O3) ratio. And the w(MgO)/w(Al2O3) ratio of experimental materials increased from 0.2 to 1.0 (Table 1).
The mixture of raw materials was placed in a corundum crucible (50 mm×80 mm), and heated in a programmable energy-saving high temperature resistance furnace. In the first stage, the heating rate was set at 4 °C/min, the heating time 50 min and the target temperature 200 °C; in the second stage, the heating rate was set at 12 °C/min, the heating ergy-saving high temperature time 50 min and the target temperature 800 °C; in the third stage the heating rate was set at 8 °C/min, the heating time 50 min and the target temperature 1200 °C; in the fourth stage, the heating rate was set at 6 °C/min, the heating time 30 min and the target temperature 1500 °C; in the fifth stage, the furnace temperature was kept at 1500 °C and heated for 60 min till the blast furnace slag samples were melted thoroughly and homogenized; in the sixth stage, as the furnace cooled, the temperature of furnace was reduced to 1100 °C, which simulated the way that blast furnace slag cooled during fiber making process. The sample was rapidly taken from the furnace in the seventh stage and rapidly cooled to the room temperature.
Table1 Chemical composition of modification slag
The air-cooled raw material was ground to less than 200 mesh and subjected to X-ray diffraction (XRD) analysis at room temperature with D/MAX2500PC X-ray diffractometer (tube voltage 40 kV, tube current 100 mA). X-ray diffraction spectrum was an angular distribution, with the scanning angle of 20–80° and scanning speed 10 (°)/min. The data were analyzed using MDI jade 6.0 and OriginPro 8.5.
About 1 mm lumps were stuck to a cylindrical test stand and subjected to surface analysis on a Hitachi S-4800 scanning electron microscope. The acceleration voltage of the electron gun was 15 kV or 20 kV, and the magnification was 1000 × or 2000 ×.
The thermal stability and crystallization performance of the samples were studied by differential scanning calorimetry (DSC) and high temperature integrated thermal analyzer. The sample was placed in a corundum crucible placed on a sample holder of a high sensitivity platinum- rhodium thermocouple and heated from room temperature to 1200 °C in a nitrogen atmosphere at a heating rate of 5 K/min, 10 K/min, 15 K/min and 20 K/min. The reference sample was α-Al2O3.
3 Results and analysis
3.1 Crystallization performance
Blast furnace slag fibers are cotton-like fibers in the glassy state. If the slag is in the cooling and fiber formation process, the growth of crystal will produce strong stress, which makes the fiber break and its thermal stability decreases. Generally, the causes of blast furnace slag crystallization phenomenon are divided into internal and external causes: the composition of the slag and the temperature, respectively. The crystallization performance of the samples was investigated by differential scanning calorimetry (DSC). The experiment was carried out in a nitrogen atmosphere with a heating rate of 5 K/min. The dynamic DSC scanning results of samples with different MgO/Al2O3 ratio are shown in Figure 1.
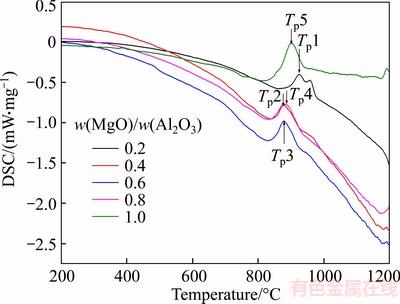
Figure 1 Dynamic DSC curves of samples with different MgO/Al2O3 ratios
As shown in Figure 1, the exothermic peaks appeared in the samples with different w(MgO)/w(Al2O3) ratios, that is, the crystallization occurred. When w(MgO)/w(Al2O3) ratio is 0.2, only two crystallization exothermic peaks appear in the DSC curve. As w(MgO)/w(Al2O3) ratio increases, only one crystallization exothermic peak appears in the DSC curve. In terms of the crystallization exothermic peak temperature of the samples, as the w(MgO)/w(Al2O3) ratio increases, the crystallization exothermic peak temperature Tp begins to decrease and then increases, but the overall change is insignificant, as shown in Figure 2. In the system, the increase of MgO content leads to the increase of the amount of “free oxygen”, resulting in the increase of the fracture of the silicon oxygen network and the contrapolarization of Si—O bonds by Mg+ to weaken the Si—O bond strength and reduce the viscosity of the system, which shows the exothermic peak temperature of crystallization decreases in Figure 2. Subsequently, the amount of “free oxygen” in the system increases with the increase of MgO content. At the same time, the content of Al2O3 in the system decreases. A small amount of Al2O3 seized “free oxygen” to form [AlO4]– to form the network structure, resulting in a slight increase in the viscosity of the system, which shows a slight increase in the crystallization exothermic peak temperature of the sample as shown in Figure 2.

Figure 2 Crystallization exothermic peak temperature curve of samples with different MgO/Al2O3 ratios
3.2 Crystallization reaction activation energy
Appearance activation energy is used to describe crystallization reaction of amorphous state materials. The crystallization reaction activation energy of samples are calculated by Kissinger equation [17]:
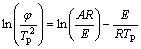
In this equation, E is appearance activation energy, kJ/mol; φ is the heating rate, K/min; Tp is the crystallization exothermic peak temperature, K; R is the gas constant, 8.314 J/(mol·K); and A is the pre-exponential factor.
According to DSC results, Tp can be collected from samples with different heating rates φ (Table 2).
Table 2 Peak temperature (Tp) of crystallization at different heating rates
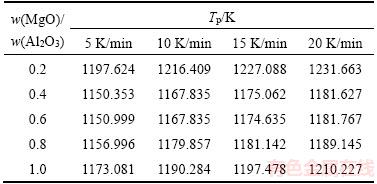
Taking  as the vertical axis and l/Tp as the horizontal axis to draw a curve, the fitting curve can be obtained by linear fitting (Figure 3). The slope of fitting line is –E/R and the intercept is ln (AR/E). The crystallization activation energy E of different samples can be calculated.
as the vertical axis and l/Tp as the horizontal axis to draw a curve, the fitting curve can be obtained by linear fitting (Figure 3). The slope of fitting line is –E/R and the intercept is ln (AR/E). The crystallization activation energy E of different samples can be calculated.

Figure 3  relationship during sample crystallization process
relationship during sample crystallization process
The fitting linear equations of samples with different Mg/Al ratios obtained through linear regression analysis are: Y=–55909.86x+34.09(0.2), Y=–58051.93x+37.95(0.4), Y=–59539.66x+39.22, Y= –55243.83x+35.21(0.8), Y=–52112.09x+31.93(1.0). The fitting linearity is very high. According to Kissinger equation, the activation energy of the crystallization reaction can be obtained (Table 3).
The crystallization activation energy of the samples with different w(MgO)/w(Al2O3) ratios increased first and then decreased (Figure 4). The activation energy indicates that the height of the crystallization energy barrier of the sample. The amount of activation energy directly reflects the degree of difficulty of the crystallization reaction occurrence. It can be seen from Figure 4 that as the MgO/Al2O3 ratio increased, the difficulty of the sample crystallization first increased and then decreased. When the ratio of w(MgO)/w(Al2O3) is 0.6, the crystallization activation energy is 495.01 kJ/mol, and the crystallization reaction is the most difficult. Meanwhile, the system is the most stable.
Table 3 Crystallization activation energy at different w(MgO)/w(Al2O3) ratios


Figure 4 Crystal activation energy change with w(MgO)/w(Al2O3) ratios
3.3 X-ray diffraction analysis
Figure 5 shows the XRD diffraction patterns for each sample, in which (a), (b), (c), (d) and (e) represent samples with w(MgO)/w(Al2O3) content ratios of 0.2, 0.4, 0.6, 0.8 and 1.0, respectively. As shown in Figure 5, the principal crystalline phases of the samples are calcium akermanite (2CaO·MgO·2SiO2) and gehlenite (2CaO·Al2O3·SiO2). Secondary crystallization phases are anorthite (CaAl2Si2O8), wollastonite minerals (WOLLA) and pyroxene minerals (cPyrA). As the w(MgO)/w(Al2O3) ratio increases, the content of MgO in the system increases, the content of Al2O3 decreases. When the content of MgO decreases, the similarities of the molecular structure between gehlenite and anorthite in the crystal phase and glass phase become larger. Therefore, the crystal is preferentially gehlenite and anorthite. With the increase of MgO content, the reaction 2CaO+MgO+2SiO2 2CaO·MgO·2SiO2 is promoted to the right, which may inhibit the precipitation of gehlenite and anorthite while promote the precipitation of calcium akermanite. With the continuous precipitation of calcium akermanite crystals, the content of CaO gradually decreases, and the excess MgO reacts with Al2O3 and SiO2 to precipitate some wollastonite and pyroxene minerals.
2CaO·MgO·2SiO2 is promoted to the right, which may inhibit the precipitation of gehlenite and anorthite while promote the precipitation of calcium akermanite. With the continuous precipitation of calcium akermanite crystals, the content of CaO gradually decreases, and the excess MgO reacts with Al2O3 and SiO2 to precipitate some wollastonite and pyroxene minerals.

Figure 5 XRD spectra of blast furnace slag at different MgO/Al2O3 ratios:
3.4 Microstructure analysis
Scanning electron microscope (SEM) was used to observe the cross section of the sample and the morphology of the crystal (Figure 6). According to the SEM results, it is found that the sample with w(MgO)/w(Al2O3) ratio of 0.2 has a layered structure with a uniform distribution (Figure 6(a)). The energy spectrum at the 1 position in the figure shows that the precipitated crystals are mainly gehlenite and anorthite. The cross section of the sample with w(MgO)/w(Al2O3) ratio of 0.4 shows a smooth glass structure with a small amount of crystal aggregation. The position 4 energy spectrum shows the precipitation of calcium akermanite (Figure 6(b)). When the w(MgO)/w(Al2O3) ratio is 0.6, more crystal aggregation occurred and the precipitation of calcium akermanite increased. The samples with w(MgO)/w(Al2O3) ratio of 0.8 and 1.0 showed more obvious crystal aggregation and the microstructure of the sample with w(MgO)/w(Al2O3) ratio of 0.8 was smaller (Figures 6(c)–(e)).
With the increase of MgO content, the content of Al2O3 decreases and the viscosity of the sample decreases gradually. In the system, [AlO4] and [SiO4] can attract Ca2+ and Mg2+ that provide non-bridging oxygen around themselves and aggregate at the interface, causing crystallization. The mass ratio of Ca2+ to Mg2+, [AlO4] and [SiO4] in the system changes due to the change of w(MgO)/w(Al2O3) ratio, which leads to the change of the type and content of the precipitated principal crystal phase, resulting in the different precipitated crystal microscopic appearances of different samples (Figure 6).
4 Conclusions
Blast furnace slag fiber is cotton-like fibers in the glassy state. If the slag is in the cooling and fiber formation process, the growth of crystal will produce strong stress, which makes the fiber break and its thermal stability decrease. The DSC curves of different samples show that the exothermic peaks appeared in the samples with different w(MgO)/w(Al2O3) ratios, that is, the crystallization occurred. As the w(MgO)/w(Al2O3) ratio increases, the crystallization exothermic peak temperature Tp begins to decrease and then increase, but the overall change is insignificant, and the crystallization temperature is below 1232 K. When the ratio of w(MgO)/w(Al2O3) in the samples is 0.6 calculated by Kissinger equation, crystallization activation energy is at the maximum value and the system is in the most stable condition. Therefore, when the blast furnace slag quenching and tempering is conducted, the w(MgO)/w(Al2O3) ratio can be controlled at 0.6 to ensure that the system of crystallization has the minimum effect on fiber-forming process and fiber performance.
Through XRD analysis of samples, it is found that the sample crystallization phases are mainly calcium akermanite (2CaO·MgO·2SiO2) and gehlenite (2CaO·Al2O3·SiO2). Secondary crystallization phases are anorthite (CaAl2Si2O8), Wollastonite minerals (WOLLA) and pyroxene minerals (cPyrA). Meanwhile, the principal crystallization phases of the samples are different types and have different contents, and the microstructures of the sample sections are different due to the difference between w(MgO)/w(Al2O3) ratios.
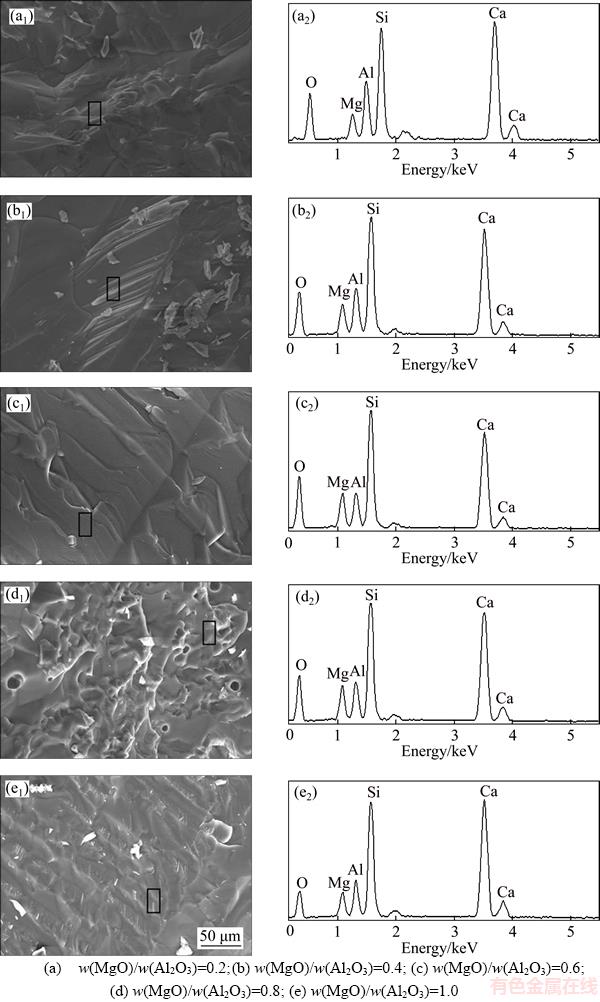
Figure 6 SEM-EDS images of samples at different MgO/Al2O3 ratios:
References
[1] MOESGAARD M, PEDERSEN H D, YUE Y Z, NIELSEN E R. Crystallization in stone wool fibres [J]. Journal of Non-crystalline Solids, 2007, 353(11, 12): 1101–1108.
[2] ZHENG Q J, YOUNGMAN R E, HOGUE C L, MAURO J C, POTUZAK M. Structure of boroaluminosilicate glasses: Impact of (Al2O3)/(SiO2) ratio on the structural role of sodium [J]. Phys Rev B, 2012, 86(5): 4583–4586.
[3] KARAMANOV A, PISCIELLA P, CANTALINI C, PELINO M. Influence of Fe3+/Fe2+ ratio on the crystallization of iron-rich glasses made with industrial wastes [J]. Cheminform, 2001, 83(12): 3153–3157.
[4] LI Zhi-hui, ZHANG Yu-zhu, LONG Yue, DU Pei-pei, Pilot-scale research on slag wool and cotton board prepared by quenched slag [J]. Journal of Materials and Metallurgy, 2017, 16(1): 63–67. (in Chinese)
[5] KARAMANOV A, TAGLIERI G, PELINO M. Sintering in nitrogen atmosphere of iron-rich glass-ceramics [J]. Journal of the American Ceramic Society, 2010, 87(7): 1354–1357.
[6] MOISEEV E A, GUTNIKOV S I, MALAKHO A P, LAZORYAK B I. Effect of iron oxides on the fabrication and properties of continuous glass fibers [J]. Inorganic Materials, 2008, 44(9): 1026–1030.
[7] XU Ji-fang, ZHANG Jie-yu, CHEN Dong, SHENG Min-qi, WENG Wen-ping. Effects of MgO content and CaO/Al2O3 ratio on surface tension of calcium aluminate refining slag [J]. Journal of Central South University, 2016, 24: 3079– 3084.
[8] SMEDSKJAER M M, SOLVANG M, YUE Y. Crystallisation behaviour and high-temperature stability of stone wool fibres [J]. Journal of the European Ceramic Society, 2010, 30(6): 1287–1295.
[9] GUTNIKOV S I, MALAKHO A P, LAZORYAK B I, LOGINOV V S. Influence of alumina on the properties of continuous basalt fibers [J]. Russian Journal of Inorganic Chemistry, 2009, 54(2): 191–196.
[10] BURKHARD D J M, SCHERER T. The effect of initial oxidation state on crystallization of basaltic glass [J]. Journal of Non-crystalline Solids, 2006, 352(38): 3961–3969.
[11] SUN Li-feng, SHI Jun-jie, ZHANG Bo, QIU Ji-yu, WANG Zhao-yun, JIANG Mao-fa. Liquidus and phase equilibria in CaO-SiO2-5%MgO-20%Al2O3-TiO2 system [J]. Journal of Central South University, 2017, 24: 48–55.
[12] MYSEN B O. The structural behavior of ferric and ferrous iron in aluminosilicate glass near meta-aluminosilicate joins [J]. Geochimica Et Cosmochimica Acta, 2006, 70(9): 2337–2353.
[13] CHENG Jin-shu, XIAO Zi-fan, XIE Jun. K2O, MgO of CaO-Al2O3-SiO2 effect of sintering and crystallization of glass ceramics [C]// Glass Branch of China Silicate Society Proceedings of 2009 Annual Meeting of National Glass Science and technology. Wuhan, China, 2009: 175–178. (in Chinese)
[14] PENG Wen-qin, XIAO Han-ning. Effect of on the crystallization behavior of CaO-MgO-Al2O3-SiO2 system glass [J]. Chinese Ceramics, 2001, 37(2): 20–22. (in Chinese)
[15] YANG Guang-yue, YANG Hui, GUO Xing-zhong, LI Wen-yan. The CaO/MgO ratio CaO-MgO-Al2O3-SiO2 effect of crystallization behavior of glass ceramics [J]. J Chin Ceram Soc, 2010, 38(11): 2045–2049. (in Chinese)
[16] SHI Pei-yang, JIANG Mao-fa, LIU Cheng-jun, WANG De-yong. Crystallization behavior and properties of CaO–MgO-Al2O3-SiO2 glass ceramics [J]. Journal of Northeastern University (Natural Science Edition), 2004, 25(12): 1169–1172. (in Chinese)
[17] FRANCISCO J T, JAVIER A. Effect of MgO/CaO ratio on the microstructure of cordierite-based glass-ceramic glazes for floor tiles [J]. Ceram Int, 2005, 31: 683–690.
(Edited by HE Yun-bin)
中文导读
Al2O3及MgO含量对高炉渣成纤过程析晶行为的影响
摘要:模拟高炉渣成分采用化学纯试剂配制了实验原料,采用DSC、XRD和SEM 等测试手段研究了Al2O3,MgO含量对高炉渣成纤过程析晶行为的影响。结果表明:随着试样中Al2O3和MgO含量的变化均呈现了析晶现象,析晶温度均在1232 K以下。通过Kissinger方程计算,当试样中镁铝比为0.6时,析晶活化能最大,此时体系最稳定。试样析出的晶相主要有钙镁黄长石(2CaO·MgO·2SiO2)和钙铝黄长石(2CaO·Al2O3·SiO2),次要晶相有钙长石(CaAl2Si2O8)、硅灰石类矿物(WOLLA)和辉石类矿物(cPyrA)。同时,由于Al2O3,MgO含量不同,试样析出的主晶相的种类及含量不同,试样断面微观形貌不同。
关键词:高炉渣;镁铝比;成纤过程;析晶
Foundation item: Project(51474090) supported by the National Natural Science Foundation of China
Received date: 2017-09-14; Accepted date: 2017-12-16
Corresponding author: ZHANG Yu-zhu, PhD, Professor; Tel: +86–315–8805001; E-mail: zyz@ncst.edu.cn; ORCID: 0000-0001-6285- 2880
Abstract: The simulation of blast furnace slag was prepared by pure chemical reagents. Test methods like DSC, XRD and SEM were used to study the effect of Al2O3 and MgO content on crystallization of blast furnace slag during fiber formation. The results show that as Al2O3 and MgO contents in the sample changed, blast furnace slag was crystallized at the average temperature below 1232 K. When the ratio of Mg/Al in the samples is 0.6 calculated by Kissinger equation, crystallization activation energy is at the maximum value and the system is in the most stable condition. The sample crystallization phases are mainly calcium akermanite (2CaO·MgO·2SiO2) and gehlenite (2CaO·Al2O3·SiO2). Secondary crystallization phases are anorthite (CaAl2Si2O8), wollastonite minerals (WOLLA) and pyroxene minerals (cPyrA). Meanwhile, the principal crystallization phases of the samples are different types and have different contents, and the microstructures of the sample sections are different due to the difference between MgO/Al2O3 ratio.


