
J. Cent. South Univ. (2021) 28: 325-337
DOI: https://doi.org/10.1007/s11771-021-4605-1
Effects of CO2 gassy-supercritical phase transition on corrosion behaviors of carbon steels in saturated vapor environment
ZENG De-zhi(曾德智)1, HUANG Zhi-yao(黄致尧)1, 2, YU Zhi-ming(喻智明)1, SHI Shan-zhi(石善志)3,
YI Yong-gang(易勇刚)3, LIU Cong-ping(刘从平)3, TIAN Gang(田刚)3, SUN Yi-cheng(孙宜成)3
1. State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Southwest Petroleum University, Chengdu 610500, China;
2. The Second Research Institute of Civil Aviation Administration of China, Chengdu 610041, China;
3. Research Institute of Engineering Technology, Xinjiang Oil Field Company, PetroChina,Karamay 834000, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
Corrosion behaviors of P110 and N80 tubular steels in CO2 gas phase and supercritical (S-CO2) phase in a saturated water vapor environment were explored in corrosion weight loss experiments by SEM, EDS, XRD, XPS and cross-section analysis techniques. With the increase in CO2 partial pressure, the average corrosion rate increased first and then decreased. The average corrosion rate reached the maximum value under the near-critical pressure. When CO2 partial pressure further increased to be above the critical pressure, the average corrosion rate gradually decreased and local aggregation of molecules was weakened.
Key words:
carbon capture and storage; supercritical carbon dioxide; corrosion product; corrosion mechanism;
Cite this article as:
ZENG De-zhi, HUANG Zhi-yao, YU Zhi-ming, SHI Shan-zhi, YI Yong-gang, LIU Cong-ping, TIAN Gang, SUN Yi-cheng. Effects of CO2 gassy-supercritical phase transition on corrosion behaviors of carbon steels in saturated vapor environment [J]. Journal of Central South University, 2021, 28(2): 325-337.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4605-11 Introduction
In the exploitation of oil and gas fields with high CO2 content, the storage and reinjection technology for capturing CO2 is an effective way to reduce carbon emissions [1]. CO2 reinjection can keep formation pressure and enhance oil and gas recovery. In recent years, the serious corrosion damage, major influencing factors, failure mechanisms, and corrosion protection measures in CO2 reinjection have been explored [2]. The corrosion under high CO2 content environment has become a hot spot in the studies on the exploitation of oil and gas fields as well as corrosion protection. Corrosion protection of tubing steel in supercritical CO2 has been explored in the exploitation of some blocks in South China Sea and Xinjiang, China. At present, the mechanism and influencing factors of CO2 corrosion have been reported [3, 4]. The mixed acid gas corrosion, corrosion prediction model, and corrosion life prediction remain to be further explored [5-7]. The corrosion of low-alloy steel in the CO2-dissolved liquid phase is mainly uniform corrosion and the average corrosion rate is much higher than that in the CO2-vapor environment [8].
However, the localized corrosion of the steel in the CO2-vapor environment may lead to corrosion perforation and corrosion failure. Therefore, it is necessary to explore the severe overall corrosion of low-alloy materials in the CO2-dissolved liquid phase and localized corrosion in supercritical CO2-vapor environments.
The supercritical point of carbon dioxide is Tc=31.1 °C, Pc=7.38 MPa (Figure 1). It is not difficult to achieve the supercritical state of carbon dioxide. In the exploitation of oil and gas fields with high content of CO2, it is possible to realize this condition for the critical point in deep wells and gas injection wells. CO2-vapor environments include the environment with high CO2 content and the environment with high vapor content. The steel corrosion rates under these two conditions are significantly different. Under normal pressure conditions, the corrosion rate in the environment with high vapor content can be tens of times higher than that in the environment with high CO2 content [9]. The supercritical CO2-saturated vapor environment belongs to the environment with high CO2 content. Physicochemical properties of CO2 in the near-supercritical state are different from those in the normal state. Therefore, the CO2-vapor corrosion behavior in near-supercritical state needs to be further explored.

Figure 1 CO2 p-T phase map
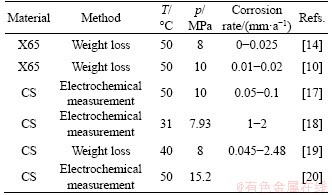
The corrosion mechanism of steels in supercritical CO2-saturated vapor environment is that supercritical CO2 dissolves in the tiny film of condensed water to produce H2CO3, which induces steel corrosion [10, 11]. Steel corrosion in non-supercritical CO2-vapor environment involves the similar corrosion mechanism. It is generally believed that there is an extreme corrosion rate of carbon steel and low alloy when the temperature in supercritical CO2-saturated vapor environment is changed. However, the influences of gassy- supercritical phase transition of CO2 on corrosion behaviors were seldom explored. Recent studies on steel corrosion in supercritical CO2-vapor environment are provided in Table 1. Water content is one of the most important factors affecting corrosion degree in supercritical CO2-vapor environment. In addition, steel corrosion in the acidic gas environment of supercritical CO2 steam mixed with H2S, SO2, NO2, etc. has been reported [5, 12, 13]. In general, the effect of water content on corrosion rate is more significant than that of other factors [12-16]. Therefore, it is necessary to control the water vapor content in supercritical CO2 environment.
Table 1 Steel corrosion in supercritical CO2-vapor environments
2 Experimental
2.1 Experimental materials
Alloy compositions and metallographic structure of P110 and N80 steels used in the experiments are shown in Table 2 and Figure 2. The methods of heat-treatment and corresponding microstructures are shown in Table 3. The corrosive medium is CO2-saturated vapor. Three parallel samples of each steel (30 mm×15 mm×3 mm) were set for each pressure. The surface roughness of the corrosion test metal samples (Ra) is 0.1-0.2 μm.
2.2 Methods
A high-temperature high-pressure (HTHP) autoclave (Figure 3) was used in the experiment. The sample was deoiled with petroleum ether, dehydrated with anhydrous ethanol, dried, and then weighed.
Table 2 Alloy compositions of P110 steel and N80 steel (wt%)
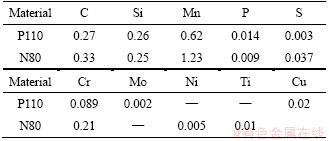
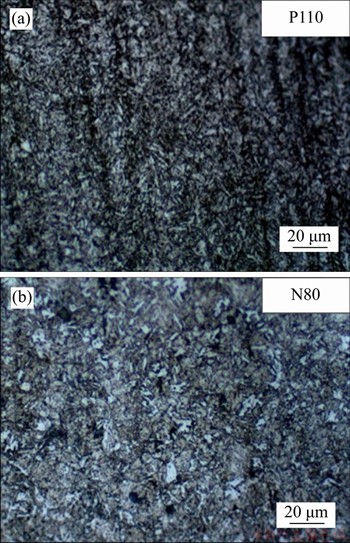
Figure 2 Metallographic structure images of P110 (a) and N80 (b) steel used in experiments
Table 3 Heat-treatment methods and microstructures

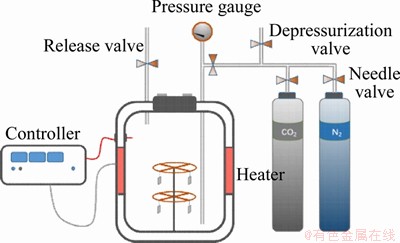
Figure 3 HTHP autoclave
Before the experiment, enough deionized water (1.75 L) was injected into the autoclave, and high-purity N2 (99.99% N2 content) was continuously purged into the autoclave to remove oxygen for 8 h. The sample was sealed and then high-purity nitrogen was purged to remove oxygen again. Then the temperature was raised to 100 °C. After water vapor in autoclave was saturated, CO2 was continuously bubbled for 2 h to remove the remaining oxygen in the autoclave and the partial pressure of CO2 was controlled at different pressures (3, 6, 8, 13 and 18 MPa). After 72 h of static experiments, the samples were taken out and immediately washed with the mixed solution (3% hydrochloric acid and 1% hexamethylenetetramine) until all corrosion products were completely removed. Then the samples were washed with deionized water and then washed in anhydrous ethanol. After drying and weighing, the weight loss was calculated.
2.3 Surface characterization
After the weight loss tests, the surface and cross-section of the samples were observed under a scanning electron microscope (SEM, JSM-7500F). The components of corrosion products were analyzed by an energy dispersive spectroscope (EDS) with an acceleration voltage of 15 kV, an X-ray diffractometer (XRD, XPert Pro MPD) and an X-ray photon spectroscope (XPS, Phi-Quantera II).
3 Results
3.1 Corrosion loss
The results of corrosion weight loss are shown in Figure 4. Due to the similar alloy composition, the corrosion rates of N80 and P110 steels were similar in CO2-saturated vapor environment. When CO2 partial pressure was increased from 3 to 8 MPa, the corrosion rate increased rapidly. When CO2 partial pressure was increased from 6 to 8 MPa, the corrosion rate was increased by 1.4 times. At 8 MPa, the corrosion rates of N80 and P110 steels respectively reached 0.21 and 0.24 mm/a. When the partial pressure of CO2 further increased, the corrosion rates of N80 and P110 steels gradually decreased to about 0.1 mm/a. The corrosion rate of carbon steel was significantly accelerated due to the increase in the saturated vapor content at high temperature. The highest corrosion rate occurred under the near-critical pressure.
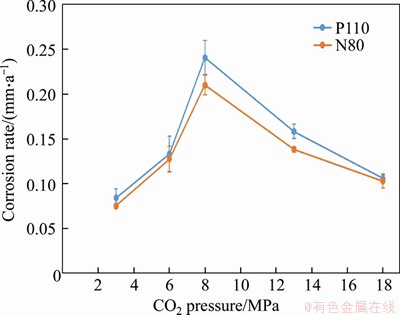
Figure 4 Corrosion rate of P110 and N80 steels in CO2-saturated vapor environment (T=100 °C) after 72 h
3.2 Surface and cross-section morphologies
SEM images of the N80 steel samples with 72-h corrosion in CO2-saturated vapor environment are shown in Figure 5 (100 times); then magnify the approximately circular condensate areas in Figure 5 to 1000 times to get Figure 6. The regions where a large number of cubic crystals accumulated are the corrosion areas. At low magnification (100 times), under the CO2 partial pressures of 8 MPa (Figure 5(c)) and 13 MPa (Figure 5(d)) the coverage of the condensed water films (corrosion area) on the surface of the samples was significantly larger than those under other partial pressures. In particular, the surfaces of the samples were almost completely covered by the condensation water films under the near-critical pressure (8 MPa) and partial corrosion areas of condensation water films were also observed under the pressure of 18 MPa (Figure 5(e)). However, the corrosion areas of condensed water films under the pressures of 3 MPa (Figure 5(a)) and 6 MPa (Figure 5(d)) were much smaller than that formed in the supercritical CO2 environment. No or few cubic crystals were found outside the condensate areas.
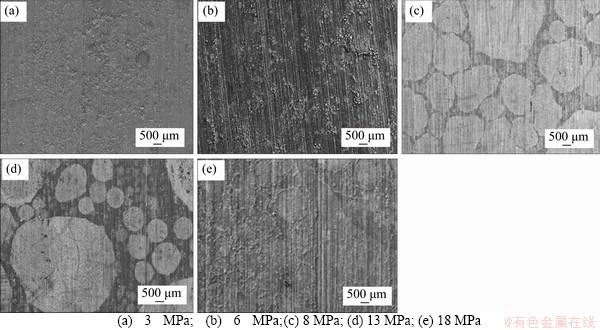
Figure 5 SEM images (100 times) of N80 steel in CO2-saturated water vapor environment (T=100 °C) under different CO2 partial pressures after 72 h:
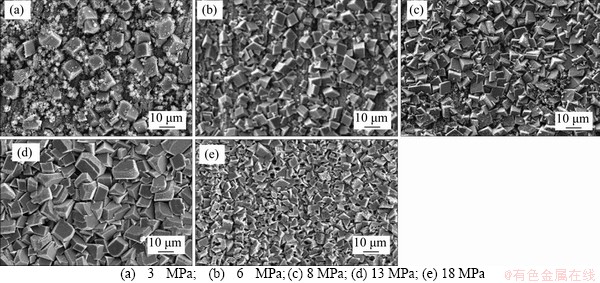
Figure 6 SEM images (1000 times) of N80 steel in CO2-saturated vapor environment (T=100 °C) under different CO2 partial pressures after 72 h:
At the magnification of 1000 times, the corrosion products formed under 5 different pressures were all cubic crystals, whereas the sizes of crystals and the intensive degree of the accumulation under 5 different pressures showed some differences. Under different CO2 partial pressures (3 MPa (Figure 6(a)), 6 MPa (Figure 6(b)) and 8 MPa (Figure 6(c))), there were significant gaps between crystals and the cubic crystal accumulation was looser than that at 13 MPa (Figure 6(d)) and 18 MPa (Figure 6(e)). The crystal accumulation became dense under the pressure of 13 MPa (Figure 6(d)). When the CO2 partial pressure was increased to 18 MPa (Figure 6(e)), the size of crystals became smaller and the accumulation became denser. The EDS of corrosion products (Figures 7(a)-(c)) showed the main elements of cubic crystals. The composition of corrosion products in the low partial pressure environment was basically consistent with that in the supercritical CO2 environment.
The cross-sectional morphology of corrosion products of N80 steel in CO2-saturated vapor environment is shown in Figure 8. Under the low CO2 partial pressure, no obvious local corrosion was observed (Figure 8(a)), so no cross-section morphologies with higher resolution are adopted. When the partial pressure of CO2 increased to the critical pressure (Figure 8(b)), the area of the observed local corrosion gradually increased. As the pressure further increased, the local corrosion phenomenon was still observed. However, the depth of single corrosion pits was gradually reduced (Figures 8(c) and (d)). The results of EDS line-scan (Figures 9 and 10) showed that the local corrosion pit of N80 steel in supercritical CO2-saturated vapor environment was composed of two layers. The C-Fe compound layer near to the base part was thinner and the Fe-C-O compound layer was above the C-Fe compound layer. The Fe-C-O compound layers formed at 18 MPa (Figure 9) and 8 MPa (Figure 10) were respectively about 20 and 3 μm thick. When the pressure was increased above the critical pressure, the thickness of C-Fe compound layer decreased significantly.
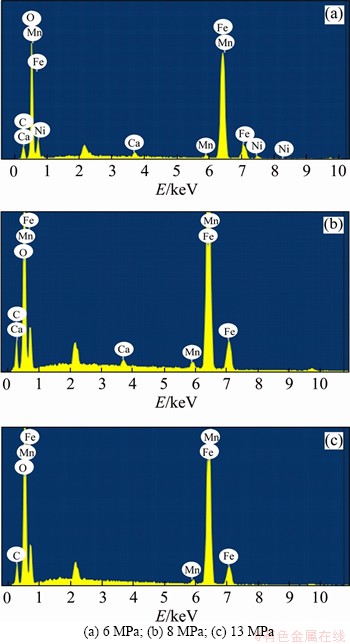
Figure 7 EDS analysis results of corrosion products of N80 steel under different CO2 partial pressures:
The XRD analysis results of corrosion products are shown in Figure 11. FeCO3, Fe2O3, and Fe3C may exist in corrosion products. Figure 12(a) shows the XPS analysis results of corrosion products of N80 steel under a CO2 partial pressure of 6 MPa. The binding energy peak of C 1s is at 284.6 eV is used to compensate for the surface charge effect. Peaks for C 1s at 288.7, O 1s at 532.3 eV and Fe 2p3/2 at 724.2.6 eV were detected, which corresponds to the presence of FeCO3. Peaks for O 1s at 530.7 eV and Fe 2p1/2 at 710.6 eV were detected, which corresponds to the presence of Fe2O3. Figure 12(b) shows the XPS analysis results of corrosion products of N80 steel under a CO2 partial pressure of 8 MPa. The corrosion components formed under low pressure conditions are the same to those formed under the supercritical condition.
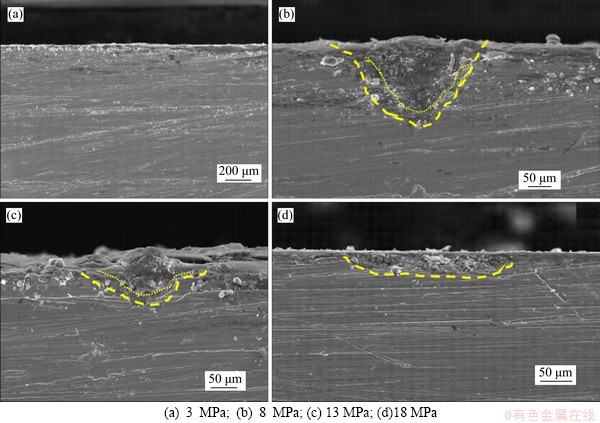
Figure 8 Cross-section morphologies of N80 steel under different CO2 partial pressures:
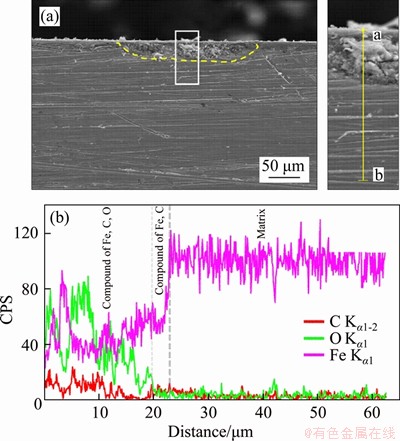
Figure 9 Line-scan EDS results of cross-section of N80 steel under a CO2 partial pressure of 18 MPa
4 Discussion
CO2 itself is not corrosive to steel, but both gaseous CO2 and supercritical CO2 dissolved in water can form H2CO3, which is corrosive to steel. The corrosion degree largely depends on the water film formed with water molecules adsorbed on the surface of the matrix. The more the water molecules adsorb on steel surface, the more the corrosion media exist.
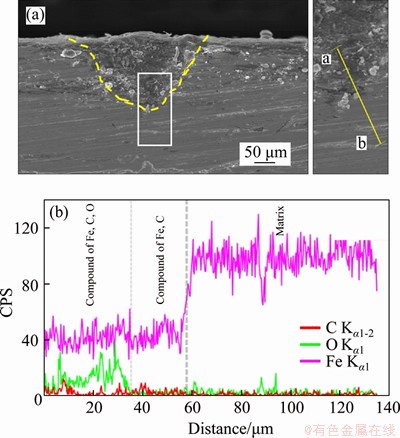
Figure 10 Line-scan EDS results of cross-section of N80 steel under the CO2 partial pressure of 8 MPa
Since the density of a supercritical fluid fluctuates abruptly near the critical point, its microscopic density is not equal to its macroscopic density, indicating the density unevenness [21]. For a rare critical fluid solution, many experimental and theoretical studies showed that in the highly compressible zone of the fluid, the density of the supercritical fluid around the solute might be much greater than the density of the solvent body due to the attraction force among molecules [22-25], thus resulting in an increase in local density. In addition to the solvent-solute agglomeration zone in the supercritical fluid, the solvent-solvent aggregation zone and solute-solute aggregation zone also exist. As the pressure further increases, due to the decrease in the fluid compressibility, the enhancement phenomenon of the local density of the molecules is weakened.
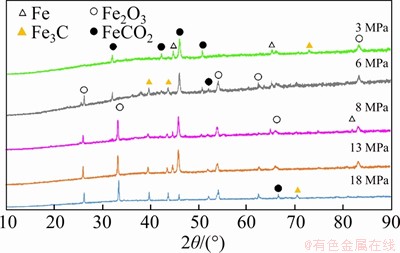
Figure 11 XRD results of corrosion products of N80 steel
The Ornstein-Zernike curve corresponding to the small-angle X-ray scattering diagram of the CO2-H2O system obtained by ZHANG et al [26], the partial molar volume of water in the supercritical CO2 and the isothermal compression coefficient [27] indicated the phenomenon of uneven molecular density in supercritical CO2-water vapor system. The change of the spatial distribution of CO2 molecules and H2O molecules with the change of pressure of CO2-H2O system is shown in Figure 13. Under low pressure conditions (Figure 13(a)), CO2 and H2O molecules are sparsely distributed. Due to the large molecular spacing, H2O molecules are difficult to be aggregated to form a water film onto steel surface. As the pressure reached the critical pressure, the enhancement phenomenon of the local density of molecules is prominent. Mixed molecules density enhancement areas (Figure 13(d), Area A), CO2 molecules density enhancement areas (Figure 13(d), Area B), H2O molecules density enhancement areas (Figure 13(d), Area C) and molecules density decrease areas (Figure 13(d), Area D) exist in the CO2-H2O system. A large number of H2O molecular density enhancement areas and the molecular density reduction areas may lead to an increase in the number of aggregated H2O molecules adsorbed onto the matrix surface, proving that there are more corrosive media formed than that under lower pressure conditions. When the pressure further increases, the molecular agglomeration is diminished (Figure 13(c)) and the number of aggregated H2O molecules adhered onto the matrix surface is also gradually reduced.
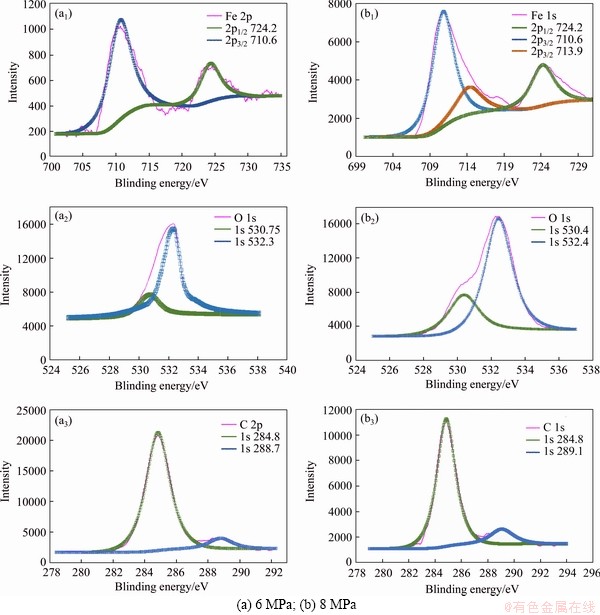
Figure 12 XPS result of corrosion products of N80 steel under different CO2 partial pressure:
Carbon steel can adsorb CO2 molecules and H2O molecules [28, 29]. According to the Langmuir isothermal adsorption method of mixed gas, the adsorption numbers of CO2 and H2O on steel surface are VCO2 and VH2O.
 (1)
(1)
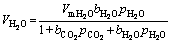 (2)
(2)
where VmCO2 and VmH2O are respectively the single- layer saturated adsorption capacity of CO2 molecules and H2O molecules; bCO2 and bH2O are respectively the adsorption coefficients of carbon steel for CO2 and H2O [30]. Equations (1) and (2) show that there is a competitive relationship between CO2 and H2O molecular adsorption. As the CO2 pressure increases, VCO2 increases and CO2 adsorption is enhanced compared to that under lower pressure conditions in competitive adsorption (Figure 13(c)). When the pressure increases to the critical pressure, H2O molecules begin to dissolve in supercritical CO2 fluid in form of solute, but the solubility is quite low. The supercritical CO2 fluid has a lower self-diffusion coefficient than that under the lower pressure of CO2 and the viscosity of the fluid is increased by about an order of magnitude. However, the absolute value of viscosity is still low and the supercritical CO2 fluid has a good mass transfer ability [31-33].
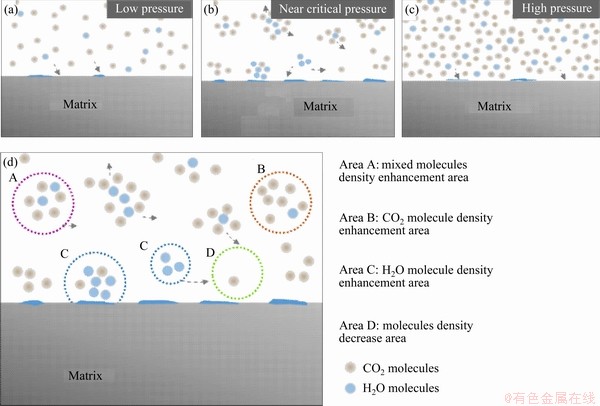
Figure 13 Schematic diagram of spatial distribution of CO2 molecules and H2O molecules under different CO2 partial pressures
The aggregation and adsorption of CO2 molecules on the matrix surface mainly depend on Van der Waals force (Figures 14(b) and (c)). The aggregation and adsorption of H2O depends on van der Waals force and hydrogen bonds. Therefore, adsorbed H2O molecules easily form water films on the surface of the substrate (Figure 14(b)). Aggregated CO2 molecules are less likely to form a continuous molecular film on the surface of the substrate due to the weaker binding of Van der Waals force. In addition, as the pressure rises, the fluid compressibility decreases. In the CO2-H2O system, H2O molecules are homogeneously dissolved in CO2. A single or small amount of H2O molecules are bound around a large number of CO2 molecules (Figure 13(c)), which weakens the molecular aggregation phenomenon of H2O and decreases the quantity of water molecules adhered onto the matrix surface.
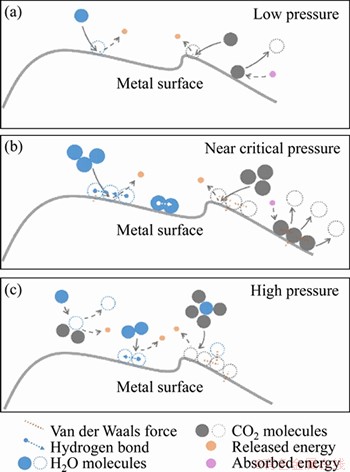
Figure 14 Adsorption model of H2O and CO2 molecules on metal surface
From the view of condensation and evaporation, under constant temperature of 100 °C, the condensation rate of water in the saturated water vapor environment is in dynamic equilibrium with the evaporation rate, and the equilibrium will not be shifted by the change of pressure of CO2. Under low or high pressure of CO2, most of the water droplets evaporate rapidly after condensation. Due to the local aggregation of water molecules near supercritical pressure of CO2, water droplets of larger size formed by the condensation of water molecules in higher local density, and the remaining part of the water droplet after evaporating is more likely to adsorb on metal surface and form a water film.
The electrochemical mechanism of the corrosion effect of H2CO3 on carbon steel is well known [2]. Corresponding cathode reactions are provided as:
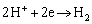 (3)
(3)
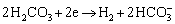 (4)
(4)
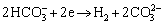 (5)
(5)
Anode reactions are provided as [39-41]:
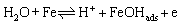 (6)
(6)
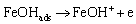 (7)
(7)
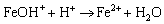 (8)
(8)
In anode reactions, H2O continuously reacts with the exposed Fe matrix to form Fe2+, whereas Fe3C (cementite) in the carbon steel pearlite is gradually exposed from the matrix in the reaction. Since Fe3C has a higher positive potential, the Fe matrix preferentially participates in the dissolution reaction [34, 35]. Due to the consumption of Fe, Fe3C is gradually stacked on the metal surface and the corrosion product FeCO3 is continuously deposited on Fe3C (Figure 15(d)). Particulate cementite is formed in N80 under CO2-containing or CO2-saturated environments, which can’t provide a grip for the protective scale or provide sufficient adhesion of corrosion scale as lamellar cementite does [36], thus allowing matrix continuously be exposed into the H2O environment. At the beginning of the corrosion process, the hydrolysis reaction of Fe2+ occurs in the water film (Eq. (9)) and formed Fe(OH)2 is inevitably exposed to air when samples taking out from autoclave and during samples’ surface treatment (Eqs. (10) and (11)):
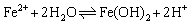 (9)
(9)
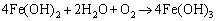 (10)
(10)
 (11)
(11)
As shown in Figure 15(a), H2CO3 generated by H2O and CO2 provides raw materials required for the occurrence of corrosion. As the reaction proceeds, 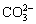 continuously combines Fe2+ to form FeCO3, and the anode reaction continuously consumes H2O. When H2O in water films is completely consumed, the FeCO3 deposition process ends. Therefore, the quantity of H2O molecules of water film determines the depth of the single corrosion region and the accumulation thickness of corrosion products. Based on Figure 5 and molecular aggregation theory, when the pressure is close to the critical pressure, more water molecules are adhered onto the matrix surface and the storage of the corrosive media are the most adequate.
continuously combines Fe2+ to form FeCO3, and the anode reaction continuously consumes H2O. When H2O in water films is completely consumed, the FeCO3 deposition process ends. Therefore, the quantity of H2O molecules of water film determines the depth of the single corrosion region and the accumulation thickness of corrosion products. Based on Figure 5 and molecular aggregation theory, when the pressure is close to the critical pressure, more water molecules are adhered onto the matrix surface and the storage of the corrosive media are the most adequate.
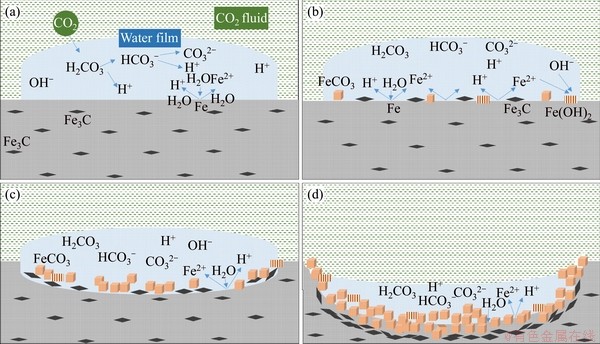
Figure 15 Local corrosion mechanism of N80 steel (a-d in chronological order)
HOU et al [37] measured the fitting curve of solubility of CO2 molecules in water with the change of CO2 partial pressure at 373.15 K (Figure 16). With the pressure increasing, the solubility of CO2 in water gradually increases; however, the increasing rate of solubility gradually decreases. More CO2 molecules per unit volume of water film on the matrix surface indicate the higher concentration of formed H2CO3 (Eq. (4)) and 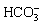 (Eq. (5)) to participate in corrosion, thus promoting the cathode reaction to generate more
(Eq. (5)) to participate in corrosion, thus promoting the cathode reaction to generate more 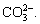 With the increase in
With the increase in 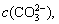 the concentration of Fe2+ and
the concentration of Fe2+ and 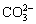 becomes larger, thus promoting the reaction (Eq. (12)) to move toward the nucleation direction and resulting in more FeCO3 nuclei on the surface of the unit matrix surface. The growth space of single crystals becomes smaller and the crystals are tightly packed together before they are completely grown, thus resulting in dense grain stacking and a small crystal size (Figure 6(e)).
becomes larger, thus promoting the reaction (Eq. (12)) to move toward the nucleation direction and resulting in more FeCO3 nuclei on the surface of the unit matrix surface. The growth space of single crystals becomes smaller and the crystals are tightly packed together before they are completely grown, thus resulting in dense grain stacking and a small crystal size (Figure 6(e)).
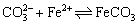 (12)
(12)
 (13)
(13)
 (14)
(14)
With the increase of CO2 partial pressure, the solubility of CO2 in water, the degree of local aggregation of molecules, the competitive adsorption between CO2 and H2O molecules on the metal surface have changed, and these changes jointly determine the corrosion degree of carbon steel (Figure 17). At lower pressures region, the factors affecting corrosion are CO2 solubility in water film and the adsorption of CO2 molecules; however, CO2 solubility in water film has a more significant effect on corrosion, which causes the increasing of corrosion rate. When the pressure reaches near the supercritical pressure, local aggregation of H2O molecules brings more corrosion media on metal surface, which significantly accelerates corrosion. During the process of increasing the partial pressure of CO2, the influence of adsorption capacity of CO2 molecules on corrosion is gradually increased. In the high-pressure region, as the molecules agglomeration is diminished, the adsorption capacity of CO2 molecules becomes the main factor affecting corrosion.
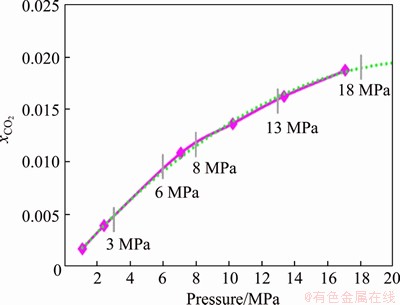
Figure 16 Fitting curve of CO2 solubility in water with change in CO2 partial pressure (T=373.15 K)
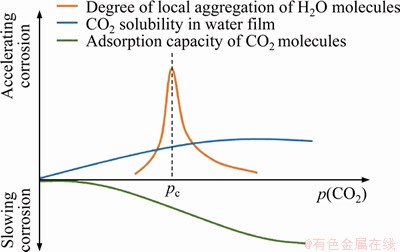
Figure 17 Schematic diagram of effect of multiple factors on corrosion rate of carbon steel in CO2-saturated vapor environment
5 Conclusions
The average corrosion rates of N80 steel and P110 steel in CO2 gas phase and supercritical phase in saturated vapor environment exceeded the oilfield corrosion control index (0.076 mm/a).
According to the surface analysis results, the corrosion mechanism of carbon steel in CO2 gas phase was basically the same to that in the supercritical phase.
The phenomenon of molecular aggregation in the supercritical CO2 phase may lead to the attachment of more water molecules on the surface of the material. When CO2 partial pressure further increased above the critical pressure, the phenomenon of molecular aggregation was weakened and the competitive adsorption relationship led to the decrease in water molecules adsorbed on the material surface.
These conclusions are based on static simulation experiments; however, the effects of shear stress generated by the gas flow on corrosion products and the adsorption of water film are not considered. Further studies can be conducted by using fluid-accelerated corrosion apparatus.
Contributors
ZENG De-zhi designed the project. HUANG Zhi-yao and YU Zhi-ming carried out data processing, performed data analysis, and contributed to the paper writing. SHI Shan-zhi, LIU Cong-ping and YI Yong-gang offered some valuable suggestions for the contents of the manuscript. SUN Yi-cheng and TIAN Gang offered the specimen, performed data analysis. All authors replied to reviewers’ comments and revised the final version.
Conflict of interest
ZENG De-zhi, HUANG Zhi-yao, YU Zhi-ming, SHI Shan-zhi, YI Yong-gang, LIU Cong-ping, TIAN Gang and SUN Yi-cheng declare that they have no conflict of interest.
References
[1] GALE J, DAVISON J. Transmission of CO2-safety and economic considerations [J]. Energy, 2003, 29(9): 1319-1328. DOI: 10.1016/j.energy.2004.03.090.
[2] ZHANG G A, LIU D, LI Y Z, GUO X P. Corrosion behaviour of N80 carbon steel in formation water under dynamic supercritical CO2 condition [J]. Corrosion Science, 2017, 120: 107-120. DOI: 10.1016/j.corsci.2017.02.012.
[3] NESIC S, POSTLETHWAITE J. Modelling of CO2 corrosion mechanisms [M]// Modelling Aqueous Corrosion. Dordrecht: Springer, 1994: 317-335. DOI: 10.1007/978-94- 011-1176-8-15.
[4] LIU Zhi-yong, ZHAO Tian-liang, LIU Ran-ke, JIA Jing-huan, DU Cui-wei, LI Xiao-gang. Influence factors on stress corrosion cracking of P110 tubing steel under CO2 injection well annulus environment [J]. Journal of Central South University, 2016, 23(4): 757-764. DOI: 10.1007/ s11771-016-3121-1.
[5] XU Ming-he, LI Wei-hong, ZHOU Yi, YANG Xiao-xian, WANG Zhe, LI Zheng. Effect of pressure on corrosion behavior of X60, X65, X70, and X80 carbon steels in water-unsaturated supercritical CO2 environments [J]. International Journal of Greenhouse Gas Control, 2016, 51: 357-368. DOI: 10.1016/j.ijggc.2016.06.002.
[6] XIANG Yong, LI Chen, HESITAO W, LONG Zheng-wei, YAN Wei. Understanding the pitting corrosion mechanism of pipeline steel in an impure supercritical CO2 environment [J]. Journal of Supercritical Fluids, 2018, 138: 132-142. DOI: 10.1016/j.supflu.2018.04.009.
[7] SUI Peng-fei, SUN Jian-bo, HUA Yong, LIU Hui-feng, ZHOU Ming-nan, ZHANG Yu-can, LIU Jia-hang, WANG Yong. Effect of temperature and pressure on corrosion behavior of X65 carbon steel in water-saturated CO2 transport environments mixed with H2S [J]. International Journal of Greenhouse Gas Control, 2018, 73: 60-69. DOI: 10.1016/j.ijggc.2018.04.003.
[8] HUA Yong, BARKER R, NEVILLE A. Comparison of corrosion behaviour for X-65 carbon steel in supercritical CO2-saturated water and water-saturated/unsaturated supercritical CO2 [J]. Journal of Supercritical Fluids, 2015, 97: 224-237. DOI: 10.1016/j.supflu.2014.12.005.
[9] SUN Chong, SUN Jian-bo, LIU Su-biao, WANG Yong. Effect of water content on the corrosion behavior of X65 pipeline steel in supercritical CO2-H2O-O2-H2S-SO2 environment as relevant to CCS application [J]. Corrosion Science, 2018, 137: 151-162. DOI: 10.1016/j.corsci.2018.03. 041.
[10] SUN Chong, WANG Yong, SUN Jian-bo, LIN Xue-qiang, LI Xue-da, LIU Hui-feng, CHENG Xiang-kun. Effect of impurity on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system [J]. Journal of Supercritical Fluids, 2016, 116: 70-82. DOI: 10.1016/ j.supflu.2016.05.006.
[11] SUN Chong, SUN Jian-bo, WANG Yong, SUI Peng-fei, LIN Xue-qiang, LIU Hui-feng, CHENG Xiang-kun, ZHOU Ming-nan. Effect of impurity interaction on the corrosion film characteristics and corrosion morphology evolution of X65 steel in water-saturated supercritical CO2 system [J]. International Journal of Greenhouse Gas Control, 2017, 65: 117-127. DOI: 10.1016/j.ijggc.2017.09.002.
[12] SUN Jian-bo, SUN Chong, WANG Yong. Effects of O2 and SO2 on water chemistry characteristics and corrosion behavior of X70 pipeline steel in supercritical CO2 transport system [J]. Industrial & Engineering Chemistry Research, 2018, 57(6): 2365-2375. DOI: 10.1021/acs.iecr.7b04870.
[13] SUN Jian-bo, SUN Chong, ZHANG Guo-an, LI Xue-da, ZHAO Wei-min, JIANG Tao, LIU Hui-feng, CHENG Xiang-kun, WANG Yong. Effect of O2 and H2S impurities on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system [J]. Corrosion Science, 2016, 107: 31-40. DOI: 10.1016/j.corsci.2016.02.017.
[14] CHOI Y S, NESIC S. Effect of water content on the corrosion behavior of carbon steel in supercritical CO2 phase with impurities [C]// Corrosion 2011. Houston, Texas: NACE International, 2011: 11377.
[15] HUA Yong, BARKER R, CHARPENTIER T, WARD M, NEVILLE A. Relating iron carbonate morphology to corrosion characteristics for water-saturated supercritical CO2 systems [J]. The Journal of Supercritical Fluids, 2015, 98: 183-193. DOI: 10.1016/j.supflu.2014.12.009.
[16] GUO Shao-pin, XU Lin-ning, ZHANG Lei, CHANG Wei, LU Ming-xu. Characterization of corrosion scale formed on 3Cr steel in CO2-saturated formation water [J]. Corrosion Science, 2016, 110: 123-133. DOI: 10.1016/j.corsci.2016. 04.033.
[17] BECK J, FEDKINA M, LVOV S, ZIOMEK-MOROZ M E, HOLCOMB G, TYLCZAK J, ALMAN D. In situ electrochemical corrosion measurements of carbon steel in supercritical CO2 using a membrane-coated electrochemical probe [J]. ECS Transactions, 2013, 45(19): 39-50. DOI: 10.1021/acs.iecr.7b04870.
[18] THODLA R, FRANCOIS A, SRIDHAR N. Materials performance in supercritical CO2 environments [C]// Corrosion 2009. Atlanta, Georgia: NACE International, 2009: 09255.
[19] SIM S, BOCHER F, COLE I S, CHEN X B, BIRBILIS N. Investigating the effect of water content in supercritical CO2 as relevant to the corrosion of carbon capture and storage pipelines [J]. Corrosion, 2014, 70(2): 185-195. DOI: 10.5006/0944.
[20] BECK J, LVOV S, FEDKIN M V, ZIOMEK-MOROZ M, HOLCOMB G, TYLCZAK J, ALMAN D. Electrochemical system to study corrosion of metals in supercritical CO2 fluids [C]// Corrosion 2011. Houston, Texas: NACE International, 2011: 11380.
[21] TUCKER S C, MADDOX M W. The effect of solvent density inhomogeneities on solute dynamics in supercritical fluids: a theoretical perspective [J]. The Journal of Physical Chemistry B, 1998, 102: 2437-2453. DOI: 10.1021/ jp972382+.
[22] NISHIKAWA K, OCHIAI H, SAITOW K, MORITA T. Static inhomogeneity of supercritical ethylene studied by small-angle X-ray scattering [J]. Chemical Physics, 2003, 286(2, 3): 421-430. DOI: 10.1016/S0301-0104(02)00935-7.
[23] TACHIKAWA T, AKIYAMA K, YOKOYAMA C, TERO- KUBOTA S. Local density effects on the hyperfine splitting constant and line width of TEMPO radical in gaseous and supercritical carbon dioxide [J]. Chemical Physics Letters, 2003, 376(3, 4): 350-357. DOI: 10.1016/S0009-2614(03) 00995-3.
[24] EGOROV S A. Local density enhancement in neat supercritical fluids: Dependence on the interaction potential [J]. Chemical Physics Letters, 2002, 354(1, 2): 140-147. DOI: 10.1016/S0009-2614(02)00129-X.
[25] MUKHOPADHYAY M, DALVI S V. Partial molar volume fraction of solvent in binary (CO2–solvent) solution for solid solubility predictions [J]. The Journal of Supercritical Fluids, 2004, 29(3): 221-230. DOI: 10.1016/S0896-8446(03) 00087-1.
[26] ZHANG Jian-liang, LIU Jun-cheng, GAO Liang, ZHANG Xiao-gang, HOU Zhen-shan, HAN Bu-xing, WANG Jun, DONG Bao-zhong, RONG Li-xia, ZHAO Hui. Small-angle X-ray scattering study on correlation length and density fluctuations in a supercritical CO2–water mixture [J]. Fluid Phase Equilibria, 2002, 198(2): 251-256. DOI: 10.1016/ S0378-3812(01)00767-1.
[27] ZHANG Jian-ling, ZHANG Xiao-gang, HAN Bu-xing, HE Jun, LIU Zhi-min, YANG Guan-ying. Study on intermolecular interactions in supercritical fluids by partial molar volume and isothermal compressibility [J]. The Journal of Supercritical Fluids, 2002, 22(1): 15-19. DOI: 10.1016/S0896-8446(01)00107-3.
[28] HESS G, FROITZHEIM H, BAUMGARTNER C. The adsorption and catalytic decomposition of CO2 on Fe (111) surfaces studied with high resolution EELS [J]. Surface Science, 1995, 331: 138-143. DOI: 10.1016/0039-6028(95) 00177-8.
[29] FERNANDES F W, CAMPOS T M B, CIVIDANES L S, SIMONETTI E A N, THIM G P. Adsorbed water on iron surface by molecular dynamics [J]. Applied Surface Science, 2016, 362: 70-78. DOI: 10.1016/j.apsusc.2015.11.143.
[30] BAI Run-sheng, YANG R T. A thermodynamically consistent Langmuir model for mixed gas adsorption [J]. Journal of Colloid and Interface Science, 2001, 239(2): 296-302. DOI: 10.1006/jcis.2001.7563.
[31] KRAVANJA G, SKERGET M, KNEZ Z, HRNCIC M K. Diffusion coefficients of water and propylene glycol in supercritical CO2 from pendant drop tensiometry [J]. The Journal of Supercritical Fluids, 2018, 133: 1-8. DOI: 10.1016/j.supflu.2017.09.022.
[32] TAYLOR R, KRISHNA R. Multicomponent mass transfer [M]. John Wiley & Sons, 1993.
[33] MAGALHAES A L, DA SILVA F A, SILVA C M. Free-volume model for the diffusion coefficients of solutes at infinite dilution in supercritical CO2 and liquid H2O [J]. The Journal of Supercritical Fluids, 2013, 74: 89-104. DOI: 10.1016/j.supflu.2012.12.004.
[34] MORA-MENDOZA J L, TURGOOSE S. Fe3C influence on the corrosion rate of mild steel in aqueous CO2 systems under turbulent flow conditions [J]. Corrosion Science, 2002, 44(6): 1223-1246. DOI: 10.1016/S0010-938X(01)00141-X.
[35] PESSU F, BARKER R, NEVILLE A. Understanding pitting corrosion behavior of X65 carbon steel in CO2-saturated environments: The temperature effect [J]. Corrosion, 2016, 72(1): 78-94. DOI: 10.5006/1338.
[36] WU Qian-lin, ZHANG Zhong-hua, DONG Xiao-ming, YANG Jian-qiang. Corrosion behavior of low-alloy steel containing 1% chromium in CO2 environments [J]. Corrosion Science, 2013, 75: 400-408. DOI: 10.1016/ j.corsci.2013.06.024.
[37] HOU S X, MAITLAND G C, TRUSLER J P M. Measurement and modeling of the phase behavior of the (carbon dioxide+ water) mixture at temperatures from 298.15 K to 448.15 K [J]. The Journal of Supercritical Fluids, 2013, 73: 87-96. DOI: 10.1016/j.supflu.2012.11.011.
(Edited by YANG Hua)
中文导读
饱和水蒸气环境中CO2气相-CO2超临界相相变对碳钢腐蚀行为的影响
摘要:通过腐蚀失重实验研究了P110和N80钢管在气相CO2 -饱和水蒸气环境和超临界CO2 -饱和水蒸气环境中的腐蚀速率变化,并通过SEM,EDS,XRD,XPS和截面技术对腐蚀产物进行了表面分析。结果表明:随着CO2分压的增加,试样平均腐蚀速率先升高后降低;在临界压力附近,试样平均腐蚀速率达到最大值;在高于临界压力后,随着CO2分压的进一步增加,分子局部聚集现象减弱,试样平均腐蚀速率降低。
关键词:碳捕获和储存;超临界CO2;腐蚀产物;腐蚀机理
Foundation item: Project(21JCQN0066) supported by the Youth Science & Technology Foundation of Sichuan Province, China
Received date: 2020-03-24; Accepted date: 2020-10-10
Corresponding author: ZENG De-zhi, PhD, Professor; Tel: +86-13880302166; E-mail: zengdezhiswpu@163.com; ORCID: https://orcid. org/0000-0002-2056-2997
Abstract: Corrosion behaviors of P110 and N80 tubular steels in CO2 gas phase and supercritical (S-CO2) phase in a saturated water vapor environment were explored in corrosion weight loss experiments by SEM, EDS, XRD, XPS and cross-section analysis techniques. With the increase in CO2 partial pressure, the average corrosion rate increased first and then decreased. The average corrosion rate reached the maximum value under the near-critical pressure. When CO2 partial pressure further increased to be above the critical pressure, the average corrosion rate gradually decreased and local aggregation of molecules was weakened.


