
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 192-198
Relationships among bioleaching performance, additional elemental sulfur, microbial population dynamics and its energy metabolism in bioleaching of chalcopyrite
XIA Le-xian1, TANG Lu1, XIA Jin-lan1, YIN Chu1, CAI Li-yuan2, ZHAO Xiao-juan1,
NIE Zhen-yuan1, LIU Jian-she1, QIU Guan-zhou1
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 14 March 2011; accepted 21 June 2011
Abstract:
To estimate the relationships among bioleaching performance, additional elemental sulfur (S0), microbial population dynamics and its energy metabolism, bioleaching of chalcopyrite by three typical sulfur- and/or iron-oxidizing bacteria, Acidithiobacillus ferrooxidans, Leptospirillum ferriphilum and Acidithiobacillus thiooxidans with different levels of sulfur were studied in batch shake flask cultures incubated at 30 °C. Copper dissolution capability (71%) was increased with the addition of 3.193 g/L S0, compared to that (67%) without S0. However, lower copper extraction was obtained in bioleaching with excessive sulfur. Microbial population dynamics during chalcopyrite bioleaching process was monitored by using PCR-restriction fragment length polymorphism (PCR-RFLP). Additional S0 accelerated the growth of sulfur-oxidizing bacteria, inhibited the iron-oxidizing metabolism and led to the decrease of iron-oxidizing microorganisms, finally affected iron concentration, redox potential and bioleaching performance. It is suggested that mixed iron and sulfur-oxidizing microorganisms with further optimized additional S0 concentration could improve copper recovery from chalcopyrite.
Key words:
bioleaching; sulfur oxidization; iron oxidization; microbial community; chalcopyrite;
1 Introduction
Chalcopyrite (CuFeS2) is the most abundant and refractory copper-bearing mineral worldwide and also the principal mineral source from which copper is recovered commercially [1, 2]. Recently, compared to traditional pyrometallurgical techniques, development of biometallurgy for bioleaching chalcopyrite has attracted more and more interests due to its lower cost, more environment friendly, less intensive labor and lower energy requirements [3].
So far, more than twenty microbial species around the world have been detected in acidic mine drainage or commercial-operation bioleaching dump by the molecular micro-ecological technique analysis [4-6]. Thus, it is very difficult to clarify the behaviors of different microorganisms in bioleaching process, the interrelationship and interaction between the microorganisms and chalcopyrite, due to the different physiological properties and functions of various species. However, these species could be divided into three groups according to their energy metabolism—iron- or/and sulfur-oxidizing metabolism. Therefore, to avoid the disturbance from various species in complex consortia, it is very necessary and meaningful to simplify the microbial consortia and clarify the interaction and interrelationship of microorganism/chalcopyrite from the view of energy metabolism.
The bioleaching of chalcopyrite at low temperatures (<40 °C) usually uses mesophilic bacteria including Acidthiobacillus ferrooxidans, Acidthiobacillus thiobacillus or Leptospirillum spp. These bacteria can oxidize iron or/and sulfur at a high rate, rapid growth and are able to tolerate high solid density and iron concentration. The main role of iron oxidizers (A. ferrooxidans and Leptospirillum spp.) is to generate ferric iron to dissolve chalcopyrite. Then, elemental sulfur, an intermediate in leaching process, may accumulate and form a layer on the chalcopyrite surface that acts as a barrier against the diffusion of oxygen or Fe (III) ions, thus, inhibiting the complete dissolution of chalcopyrite [7]. However, sulfur oxidizer can remove elemental sulfur which has accumulated on the mineral’s surface and decrease the pH value due to their ability to oxidize the elemental sulfur to sulfuric acid. Thus, sulfur- or/and iron-oxidizing microorganisms are often mixed and inoculated into leaching systems of sulfide ores by means of their cooperative bioleaching [8, 9]. From the reports of LIU et al [10] and XIA et al [11], S0 was added to promote the growth of A. thiooxidans and recovery of metal from sulfide ores in some bioleaching process.
In this study, to elucidate the relationships among additional sulfur, bioleaching performance, microbial population dynamics and its energy metabolism, and the feasibility of enhancing the bioleaching, the microbial consortia are simplified into three typical species with iron- and/or sulfur-oxidizing metabolisms for A. ferrooxidans, A. thiooxidans and L. ferriphilum. And the influence of chalcopyrite bioleaching by different levels of additional sulfur is investigated.
2 Experimental
2.1 Microorganisms and minerals
The mesophilic microorganisms used in this study were Acidithiobacillus ferrooxidans, Leptospirillus ferriphilum and Acidithiobacillus thiooxidans based on their known properties. Microorganisms were grown on appropriate liquid media in pure culture at 30 °C and were used as inocula in mixed culture bioleaching experiments.
A. ferrooxidans and L. ferriphilum were grown in 9K medium with initial pH of 2.0 and 1.6, complemented with FeSO4·7H2O of 44.7 g/L and 89.4 g/L, respectively. A. thiooxidans was maintained in Starky basal salt medium with sulfur as the energy source. The 9K medium contained: (NH4)2SO4 3 g/L, K2HPO4 0.5 g/L, KCl 0.1 g/L, MgSO4·7H2O 0.5 g/L, and Starky-S medium was prepared as follows: (NH4)2SO4 3 g/L, KH2PO4 3 g/L, MgSO4·7H2O 0.5 g/L, CaCl2·2H2O 0.25 g/L, S0 10 g/L.
The minerals used in this study were provided by the School of Minerals Processing and Bioengineering, Central South University, China. X-ray diffraction (XRD) analysis of the mineral sample showed that chalcopyrite was the major component, and galena and gypsum were minor components. Chemical analysis revealed that it contained 32.6% Cu, 25.3% Fe, 30.6% S, 3.7% Pb, 0.2% Ca, according to inductively coupled plasma-atomic emission spectroscopy (ICP-AES). The mineral was ground to the particle size of 45 μm over 90%.
2.2 Bioleaching experiment
Bioleaching experiments were carried out in 250 mL shake flasks containing 150 mL iron-free 9K medium, which were incubated at 30 °C, and stirred at 170 r/min. The initial pH was adjusted to 1.8 by 0.5 mol/L H2SO4, and a pulp density of 4% (w/v) was employed in this experiment.
Pure cultures of A. ferrooxidans, L. ferriphilum and A. thiooxidans were harvested by centrifugation and washed twice in sterilized water, which were adjusted to pH 2.0 with sulfuric acid, and then they were mixed and inoculated (initial cell number of each species was 4×106 cell/mL) into the shake flasks. Redox potentials, pH values, and the concentrations of Cu2+, SO42-, Fe2+ and total iron were analyzed every three days.
Bioleaching experiments were performed using A. ferrooxidans, L. ferriphilum and A. thiooxidans with the following systems: 1) Abiotic control; 2) Consortia without sulfur (CWOS); 3) Consortia with moderate sulfur (CWMS, 3.193 g/L S0, such extra elemental sulfur could make all copper and iron in chalcopyrite oxidized to ferric and copper iron); 4 Consortia with excessive sulfur (CWES, 6.386 g/L S0).
2.3 Analytical method
The concentrations of Cu2+ and total Fe in solution were determined by atomic absorption spectrophotometer. The pH values were measured with PhS-3C acid meter. The redox potentials, which indicated the ratio of Fe(III)/Fe(II), were measured with Pt electrode, and a saturated calomel electrode as the reference electrode. Sulfate ions were quantified by a barium sulfate turbidimetric colorimetric method [12]. Bacterial counting in ore was done by agitating the ore material in 0.1% Tween 20 solution for detachment of cells from mineral particles [13].
2.4 Bacterial population analysis
Bacteria in solution and adsorbed on mineral were harvested by agitation with 0.1% Tween 20, sedimentation and centrifugation. Total DNA was extracted by using a TIANamp genomic DNA purification Kit (Tiangen Biotech, Co., Ltd., China). Community 16S rDNA genes were first amplified by using the universal primer set 1492R (5’-CGGCTACCTTGTTACGACTT-3’) and 27F (5’-AGAGTTTGATCCTGGCTCAG-3’). The PCR product was separated by gel electrophoresis on a 1% agar gel in Tris/acetate-buffer and analyzed by staining with Ethidium Bromide (EB) under UV light. The band of the expected size (~1460 bp) was cut-off and purified with a commercial Kit (Gel Extraction Kit, Promega, USA). The purified 16S rDNA was cloned into the PUM-T vector (Biobasic, Co., Ltd, Canada) and transformed into E.coli DH5α competent cells (Tiangen Biotech, Co., Ltd., China) for blue-white screening. About 50 white clones were randomly selected from each library, and the inserted fragments were amplified with the vector specific M13R and M13F primers and digested by the restriction enzymes RsaI and MspI overnight at 37 °C. The product was detected by 3% (w/v) agarose gel electrophoresis.
3 Results and discussion
3.1 Copper extraction by mixed microorganisms with or without additional sulfur
The results of bioleaching chalcopyrite by mixed sulfur- and iron-oxidizing microorganisms are shown in Fig. 1. The copper recovery increased quickly from the 3rd day to the 9th day, achieved to 31% in CWOS, after that it changed slowly and finally reached 67%. However, the copper recovery in CWMS increased slowly before the 12th day, thereafter, the soluble copper increased continuously and the final leaching ratio was 71% after bioleaching for 30 d. The abiotic control and the CWES showed the slight leaching ability and only 9% and 44% copper were dissolved in solution, respectively. Under the present additional moderate sulfur concentration, although the bioleaching ratio had only a light increase in CWMS, compared to that in CWOS, a further optimized moderate additional sulfur could improve the bioleaching ratio of chalcopyrite. In contrast, copper dissolution could be inhibited due to bacteria utilizing excessive sulfur prior to chalcopyrite.
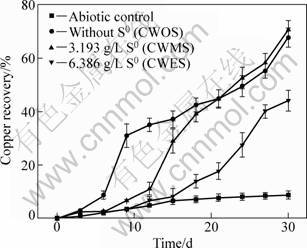
Fig. 1 Copper recovery from chalcopyrite with additional elemental sulfur
3.2 Impact of additional sulfur on iron oxidation activities of mixed microorganisms
Many researchers [14] reported that iron (Fe3+ and Fe2+) played a key role in the bioleaching of chalcopyrite. The distinguishing feature of both direct and indirect mechanisms of sulphide oxidation was necessary for ferric ions to participate in the mineral dissolution. Therefore, the concentrations of Fe3+, total iron and redox potential were measured and analyzed. Ferric iron concentrations in CWMS were initially kept constant (Fig. 2(a)), subsequently increased from the 12th day and finally reached 2.69 g/L, which were towered over that in CWOS and CWES. This indicated that ferric ions, as an oxidant, could accelerate the dissolution of chalcopyrite (Fig. 1 and Fig. 2(a)). Furthermore, the difference of Fe3+ concentration could be attributed to adding different levels of sulfur.
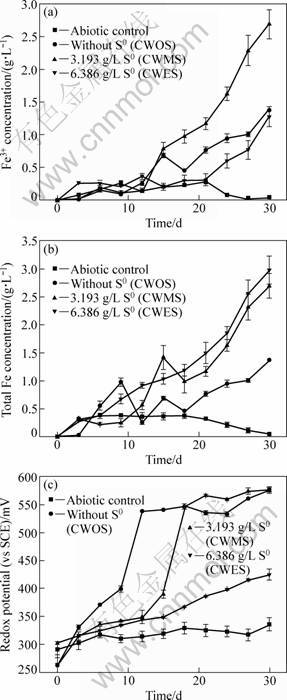
Fig. 2 Variation of ferric iron concentration (a), total iron concentration (b) and redox potential (c) during bioleaching of chalcopyrite by mixed cultures of mesophiles
A decrease of Fe3+ concentration and total iron in CWOS from the 9th day to the 12th day suggested that the Fe3+ precipitation (such as jarosite) was generated (Reaction (1), Figs. 2(a) and (b)). The formed jarosite caused an obstruction to mineral-microbe and mineral-oxidation reagent contact and formed mass transfer barrier to nutrients [15, 16]. Consequently, the decrease of Fe3+ and total iron may result in the reduction of chalcopyrite dissolution (Fig. 1, Figs. 2(a) and (b)). However, the increase of redox potential in CWOS was detected from the 9th day to 12th day (Fig. 2(c)), indicating that Fe2+ was rapidly oxidized to Fe3+ due to the high iron-oxidizing activity of the microbial consortia from the 9th day to 12th day (Reaction (2)). As a result, it led to the rapid Fe3+ accumulation and precipitation. The change of Fe3+ , total iron and redox potential in CWMS displayed the same tendency from the 15th day to 18th day as those in CWOS from the 9th day to 12th day (Figs. 2(a), (b) and (c)). In other words, additional sulfur could limit or reduce iron-oxidizing activity in the microbial consortia. Consequently, it postponed and limited the Fe3+ accumulation and subsequently Fe3+ precipitation. The postponed generation of Fe3+ precipitation may be a main reason for the higher leaching ratio in CWMS compared with that in CWOS.
3Fe3++K++2HSO4-+6H2O→KFe3(SO4)2(OH)6+8H+ (1)
4Fe2++O2+4H+![]() 4Fe3++2H2O (2)
4Fe3++2H2O (2)
When excessive sulfur was added in CWES, the inhibition of iron-oxidizing activity became more obvious. This could be further testified by the evidence of the highest total iron and ferrous ions concentrations and the lowest redox potential (excluding abiotic control) in CWES. This indicated that although iron precipitation was inhibited, poor iron-oxidizing activity of mixed bacteria led to reduction of copper extraction.
3.3 Impact of additional sulfur on sulfur oxidation activities of mixed bacteria
The sulfur-oxidizing activity of the microbial consortia could be determined by measuring the SO42- ions concentration. It can be seen from Fig. 3(a) that sulfate production increased at various rates throughout the bioleaching experiments. Although the sulfate production initially increased in CWOS, the sulfur- oxidizing rate was greater in CWMS than in CWOS from the 12th day, and the final sulfate production of CWOS, CWMS, CWES was 24.27, 32.99 and 34.70 g/L, respectively. The results suggested that the more the additional sulfur was, the higher the SO42- ions concentration was, and the higher the sulfur-oxidizing activity was. The enhancement of sulfur-oxidizing activity under additional sulfur was also denominated by the change of pH in Fig. 3(b). The pH value was initially increased due to protonic attack during acid dissolution of chalcopyrite and the consumption of H+ ion by ferrous ion oxidation (Reactions (2) and (3)). After that, the generation of H+ ion from sulfur oxidization surpassed its consumption from iron oxidization and acid dissolution (Reaction (4)). Finally, the whole H+ ion concentration increased due to the improvement of sulfur-oxidizing activity in the microbial consortia, and the bioleaching then proceeded more efficiently [17]. Thus, it was concluded that additional sulfur could limit the iron-oxidizing activity and improve the sulfur-oxidizing activity of the microbial consortia including the sulfur- and iron-oxidizing metabolism based on the analysis of Figs. 2 and 3. Further, the improved sulfur-oxidizing activity could maintain a low pH and increase the acid dissolution of chalcopyrite.
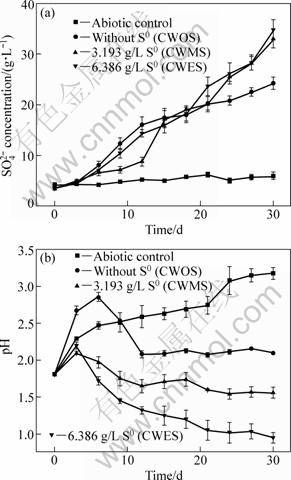
Fig. 3 Variation of SO42- ions (a) and pH (b) during chalcopyrite bioleaching
CuFeS2+4H+→Fe2++Cu2++2H2S (3)
2S0+2H2O+3O2![]() 2SO42-+4H+ (4)
2SO42-+4H+ (4)
Additionally, it was reported that the ferric iron at pH 2.0-2.6 was more easy to form jarosite precipitate on the mineral surface than at pH 0.9-2.0 [18-21]. VILCAEZ et al [22] also testified that providing excess sulfuric acid concentration could avoid the formation of iron salt precipitates and improve the bioleaching performance. For CWES, the additional elemental sulfur enhanced the sulfur-oxidizing activity of microbial consortia, and the medium exhibited the lowest pH value at the early stage of bioleaching (Fig. 3(a)), which should accelerate the bioleaching process based on the above reports. However, the dissolution rates of copper and iron were lower than those without elemental sulfur. This could be explained as that the lower pH (<1.0) and redox potential (<400 mV) damaged and reduced the sulfur and iron-oxidizing activity of bacteria. Thus, although additional sulfur inhibited the iron-oxidizing activity of microbial consortia, moderate additional sulfur could improve the bioleaching performance by means of inhibiting or decreasing the jarosite formation, which could be attributed to the increase of sulfur-oxidizing activity and decrease of pH value.
3.4 Monitoring of bacterial population without additional elemental sulfur
In this study, we constructed a simple bioleaching ecological community consisting of three typical energy metabolism microbial species, A. ferrooxidans, A. thiooxidans and L. ferriphilum, which exist widely in natural acidic mineral drainage and mesophilic commercial bioleaching operation.
Among the RFLP analyzed, the community structure of CWOS varied dramatically during the bioleaching process (Fig. 4(a)), the iron- and sulfur- oxidizing microorganisms, A. ferrooxidans, were largely dominant (64.7%) over A. thiooxidans (less than 26.5%) at the early stage of the bioleaching process. At the late period of process, however, A. thiooxidans, a special sulfur-oxidizing microorganism, gradually became the most dominant bacteria (52.6%) in the microbial community, while A. ferrooxidans decreased to 44.7%. Actually, at the early stage of bioleaching, there existed no elemental sulfur in the system, and A. thiooxidans, the special sulfur-oxidizing species, grew slowly due to no energy resources—elemental sulfur. Later, sulfur, an intermediate of leaching (Reaction (5)), was generated, and only at this time, A. thiooxidans could exuberantly grow and become the dominant species in consortia due to its supplementary energy.
CuFeS2+4Fe3+→5Fe2++Cu2++2S0 (5)
Likewise, A. ferrooxidans mainly grew by its iron-oxidizing metabolism due to no elemental sulfur at the early stage of bioleaching and its main function in this stage was to enhance the dissolution of chalcopyrite by oxidizing Fe2+ to Fe3+. The gradually increased proportion of A. thiooxidans showed that elemental sulfur was continuously formed as a function of bioleaching and continuously oxidized to sulfate by its sulfur oxidizing metabolism. Thus, if there did not exist sulfur-oxidizing metabolism, a passivation layer containing sulfur would be formed and covered on mineral surface, which consequently inhibited the bioleaching. Therefore, the analysis of microbial ecology in this simply consortia also supported the report on the function of A. thiooxidans to remove the passivation layer and promote the dissolution of chalcopyrite [23]. The proportion of L. ferriphilum was below 10% and gradually decreased during bioleaching of chalcopyrite. This indicated that L. ferriphilum was less important in the investigated system without elemental sulfur.
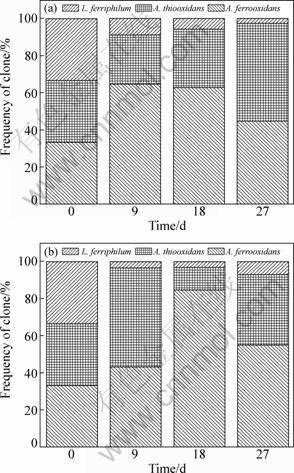
Fig. 4 Changes in community composition of three microbial species without additional sulfur (a), and microbial community shifts with additional 3.193 g/L sulfur (b)
3.5 Monitoring of bacterial population with additional moderate sulfur
When elemental sulfur was added, the microbial consortia changed rapidly in CWMS (Fig. 4(b)), especially for A. ferrooxidans and A. thiooxidans. Examination of both solid and liquid phases showed that A. thiooxidans was the dominant microorganism (53%) due to the oxidation of elemental sulfur at the first stage, then with the elemental sulfur consumption, A. ferrooxidans proportion was significantly increased up to 85% while the presence of A. thiooxidans was decreased to approximately 12%. At the last stage, however, the proportion of A. ferrooxidans decreased to 50%, and A. thiooxidans gradually increased to 38%. The proportion of L. ferriphilum stabilized at approximately 3% during the first 18 days, followed by an increase thereafter. Compared to CWOS, sulfur oxidizer utilized additional elemental sulfur rather than chalcopyrite at the first stage. Therefore, the proportion of A. thiooxidans increased and became the dominant species. The proportion of L. ferriphilum decreased in CWMS. This reduction was much more obvious for CWMS than for CWOS, which also indicated that additional sulfur inhibited the iron-oxidizing metabolism and led to the decease of special iron-oxidizing microorganisms, similar to the evidence from the analysis of total iron, redox potential and Fe3+. Due to the existence of two kinds of energy metabolism (iron/sulfur metabolism) in A. ferrooxidans, it could evade the inhibition of iron-oxidizing metabolism by means of the alternative sulfur- metabolism function, thus, its proportion did not decrease but increased at the middle of process. Furthermore, the results showed that A. thiooxidans together with A. ferrooxidans initially oxidized the elemental sulfur to sulfuric acid in bioleaching system with additional sulfur, which could inhibit the formation of passivation layer, then an exuberant growth of A. ferrooxidans maintained the high concentration of ferric iron (Fig. 2(a)), and promoted the dissolution of chalcopyrite [24]. A. thiooxidans also played a key role in dispelling intermediate including jarosite, sulfur and other reduced sulfide at the last stage of bioleaching process. Similar to CWOS, the low proportion of L. ferriphilum also showed its less importance compared to A. ferrooxidans and A. thiooxidans.
During the two bioleaching system processes, the cell density in CWOS, CWMS and CWES was maintained at very large number (>108 cell/mL) at later stage, indicating that the mixed culture had high growth activity. The population of L. ferriphilum was maintained at a low level, which may be due to its low copper toleration (<5 mmol/L), preferring higher redox potential (>690 mV) and temperature (>30 °C) [25, 26].
4 Conclusions
1) Additional sulfur could inhibit the iron-oxidizing activity, therefore, modify the distribution of iron- and sulfur-oxidizing activity and the proportion of sulfur- and/or iron-oxidizing species in microbial consortia, consequently, had a significant influence on the pH, dissolution of iron, passivation layer, redox potential and bioleaching performance.
2) A. thiooxidans played a significant role in inhibiting and dispelling passivation layer and improving the acid dissolution of chalcopyrite in bioleaching process. The role and the presence of L. ferriphilum seemed less importance.
3) Culture including iron- and sulfur-oxidizing metabolism with further optimized moderate elemental sulfur could promote the bioleaching performance of chalcopyrite.
References
[1] BEVILAQUA D, DIEZ-PEREZ I, FUGIVARA C S, SANZ F, BENEDETTI A V, GARCIA O. Oxidative dissolution of chalcopyrite by Acidithiobacillus ferrooxidans analyzed by electrochemical impedance spectroscopy and atomic force microscopy [J]. Bioelectrochemistry, 2004, 64(1): 79-84.
[2] DEVI N B, MADHUCHHANDA M, RAO K S, RATH P C, PARAMGURU R K. Oxidation of chalcopyrite in the presence of manganese dioxide in hydrochloric acid medium [J]. Hydrometallurgy, 2000, 57(1): 57-76.
[3] WATLING H R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review [J]. Hydrometallurgy, 2006, 84(1-2): 81-108.
[4] BOND P L, DRUSCHEL G K, BANFIELD J F. Comparison of acid mine drainage microbial communities in physically and geochemically distinct ecosystems [J]. Applied and Environmental Microbiology, 2000, 66: 4962-4971.
[5] BAKER B J, BANFIELD J F. Microbial communities in acid mine drainage [J]. Fems Microbiology Ecology, 2003, 44: 139-152.
[6] JOHNSON D B, HALLBERG K B. The microbiology of acidic mine waters [J]. Research in Microbiology, 2003, 154: 466-473.
[7] NAVA D, GONZALEZ I, LEINEN D, RAMOS-BARRADO J R. Surface characterization by X-ray photoelectron spectroscopy and cyclic voltammetry of products formed during the potentiostatic reduction of chalcopyrite [J]. Electrochimica Acta, 2008, 53(14): 4889-4899.
[8] SASAKI K, TSUNEKAWA M, OHTSUKA T, KONNO H. The role of sulfur-oxidizing bacteria Thiobacillus thiooxidans in pyrite weathering [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1998, 133(3): 269-278.
[9] SCHIPPERS A, SAND W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur [J]. Applied and Environmental Microbiology, 1999, 65(1): 319-321.
[10] LIU Y G, ZHOU M, ZENG G M, WANG X, LI X, FAN T, XU W H. Bioleaching of heavy metals from mine tailings by indigenous sulfur-oxidizing bacteria: Effects of substrate concentration [J]. Bioresource Technology, 2008, 99(10): 4124-4129.
[11] XIA L X, DAI S L, YIN C, HU Y, LIU J S, QIU G Z. Comparison of bioleaching behaviors of different compositional sphalerite using Leptospirillum ferriphilum, Acidithiobacillus ferrooxidans and Acidithiobacillus caldus [J]. Journal of Industrial Microbiology & Biotechnology, 2009, 36(6): 845-851.
[12] DING Jian-nan. Isolation, identification of thermophilic leaching microorganisms and studies on their bioleaching mechanism and potential [D]. Changsha: Central South University, 2007: 70-72. (in Chinese)
[13] MARHUAL N P, PRADHAN N, KAR R N, SUKLA L B, MISHRA B. Differential bioleaching of copper by mesophilic and moderately thermophilic acidophilic consortium enriched from same copper mine water sample [J]. Bioresource Technology, 2008, 99(17): 8331-8336.
[14] CRUNDWELL F K. How do bacteria interact with minerals? [J]. Hydrometallurgy, 2003, 71(1-2): 75-81.
[15] KODALI B, RAO M B, NARASU M L, POGAKU R. Effect of biochemical reactions in enhancement of rate of leaching [J]. Chemical Engineering Science, 2004, 59(22-23): 5069-5073.
[16] TSHILOMBO A F, PETERSEN J, DIXON D G. The influence of applied potentials and temperature on the electrochemical response of chalcopyrite during bacterial leaching [J]. Minerals Engineering, 2002, 15(11): 809-813.
[17] DOPSON M, LINDSTROM E B. Analysis of community composition during moderately thermophilic bioleaching of pyrite, arsenical pyrite, and chalcopyrite [J]. Microbial Ecology, 2004, 48(1): 19-28.
[18] POGLIANI C, DONATI E. Immobilisation of Thiobacillus ferrooxidans: Importance of jarosite precipitation [J]. Process Biochemistry, 2000, 35(9): 997-1004.
[19] DAOUD J, KARAMANEV D. Formation of jarosite during Fe2+ oxidation by Acidithiobacillus ferrooxidans [J]. Minerals Engineering, 2006, 19(9): 960-967.
[20] KOTSIOPOULOS A, HANSFORD G S, RAWATLAL R. An investigation into the dynamics of chalcopyrite bioleaching [J]. Aiche Journal, 2010, 56(10): 2650-2661.
[21] STOTT M B, WATLING H R, FRANZMANN P D, SUTTON D. The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching [J]. Minerals Engineering, 2000, 13(10-11): 1117-1127.
[22] VILCAEZ J, YAMADA R, INOUE C. Effect of pH reduction and ferric ion addition on the leaching of chalcopyrite at thermophilic temperatures [J]. Hydrometallurgy, 2009, 96(1-2): 62-71.
[23] LARA R H, VALDEZ-PEREZ D, RODRIGUEZ A G, NAVARRO-CONTRERAS H R, CRUZ R, GARCIA-MEZA J V. Interfacial insights of pyrite colonized by Acidithiobacillus thiooxidans cells under acidic conditions [J]. Hydrometallurgy, 2010, 103(1-4): 35-44.
[24] KINNUNEN P, HEIMALA S, RIEKKOLA-VANHANEN M L, PUHAKKA J A. Chalcopyrite concentrate leaching with biologically produced ferric sulphate [J]. Bioresource Technology, 2006, 97(14): 1727-1734.
[25] TIAN J, WU N F, LI J, LIU Y J, GUO J, YAO B, FAN Y L. Nickel-resistant determinant from Leptospirillum ferriphilum [J]. Applied and Environmental Microbiology, 2007, 73(7): 2364-2368.
[26] JOHNSON D B, GHAURI M A, SAID M F. Isolation and characterization of an acidophilic, heterotrophic bacterium capable of oxidizing ferrous iron [J]. Applied and Environmental Microbiology, 1992, 58(5): 1423-1428.
元素硫对黄铜矿生物浸出行为及群落结构的影响
夏乐先1,汤 露1,夏金兰1,尹 礎1,柴立元2,赵小娟1,聂珍媛1,柳建设1,邱冠周1
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 冶金科学与工程学院,长沙 410083
摘 要:研究3种典型铁/硫代谢菌—Acidithiobacillus ferrooxidans, Leptospirillum ferriphilum及 Acidithiobacillus thiooxidans 混合浸出黄铜矿过程中铁/硫氧化活性、群落结构(PCR-RFLP)的变化,以及不同浓度的元素硫对其影响。结果发现,加入3.193 g/L元素硫能促进细菌的表观硫氧化活性,改变浸矿体系的群落结构,并进一步影响钝化层的形成、金属离子的溶出,其浸出率(71%)较未添加硫的(67%)有一定程度的提高。而过量的元素硫会抑制铜的浸出(浸出率44%)。
关键词:生物浸出;硫氧化;铁氧化;群落结构;黄铜矿
(Edited by YUAN Sai-qian)
Foundation item: Project (20803094) supported by the National Natural Science Foundation of China; Project (20100471233) supported by the Postdoctoral Foundation of China and the Postdoctoral Foundation of Central South University
Corresponding author: XIA Le-xian; Tel: +86-731-86504165; Fax: +86-731-88879815; E-mail: xialex888@163.com
DOI: 10.1016/S1003-6326(11)61160-6
Abstract: To estimate the relationships among bioleaching performance, additional elemental sulfur (S0), microbial population dynamics and its energy metabolism, bioleaching of chalcopyrite by three typical sulfur- and/or iron-oxidizing bacteria, Acidithiobacillus ferrooxidans, Leptospirillum ferriphilum and Acidithiobacillus thiooxidans with different levels of sulfur were studied in batch shake flask cultures incubated at 30 °C. Copper dissolution capability (71%) was increased with the addition of 3.193 g/L S0, compared to that (67%) without S0. However, lower copper extraction was obtained in bioleaching with excessive sulfur. Microbial population dynamics during chalcopyrite bioleaching process was monitored by using PCR-restriction fragment length polymorphism (PCR-RFLP). Additional S0 accelerated the growth of sulfur-oxidizing bacteria, inhibited the iron-oxidizing metabolism and led to the decrease of iron-oxidizing microorganisms, finally affected iron concentration, redox potential and bioleaching performance. It is suggested that mixed iron and sulfur-oxidizing microorganisms with further optimized additional S0 concentration could improve copper recovery from chalcopyrite.


