文章编号:1004-0609(2007)02-0303-05
Ni对Mg-Cu-Tb非晶合金形成及力学性能的影响
张青绒,李金山,王一川,寇宏超,胡 锐,周 廉,傅恒志
(西北工业大学 凝固技术国家重点实验室,西安 710072)
摘 要:
利用熔体铜模喷铸法制备出直径为3 mm的Mg65Cu25-xNixTb10(x=0, 5, 10) 非晶合金。利用X射线衍射、差热分析、压缩实验分析和扫描电镜分析了添加Ni元素对Mg-Cu-Tb非晶合金形成能力及力学性能的影响。研究表明:随着Ni含量的增加,合金的玻璃转变温度Tg增大;开始结晶温度Tx降低;过冷液相区宽度ΔTx减小, 约化玻璃转变温度Trg从0.562降至0.530,非晶形成能力逐渐降低。压缩实验结果表明:当Ni含量增加到5%时可以明显提高Mg-Cu-Tb-Ni非晶合金的断裂强度。
关键词:
中图分类号:TG139.8 文献标识码:A
Effect of substitution of Ni for Cu on glass-forming ability and
mechanical properties of Mg-Cu-Tb metallic glass alloys
ZHANG Qing-rong, LI Jin-shan, WANG Yi-chuan, KOU Hong-chao,
HU Rui, ZHOU Lian, FU Heng-zhi
(State Key Laboratory of Solidification Processing,
Northwestern Polytechnical University, Xi’an 710072, China)
Abstract: The Mg65Cu25-xNixTb10(x=0, 5, 10) bulk amorphous alloy rods with 3 mm diameters were prepared by copper mold casting technique, and the effect of Ni addition on the glass forming ability ( GFA) and mechanical properties of Mg65Cu25-xNixTb10(x=0, 5, 10) alloys were studied by to X-ray diffractometry (XRD), differential scanning calorimeter (DSC) , compression tests and scanning electron microscopy (SEM). The results show that the glass transition temperature (Tg) of the Mg65Cu25-xNixTb10 alloys increases slightly whereas the onset temperature of crystallization (Tx), decreases slightly with the increase of Ni content, which results in the decrease of the supercooled liquid region and the glass forming ability. However, an appropriate substitution of Cu by Ni(5%, mole fraction) in Mg65Cu25Tb10 significantly improves the mechanical properties.
Key words: Mg-based metallic glasses; glass-forming ability; thermal stability; mechanical properties
块体非晶合金具有高强度及高耐磨性等一系列优异的性能,在体育器材、电子器件、航空航天等领域具有广泛的应用前景,而且还可作为初始材料制备块体纳米材料,在基础研究方面具有很大的意义。在众多多组元非晶合金体系中,镁基非晶合金具有较高的比强度和贮氢特性,是一种极具应用潜力的轻质结构及功能材料。1990年,Inoue等[1]成功制备出直径达4 mm的Mg65Cu25Y10块体非晶合金。后来,人们又成功地在空气中制备出一系列块体镁基非晶,如Mg-Ni-Pr[2]、Mg65Cu25Tb10[3]和Mg-Cu-Gd[4]等,这些非晶合金具有较强的抗氧化性,采用铜模铸造法可以在空气中得到直径为5 mm的非晶合金棒材。最近,在这些三元合金的基础上,发展了四元合金Mg-Cu-Ni-G[5]和Mg-Cu-Ag-Er[6]等,研究表明,用Ni部分替代Cu可以在不降低Mg65Cu25Gd1非晶形成能力的同时,提高该非晶合金的力学性能;用Ag部分替代Cu可以明显提高Mg65Cu25Er10的硬度和断裂强度。虽然这些合金元素的添加对镁基非晶形成能力和力学性能的影响因素尚不明确,但是,合金多组元间的交互作用仍然是提高镁基非晶形成能力和改善力学性能的有效途径之一[7-8]。
本文作者在Mg65Cu25Tb10非晶合金的基础上,研究了Mg65Cu25-xNixTb10(x=0, 5, 10)非晶合金的形成能力、热稳定性及性能,探讨了添加元素Ni对Mg-Cu-Ni-Tb非晶合金的各种影响及原因。
1 实验
实验原料为纯度高于99.8%的Mg、Cu、Ni和Tb块状纯金属,合金设计成分为Mg65Cu25-xNixTb10(x=0, 5, 10)。采用电弧熔炼法制备中间合金锭Cu-Tb和Cu-Ni-Tb,感应熔炼法制备母合金锭,铜模喷铸法制备直径为3mm 的合金棒。沿轴向打磨样品至中心平面,使用PHI-5400型X射线衍射仪(采用单色Cu Kα辐射源)分析样品的组织结构;使用2910型MDSC设备进行DSC分析,在加热速率为0.67 K/s的条件下,研究了玻璃转变、晶化和熔化行为;使用ZMT5305型电子万能试验机进行压缩实验(长度与直径比为2,压缩应变速率为3×10-4 s-1);使用JEOLJSM-5610LV型扫描电镜进行断口形貌观察。
2 结果与分析
图1所示为d 3 mm Mg65Cu25-xNixTb10(x=0, 5, 10)合金的X射线衍射谱。从图1中可看出,所有样品仅有一个宽化的衍射峰,这是非晶结构的衍射特征,表明所有样品为完全非晶态结构。
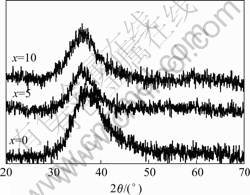
图1 Mg65Cu25-xNixTb10(x=0, 5, 10)铸态合金的X射线衍射谱
Fig.1 XRD patterns of as-cast Mg65Cu25-xNixTb10(x=0, 5, 10) alloy
图2所示为Mg65Cu25-xNixTb10(x=0, 5, 10)非晶在连续加热(加热速率为0.67 K/s)条件下的DSC曲线。由图2中可看出, 3个合金都表现出明显的玻璃转变,有一个宽的过冷液相区和结晶化的放热反应,晶化焓(ΔHx)及其它热力学参数分别列入表1。由表1可看出,Mg65Cu25Tb10非晶合金的玻璃转变温度Tg在423 K,这与文献[3]中d5 mm Mg65Cu25Tb10非晶合金的Tg点相差8 K,且随着Ni含量的增多,Mg65Cu25-xNixTb10合金的玻璃转变温度Tg逐渐增加,这种变化与原子间结合力的增大有关[9];另一方面,开始结晶温度Tx随着Ni含量的增大而降低,从而导致过冷液相区宽度ΔTx(ΔTx=Tx-Tg)变小。Inoue等[10]认为,ΔTx值越大,过冷液相区可以在更宽的温度范围存在而不结晶,并对结晶的形核与长大有更高的抵抗力,从而提高了非晶合金的热稳定性。由此可以得出,Ni超过5%时会明显降低Mg65Cu25-xNixTb10(x=0, 5, 10)非晶的形成能力和热稳定性。这与文献[5]中所得出的Ni对Mg65Cu25-xNixGd10非晶合金的影响结果一致。

图2 Mg65Cu25-xNixTb10(x=0, 5, 10)非晶合金的DSC曲线
Fig.2 DSC curves of Mg65Cu25-xNixTb10(x=0,5,10) alloy at heating rate of 0.67 K/s
表1 Mg65Cu25-xNixTb10(x=0, 5, 10)非晶合金的热力学参数
Table 1 Thermodynamic properties of several Mg65Cu25-xNixTb10(x=0, 5, 10) amorphous alloys

从图2还可明显看出,当x=0时,有一熔化区间较小(47 K)的单一熔化吸热峰;相比之下,当x为5和10时,合金的熔化区间越来越宽,放热峰从2个变成3个,这说明随着Ni含量的增加,四元的Mg-Cu-Ni-Tb非晶合金越来越偏离了共晶成分点。液相线温度Tl随着Ni含量的增加陡然升高,依次为752、782和812 K,这与合金成分远离共晶点以及所添加元素Ni的熔点很高有关。结果使得约化玻璃转变温度Trg(Trg=Tg/Tl) 从Mg65Cu25Tb10和Mg65Cu20Ni5Tb10的0.562和0.542降低至Mg65Cu15Ni10Tb10的0.530。如果用Trg值的大小表征镁基合金的非晶形成能力,那么随着Ni含量的增大,Mg65Cu25-xNixTb10(x=0,5,10)非晶的形成能力明显降低。合金成分远离共晶点是降低非晶形成能力的主要原因。
图3所示为Mg65Cu25-xNixTb10(x =0, 5, 10)非晶合金的压缩应力—应变曲线。从图2中可看出,所有试样在最终断裂前都没有明显的塑性应变,仅表现出1.5%的压缩弹性应变,没有出现文献[4,11]中Mg65Cu25-xNixTb10和Mg65Cu 25Y10非晶在断裂之前表现出的塑性应变(0.15%)。Mg65Cu25-xNixTb10 (x=0,5,10)试样的压缩断裂强度δcf分别为756 MPa、867 Mpa和712 MPa,弹性模量E分别为54、56和57 GPa。当Ni含量为到5%时,压缩断裂强度最高。根据Epark等[12]的分析,强度的增加是由于Ni元素替代部分Cu后,增加了原子之间的结合能(Ni-Mg: -4 kJ/mol; Cu-Mg: -3 kJ/mol; Ni-Tb: -32 kJ/mol; Cu-Tb: -23 kJ/mol)。当Ni含量为到10%时,原子间的结合能更强,压缩断裂强度却最低,其原因主要为:一方面,合金成分在x=10时已远离了共晶成分点,Tl值很高,急冷时降低了液态原子的堆垛密度,影响了微结构中化学短程序的分布[13];另一方面, Ni元素的原子半径最小(Tb: 1.90 nm; Mg: 1.60 nm; Cu: 1.28 nm; Ni: 1.24 nm),且易于扩散,随着Ni含量的提高,微结构中的成分不均匀性加剧[14]。Madge等[15]已通过能量过滤透射式电子显微镜(EFTEM)证实在Mg65-xNixNd10非晶中,随着Ni含量的提高,微结构中的成分不均匀性加剧,出现Ni元素的富集。
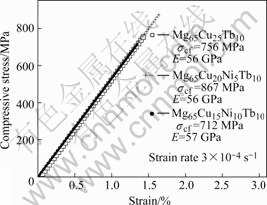
图3 Mg65Cu25-xNixTb10(x=0, 5, 10)非晶合金室温压缩应 力—应变曲线
Fig.3 Compressive stress—strain curve of Mg65Cu25-xNix- Tb10(x=0, 5, 10) alloy at room temperature
图4所示为Mg65Cu25-xNixTb10(x=0, 5, 10)非晶合金的断口形貌。图4(a)所示为Mg65Cu25Tb10压缩试样断口的宏观型貌,起裂源位置和放射状的裂纹扩展区清晰可见,将方框位置(初始裂纹扩展区)放大的微观断口形貌如图4(b)所示,断口表面出现的光滑区域与脉状纹理是剪切带的滑移与变形造成的。从图4(b)可以看出,剪切带几乎完全平行受力方向,使得断裂源快速传播,造成脆断。图4(c)和(d)所示分别为Mg65Cu20Ni5Tb10和Mg65Cu15Ni10Tb10在相应裂纹扩展区位置上的微观形貌。与图4(b)相比,图4(c)表现出更多的剪切变形和脉状纹理,这是由于在裂纹扩展阶段,更大的结合能导致剪切带的滑移阻力增大,产生的局部绝热造成了明显的粘性流动,这可以解释添加5%的Ni后,Mg-Cu-Tb非晶断裂强度的明显提高。在图4(d)中,剪切带数量较少,出现尖锐的断裂棱角,这说明剪切带在发展的过程中出现了局部的脆断。造成这种断裂方式的原因是剪切带在滑移的过程中,常常首先从微结构上化学不均匀性区域的边界脆断[6, 16-18]。

图4 Mg65Cu25-xNixTb10(x=0,5,10)非晶合金的断口形貌
Fig.4 Fracture morphologies of Mg65Cu25-xNixTb10 alloys: (a), (b) x=0; (c) x=5; (d) x=10
3 结论
1) 随着Ni含量的提高,Mg65Cu25-xNixTb10(x=0,5,10)非晶合金的玻璃转变温度Tg增大,开始结晶温度Tx降低,过冷液相区宽度ΔTx减小,热稳定性下降。
2) 随着Ni含量的提高,Mg65Cu25-xNixTb10(x=0,5,10)合金逐渐远离共晶成分点,导致约化玻璃转变温度Trg值从0.562降至0.542和0.530,非晶形成能力降低。
3) Mg65Cu20Ni5Tb10块体非晶合金的断裂强度约为867 MPa,添加适量的Ni元素增强了原子间结合能为其断裂强度高于Mg65Cu25Tb10非晶合金的主要原因。
REFERENCES
[1] Gyoo K S, Inoue A, Masumoto T. High mechanical strengths of Mg-Ni-Y and Mg-Cu-Y amorphous alloys with significant supercooled liquid region[J]. Mater Trans JIM, 1990, 11: 929-934.
[2] Wei Y X, Xi X K, Zhao D Q, Pan M X, Wang W H. Formation of MgNiPr bulk metallic glasses in air[J]. Materials Letters, 2005, 59: 945-947.
[3] Xi X K, Zhao D Q, Pan M X. Highly processable Mg65Cu25Tb10 bulk metallic glass[J]. Journal of Non-Crystalline Solids, 2004, 344: 189-192.
[4] Xi X K, Wang R J, Zhao D Q, Pan M X, Wang W H. Glass-forming Mg-Cu-RE(RE=Gd, Pr, Nd, Tb, Y, and Dy) alloys with strong oxygen resistance in manufacturability[J]. Journal of Non-Crystalline Solids , 2004, 344: 105-109.
[5] Guangyin Y, Inoue A. The effect of Ni substitution on the glass-forming ability and mechanical properties of Mg-Cu-Gd metallic glass alloys[J]. Journal of Alloys and Compounds, 2005, 387: 134-138.
[6] Liu W Y, Zhang H F, Hu Z Q, Wang H. Formation and mechanical properties of Mg65Cu25Er10 and Mg65Cu15- Ag10Er10 bulk amorphous alloys[J]. Journal of Alloys and Compounds, 2005, 397: 202-206.
[7] lnoue A, Zhang T, Masumoto T. Glass-forming ability of alloys[J]. J Non-Cryst Solids, l993, 156-158: 473-480.
[8] 杨元政,仇在宏,谢致薇,白晓军.合金元素对Cu60Zr30Ti10合金的非晶形成能力与显微硬度的影响[J]. 中国有色金属学报, 2006, 16(7): 1155-1160.
YANG Yuan-zheng, QIU Zai-hong, XIE Zhi-wei, BAI Xiao-jun. Effects of alloying elements on glass forming ability and microhardness of Cu60Zr30Ti10 alloy[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(7): 1155-1160.
[9] FANG Shou-shi, XIAO Xue-shan, XIA Lei, LI Wei-huo, DONG Yuan-da. Relationship between the widths of supercooled liquid regions and bond parameters of Mg-based bulk metallic glasses[J]. Journal of Non-Crystalline Solids, 2003, 321: 120-125.
[10] Inoue A, Zhang Tao, Kato M T. Mg-Ni-Y amorphous alloys with high mechanical strengths produce by a metallic mold casting method[J]. Mater Trans JIM, 1991, 32: 609-616.
[11] Inoue A, Nakamura T, Nishiyama N. Mg-Ni-Y bulk amorphous alloys with high tensile strength produced by a high-pressure die casting method[J]. Mater Trans JIM, 1992, 33: 937-945.
[12] Park E S, Chang H J, Kim D H, Ohkubo T, Hono K. Effect of the substitution of Ag and Ni for Cu on the glass forming ability and plasticity of Cu60Zr30Ti10 alloy[J]. Scripta Materialia, 2006, 54: 1569-1573.
[13] Ren Y L, Zuoa J H, Qiu K Q, Zhang H F, Hu Z Q. Eutectic structure and bulk glass formation in Mg-based alloys[J]. Intermetallics, 2004, 12: 1205-1209.
[14] FuKunaga T, Sugiura H, TaKeichi N, Aoki K. Experimental studies of atomic structure, electronic structure, and the electronic transport mechanism in amorphous Al-Cu-Y and Mg-Cu-Y ternary alloys[J]. Physical Review B, 1996, 54: 5-8.
[15] Madge S V, Alexander D T L. An EFTEM study of compositional variations in Mg-Ni-Nd bulk metallic glasses[J]. Journal of Non-Crystalline Solids, 2003, 31: 723-29.
[16] 程 明, 张士宏, Wert J A. 镁基大块非晶合金在过冷液相区流变行为本构关系[J]. 中国有色金属学报, 2005, 15(11): 1682-1686.
CHENG Ming, ZHANG Shi-hong, Wert J A. Constitutive equation for viscous flow behavior of Mg-based bulk metallic glass in supercooled liquid region[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(11): 1682-1686.
[17] Bailey N P. Atomistic simulations of Mg-Cu metallic glasses: mechanical properties[J]. Mater Sci Eng A, 2004, A387/389: 996-1000.
[18] Lu Z P, Liu C T, Dong Y D. Effects of atomic bonding nature and size mismatch on thermal stability and glass-forming ability of bulk metallic glasses[J]. Journal of Non-Crystalline Solids, 2004, 341: 93-100.
基金项目:陕西省科技攻关资助项目(2005k06-G2); 新世纪优秀人才支持计划资助项目;西北工业大学科技创新计划资助项目; 英才计划支持资助项目
收稿日期:2006-06-27;修订日期:2006-11-06
通讯作者:李金山,教授;电话:029-88491074;E-mail:ljsh@nwpu.edu.cn
摘 要:利用熔体铜模喷铸法制备出直径为3 mm的Mg65Cu25-xNixTb10(x=0, 5, 10) 非晶合金。利用X射线衍射、差热分析、压缩实验分析和扫描电镜分析了添加Ni元素对Mg-Cu-Tb非晶合金形成能力及力学性能的影响。研究表明:随着Ni含量的增加,合金的玻璃转变温度Tg增大;开始结晶温度Tx降低;过冷液相区宽度ΔTx减小, 约化玻璃转变温度Trg从0.562降至0.530,非晶形成能力逐渐降低。压缩实验结果表明:当Ni含量增加到5%时可以明显提高Mg-Cu-Tb-Ni非晶合金的断裂强度。


