SnAg及SnAgCu无铅焊料接头中金属间化合物
在时效中的演变
李晓延1,杨晓华2,吴本生2,严永长1
(1. 北京工业大学 材料科学与工程学院,北京,100022;2. 福州大学 测试中心,福建 福州,350002)
摘要:对SnAg共晶合金及SnAgCu共晶合金无铅焊料与Cu或Ni/Cu或Au/Ni/Cu衬底经钎焊方法焊接后,在焊接界面和焊料内部形成的金属间化合物(IMC)的类型、形貌和分布形式,以及焊接接头在随后时效过程中IMC的类型、成分和形貌的演变规律进行研究。分析结果表明,在钎焊过程中,IMC的类型与焊料成分有关,与衬底金属在焊料合金中的溶解度及扩散速度有关;IMC的形貌与加热温度、冷却速度及焊接界面的温度梯度有关;IMC的分布与焊料成分及接头中金属元素的扩散能力有关;焊料接头的断裂机理与接头合金成分、时效温度、时效时间、载荷方式有关;在时效过程中,焊料共晶组织粗化,焊料强度下降,断裂会在焊料内部发生;当IMC厚度增大到临界尺寸时,应力集中严重,多层IMC形成,空穴形成及长大,在IMC界面层断裂;若两者强度接近,则断裂部分发生在焊料,部分发生在界面IMC处。
关键词:
中图分类号:TN604 文献标识码:A 文章编号:1672-7207(2007)01-0030-06
Evolution of intermetallic compounds in SnAg and SnAgCu lead-free solder joints during aging
LI Xiao-yan1, YANG Xiao-hua2, WU Ben-sheng2, YAN Yong-chang1
(1. School of Materials Science and Engineering, Beijing University of Technology, Beijing 100022, China;
2. Testing Center, Fuzhou University, Fuzhou 350002, China)
Abstract: The types, morphology and distribution of intermetallic compounds (IMCs), and the evolution of the types and composition and morphology of IMC in the joints during the following aging process were studied. The analysis results show that the types of IMCs mainly depend on solder composition and the solubility and diffusion rate of metal substrate in solder alloy; the morphology of IMC is closely related with the soldering peak temperature, cooling rate and the thermal gradient of the interface of joints; the distribution of IMC is related with solder composition and the diffusivity of metal elements in the joints. The analysis results also show that the fracture mechanism of the joints is mainly controlled by the solder composition, the peak temperature and duration of aging as well as the applied loads. The eutectic structures of the solder are coarsened during the aging process and the strength of the solder decreases, thus the fracture occurrs inside the solder. If the thickness of IMC increases to critical size, the stress concentration wil be serious. Some time multilayer IMCs will be formed and cavity will be formed and grow, the fracture will occur in the interfacial layer of IMCs. If the strengths of the solder and IMC are close during aging, the fracture will occur both inside the joints and in the interfacial layer of IMCs.
Key words: lead-free solders; intermetallic compounds; aging
在电子封装技术中,焊料起着连接和支撑电子元件和电路板的作用。在焊料和衬底的连接界面上或在焊料内部,常常会形成各种各样的金属间化合物(IMC)。IMC对接头的可靠性至关重要,适量的IMC可以起到提高接头强度、润湿焊料及阻碍焊料扩散及氧化的作用。然而,若IMC量过大,IMC层过厚或者分布不均,会对接头的性能造成危害。不同的焊料合金和衬底金属形成的IMC的类型、成分、形貌及形核地点都不同,在随后的时效过程中的变化也不同,最后导致接头断裂的机理不同。人们对各种成分的再流焊接头在不同的焊接工艺参数下形成的微观组织及性能[1-4],以及各种再流焊接头在不同的时效条件下微观组织和性能的演变规律[5-7]、对各种性能的影响[8-10]进行了研究,在此,本文作者对目前研究很多的SnAg共晶合金焊料及SnAgCu共晶合金焊料与Cu或Ni/Cu或Au/Ni/Cu衬底经1次或多次再流焊方法焊接后,在焊接界面和焊料内部形成的IMC的类型、形貌及分布形式及其随后在150 ℃左右时效不同时间后,IMC的成分和形貌的演变规律进行研究,就IMC对焊料接头的断裂机制的影响进行分析。
1 再流焊接过程中IMC的形成规律
1.1 SnAg共晶焊料中的IMC
Sn3.5Ag共晶焊料的熔点为221 ℃,它的焊接峰值温度通常在240 ℃左右,比SnPb共晶焊料的高很多。金属在该焊料中的溶解能力也比在Sn3.5Pb共晶焊料中的强,所形成的界面IMC晶粒较SnPb共晶焊料中的粗大[8, 11]。当金属衬底为Cu时,Sn3.5Ag 合金在Cu 基体上会形成金属间化合物。且近Cu 侧为很少量的Cu3Sn 相,近焊料侧为扇贝状的Cu6Sn5 相,Ag几乎不进入界面的金属间化合物层,焊料组织为部分Sn的树枝晶粒和富Sn相与Ag3Sn颗粒弥散分布组成的共晶组织[11]。
当在Cu衬底上镀上Ni(P)的保护层后,在界面上首先得到的IMC是具有小平面状的Ni3Sn4相,若Ni层厚度大于5 μm,则在焊接过程中Ni层可以起到很好的保护作用,如图1所示[12-13]。在Ni层上除了Ni3Sn4外还有Ni3Sn和 Ni3Sn2 2种IMC[14]。若是Cu/Ni/Cu衬底,由于Cu的存在改变了界面IMC的显微组织,当上层的Cu层较薄时,接头中扇贝状(Ni,Cu)6Sn5是主要形成相;对于厚Cu层,扇贝状(Cu,Ni)6Sn5是主要形成相, 三元(Ni,Cu)6Sn5相的生长比二元CuSn或NiSn金属间化合物具有更强的侵入性[15]。
对于Au/Ni/Cu衬底和Sn3.5Ag接头,Au层在第1次焊接循环过程中就完全熔入焊料中,Ni与Sn在界面形成Ni3Sn4相,在经过多次焊接循环过程后,AuSn4在焊料中溶解最后弥散析出,由于Au的溶解能力比Ag的强,所以,没有观察到明显的Ag3Sn相[16]。
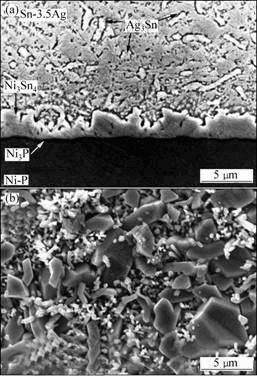
(a) 截面图;(b) 顶视图
图1 Sn3.5Ag焊料与Ni(P)焊接接头中IMC的形貌及分布
Fig. 1 Morphologies of IMC in Sn3.5Ag solder and Ni(P)
1.2 SnAgCu焊料中的IMC
SnAgCu系焊料是目前研究较多的无铅焊料,共晶成分为Sn3.5Ag0.9Cu,熔点为216 ℃,再流焊的峰值温度一般为230 ℃。在Sn3.5Ag合金焊料中加入小于1%的Cu,在与Cu衬底的焊接过程中,扇贝状的Cu6Sn5很快在焊料与衬底界面上形成,Cu在焊接初期对IMC形核有贡献。所以,含Cu的焊料容易形成IMC,对提高电子元件的可靠性有利。Cu的加入在IMC形成后期阻碍晶粒长大,起到细化晶粒的作用。采用XRD检测到在刚刚焊接完成后的界面上有少量的Cu3Sn形成,但在扫描电镜照片中未观察到[17]。若钎焊峰值温度太高,如在260 ℃左右,则所有的接近共晶成分的SnAgCu焊料与Cu的接头中都形成了晶须或管状Cu6Sn5相,形核地点为扇贝状Cu6Sn5上方或大的Ag3Sn处或孔穴处。Ag含量越高,晶须状Cu6Sn5相越多。晶须的形成是由于在上述形核地点有过饱和的Cu存在,而且当从较高的钎焊峰值温度冷却时,有足够的温度梯度和时间使晶须形核并长大。晶须状Cu6Sn5对强度和断裂性能无明显的影响[18]。
对于Sn3.5Ag合金,当加入小于或等于1%的Cu焊料时,将焊料与Al/Ni(V)/Cu薄膜结构分别进行回流焊接1,5,10和20次后,在焊球内部发现2种IMC,分别为Cu6Sn5(或(Cu,Ni)6Sn5)及Ag3Sn。Cu6Sn5主要存在于界面,也有部分大的Cu6Sn5存在于焊料内部。细小的Ag3Sn颗粒均匀分布在焊料内部与富Sn相组成弥散分布的共晶组织,偶尔发现大的板状Ag3Sn。由于Cu在SnAgCu焊料中的溶解度远大于在SnPb焊料的溶解度,Ni层上的Cu层若较薄,且全部熔入焊料后的浓度小于它在该焊料中的饱和溶解度1.54%,则Cu在焊接过程中会全部熔入焊料,经过1次回流焊接后,IMC呈厚度1~3 μm的半球形扇贝状,当Cu层被消耗完后, Ni层仍然完好。经过5次回流后,IMC的形态从球形扇贝状转变为拉长的扇贝状或杆状,长径比也随之增加。Ni在SnAgCu共晶焊料中的扩散速度比在SnPb中的快很多,在随后的多次焊接循环过程中,部分Cu6Sn5以碎片形式进入焊料,使Ni层暴露在焊料中,Ni与Cu共同与Sn反应,形成(Cu,Ni)6Sn5。随着焊接循环次数的增加,Ni在(Cu,Ni)6Sn5中的含量增加,Ni层被(Cu,Ni)6Sn5逐渐消耗。经过5次焊接循环后,(Cu,Ni)6Sn5中的Ni达到5%,经过20次焊接循环后,(Cu,Ni)6Sn5中的Ni达到6%,如图2所示[15]。若衬底表面没有Cu,则界面IMC的类型和形貌取决于焊料的含Cu量。当Cu的(质量分数)小于等于0.2%时,在界面形成连续层状的(Ni,Cu)3Sn4;当Cu的质量分数大于0.6%时,界面形成扇贝状(Cu,Ni)6Sn5;当Cu质量分数在二者之间时,不连续的(Cu,Ni)6Sn5层在连续的(Ni,Cu)3Sn4层上面形成。若用在高温停留5,10和20 min来代替多次再流焊热循环,IMC的组织变化与再流焊过程的相似,但组织演变要比再流焊时的快。在一般情况下,在再流焊过程中,在峰值温度停留时间大约为1 min。在SnAgCu与Al/Ni/Cu的接头中,当在260 ℃停留10 min时,Ni层已经全部消耗完毕,而经过焊接循环20次后,Ni层才消失[15]。
当薄膜层中有Au时,如Au/Ni/Cu金属薄膜,Au的存在促进了Ni层的溶解,在第1次焊接循环过程中,50%Ni层被消耗形成(Cu,Ni)6Sn5,在经过3次焊接循环后Ni层100%溶解[19],Au在第1次循环过程中就熔入焊料,在5 min内就可以扩散到整个焊接接头,形成AuSn4金属间化合物,其形貌和分布与焊接循环的峰值温度和循环次数有关。当焊接循环温度高、冷却速度慢时,AuSn4由于有择优取向而易于形成薄板状,随着循环次数的增加,AuSn4不断溶解和析出最后弥散分布在焊料中[16, 20]。
Ag3Sn化合物很少在界面层中出现,一般为粒状与富Sn相形成的焊料共晶组织。在Ag含量较高的焊料中,根据加热速度和焊料温度梯度变化,Ag与Sn反应形成颗粒状、树枝状、片状、网状和板状的Ag3Sn相[21-22]。而且当Ag含量越高时,加热温度越高,板状的Ag3Sn相越大、越多。对于Ag3.8系列和Ag3.9系列合金,在降低焊后冷却速率时,大的板状Ag3Sn相可穿过整个焊接接头的横截面,如图3所示[18],严重影响接头在承受热应力时的机械性能[23]。然而,在Ag含量小于3.2%的SnAgCu焊料中,即使慢冷也不会析出大的板状Ag3Sn相[18, 23]。当Ag含量为3.5%时,在三元合金系中最接近共晶熔化反应,容易得到弥散的共晶组织,而且快速冷却可以避免板状的Ag3Sn相析出。所以,最佳Ag含量为3.2%~3.5%。
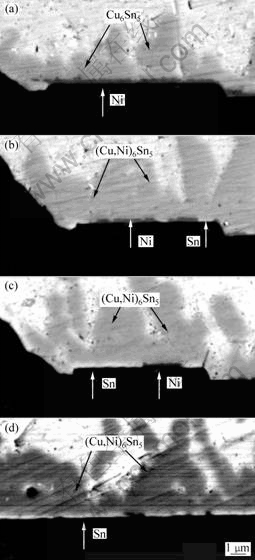
(a) 1次;(b) 5次;(c) 10次;(d) 20次
图2 经过再流焊后共晶SnAgCu焊料与Al/Ni(V)/Cu衬底接头中IMC的背散射扫描电镜照片
Fig.2 Back-scattering SEM images of IMC in Al/Ni(V)/Cu
and SnAgCu solder reflowed for different times
当焊料与不同的Ni层反应时,形成的化合物不同。若是无电镀的Ni-P层,通常为无定形组织,晶化温度为250 ℃,与SnAgCu焊料在230 ℃的焊接峰值温度下,在界面上除了形成(Cu,Ni)6Sn5 IMC层外,在Ni(P)与(Cu,Ni)6Sn5层之间也形成Ni3P层和很薄NiSnP无定形层。Ni3P比较脆,具有很完整的柱状结构。在高温停留时,Sn从NiSnP层通过Ni3P的柱状晶界向Ni3P中扩散,在NiSnP层中形成Kirkendall孔穴,使NiSnP层变成不连续状[24]。Ni(V)层中的V对接头界面的IMC没有明显的影响[15]。
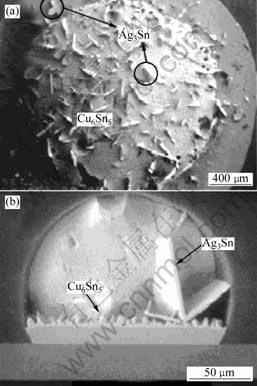
(a) Sn3.9Ag0.6Cu的顶视图;(b) Sn3.8Ag0.7Cu的侧视图
图3 Sn3.9Ag0.6Cu和Sn3.8Ag0.7Cu焊料中
AgSn3的SEM图
Fig.3 SEM images of Ag3Sn in Sn3.9Ag0.6Cu
and Sn3.8Ag0.7Cu
2 时效过程中IMC的演变
2.1 SnAg共晶焊料中IMC的演变
Sn3.5Ag焊料与Cu衬底接头中在靠近Cu的一侧形成很少量的Cu3Sn 相,在靠近焊料侧扇贝状的Cu6Sn5 相,在回流过程中的变化与共晶SnPb焊料的变化相似,在Ni与Sn3.5Ag焊料的接头中,锯齿状的Ni3Sn4层的厚度在回火过程中增加,但增加速度较慢。在150 ℃时效 1 000 h后,Ni3Sn4层没有长大到临界厚度[16]。对于Cu/Ni/Au和Sn3.5Ag的接头,刚刚焊接后,Au熔入焊料形成粒状的AuSn4相,界面为不规则齿状的Ni3Sn4层,在150 ℃以下时效时,界面化合物长大速度较慢,但在高温190 ℃时效时,伴随着界面IMC的厚度增加,在IMC层上析出Ag3Sn和AuNiSn化合物,而且在焊料内部也析出大的板状Ag3Sn和AuSnNi化合物,如图4所示[14]。总的来说,Sn3.5Ag焊料中的界面IMC与SnAgCu系焊料相比,在时效过程中的变化较慢,在无铅焊料中高温性能是最稳定的。
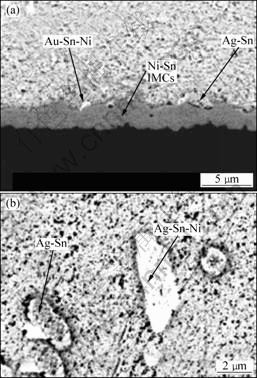
图4 Sn3.5Ag与Cu/Ni/Au接头在190 ℃时效4 d后的
IMCs(a)及焊料内部在190 ℃时效8 d的IMCs的SEM照片(b)
Fig.4 SEM images of IMCs of CuNiAu and Sn3.5Ag
solder after aging for 4 d at 190 ℃ (a) and after aging
for 8 d at 190 ℃ (b)
2.2 SnAgCu焊料中IMC的演变
SnAgCu焊料与Cu的接头在焊接后形成界面扇贝状的Cu6Sn5相,在随后的时效过程中,扇贝状的Cu6Sn5相厚度略有增加[25]。随着时效温度的升高,Cu3Sn在Cu和Cu6Sn5相之间析出,并且温度越高,Cu3Sn层越厚[26],在Sn3.5Ag0.9Cu焊料与Cu /Ni/Au的接头于150 ℃时效18 d后可观察到Kirkendall孔 穴[22]。SnAgCu焊料与Cu /Ni(V)/Al薄膜的焊接接头在150 ℃下时效24 h后, Cu6Sn5仍然为扇贝状。当时效达到500 h后,扇贝状的Cu6Sn5转变成层状。需要指出的是,虽然在150 ℃时效时,IMC的形态发生改变,但是仍然保持着同样的体积。Ni(V)层在150 ℃时效1 000 h后,仍然与Cu6Sn5连接良好。在Cu6Sn5里面没有发现Ni,表明在150 ℃时Ni在固态Cu6Sn5中扩散非常缓慢[15]。
当SnAgCu焊料与Ni焊接后,根据焊料中Cu含量不同在界面形成扇贝状(Cu,Ni)6Sn5或(Ni,Cu)3Sn4或它们的混合层状化合物。在随后的时效过程中,界面IMC层逐渐长大,但长大速度较慢。当时效温度较高、时间较长时,(Cu,Ni)6Sn5扇柱或(Ni,Cu)3Sn4长大并碎化成颗粒进入焊料中,但是,IMC的演化过程较缓慢,并不会出现“失润”现象[22]。
当SnAgCu焊料与Au/Ni/Cu焊接后,界面形成扇贝状(Ni,Cu)6Sn5相和焊料内部形成粒状的Cu6Sn5相,在时效过程中,在界面层上形成复杂的SnNiCuAu和SnNiCuAg金属间化合物。能谱分析结果表明,该化合物为(Cu,Au,Ni)6Sn5相。界面层的化合物在150 ℃时效8~500 h期间稳定长大,500~1 000 h之间快速长大。焊料内部的Cu6Sn5相粗化。在时效过程中在焊料内部析出细层片状的Cu6Sn5相[6]。在高温190 ℃时效时,除了上述变化加速外,在焊料内部和界面IMC层上还会析出板状的Ag3Sn。在时效16 d后,5 μm的Ni层被消耗完。所以,在高温下Ni保护层需要加厚[14]。
Ag3Sn主要在再流焊过程中形成,由于其化学稳定性强,在150 ℃以下的时效阶段没有变化,它的形态主要取决于回流过程中的液态反应及随后的冷却速率[22]。Ag3Sn硬而脆,小的Ag3Sn颗粒弥散分布在焊料中有利于焊接强度的提高,但是树枝状和板状的Ag3Sn相则破坏了焊料的连续性,在焊接过程中加快冷却速度可以控制大的Ag3Sn相析出。
3 IMC对焊料接头的断裂过程影响
当Sn3.5Ag0.75Cu焊料与Cu接头在125 ℃时效后,因为Sn3.5Ag0.75Cu共晶焊料的强度较高,而且在125 ℃时效过程中比较稳定,所以,在拉伸过程中,薄弱区域出现在焊料与IMC层界面处。Sn3.5Ag0.75Cu焊料与Ni的接头在时效前、后的断裂都发生在焊料内部,界面的IMC层在时效过程中长大较慢, 在时效前、后接头强度都比与焊料与Cu的结合强度高,所以,焊料成为拉伸过程中的薄弱区。Sn3.5Ag0.75Cu焊料与AuNi接头的断裂机理与该焊料与Ni接头的断裂机理相似,但由于AuSn4弥散分布在焊料中,使焊料强度提高,所以该接头的抗拉强度最高,且不随时效时间变化,而Sn3.5Ag0.75Cu焊料与Cu的接头抗拉强度最低[27]。
当然,上述断裂机理还与时效温度有关。时效温度升高,焊料组织和IMC的形貌、成分及尺寸都会发生变化,断裂机理也会发生变化。例如,对于Sn3.5Ag0.75Cu焊料与Cu的接头,若时效温度从125 ℃增加到150 ℃或更高, Cu6Sn5厚度增加,在Cu3Sn中会形成新的Kirkendall孔穴或者原来的孔穴长大。随着界面IMC厚度增加,焊料与IMC间的热应力增加,在外力作用下的变形不协调性增加,当应力集中时焊料与IMC界面开裂,若时效时间增加,IMC在应力作用下自身断裂或在Kirkendall孔穴处断裂 [9]。另外,在拉伸载荷和剪切载荷作用下,断裂机理不同。文献[22]介绍了几种焊料与AuNiCu在150 ℃时效过程中,接头的剪切断裂机理。对于Sn3.5Ag焊料,断裂都发生在焊料内部,与时效时间无关。微观分析结果表明,界面IMC长大缓慢,在时效1 000 h后仍然没有达到临界尺寸。对Sn3.8Ag0.7Cu焊料,当时效时间为0~192 h时,断裂全部发生在焊料内部;经过500 h时效后,有30%的断裂发生在界面IMC处;经过 1 000 h时效后,有40%的断裂发生在界面IMC处。微观分析结果表明,细小扇贝状的Cu6Sn5层与焊料结合强度较高,随着时效时间延长,Cu6Sn5层的扇柱渐渐长大,与焊料的结合强度逐渐下降,所以,断裂从焊料内部向IMC界面处移动。除了Sn3.5Ag焊料外,随着时效时间的延长,断裂从焊料内部向界面IMC层移动,哪里的应力最大,哪里的组织退化最严重,断裂就在哪里发生。
在焊料内部或界面IMC层上有时会析出晶须状或管状的Cu6Sn5或大的板状的Ag3Sn相,晶须状或管状的Cu6Sn5对焊接接头的机械性能没有明显的影响,界面层上析出的大的板状Ag3Sn会增加界面层的脆性断裂倾向,焊料内部的大的板状Ag3Sn提供裂纹扩展路径及二次裂纹源,使拉伸强度降低,但对剪切强度没有明显的影响,只会改变裂纹起源及扩展路径,降低接头的延展性并导致脆性断裂[18]。
4 结 论
a. 无铅焊料SnAg共晶合金及SnAgCu共晶合金与Cu或Ni/Cu或Au/Ni/Cu衬底经钎焊形成焊接接头后,在焊料与衬底界面及焊料内部形成各种金属间化合物,化合物主要与焊料成分及衬底合金的成分、焊接工艺及时效温度和时间有关。焊料与Cu界面通常会析出层状Cu3Sn和扇贝状Cu6Sn5化合物; 焊料与Ni的界面上通常会析出(Cu,Ni)6Sn5,(Ni,Cu)3Sn4和不规则齿状的Ni3Sn4化合物层。界面IMC层在时效过程中晶粒粗化,层厚度增加。
b. 焊料内部的IMC一般有粒状、晶须状Cu6Sn5或颗粒状、树枝状、片状、网状和板状的Ag3Sn及AuSn4,焊接温度越高,冷却速度越慢,焊料中的IMC尺寸越大;当界面到焊料的温度梯度较大时,容易从界面IMC上长出一维方向很长的IMC。Ag含量越高,越容易形成大板状的Ag3Sn,焊料中的Ag3Sn在150 ℃以下时效过程中长大不明显。
c. 焊接接头的断裂会发生在焊料内部、焊料与IMC界面、界面IMC层中,或者上述断裂机制各占一定比例。在时效过程中,哪里最薄弱,哪里强度退化严重,断裂就在哪里发生,若相差不多,则会发生混合断裂。
参考文献:
[1] Abtew M, Selvaduray G. Lead-free solders in microelectronics[J]. Mater Sci Eng Rep, 2000, 27(5/6): 95-141.
[2] He M, Kumar A, Yeo P T, et al. Interfacial reaction between Sn-rich solders and Ni-based metallization[J]. Thin Solid Films, 2004,462/463: 387-394.
[3] Choi W K, Lee H M. Prediction of primary intermetallic compound formation during interfacial reaction between Sn-based solder and Ni substrate[J]. Scripta Materialia, 2002, 46(11): 777-781.
[4] Zeng K, Tu K N. Six cases of reliability study of Pb-free solder joints in electronic packaging technology[J]. Materials Science and Engineering: R, 2002, 38(2): 55-105.
[5] Tu P L, Chan Y C, Lai J K L. Effect of intermetallic compounds on the thermal fatigue of surface mount solder joints[J]. IEEE Transactions on Components, Packaging, and Manufacturing Technology: Part B, 1997, 20(1): 87-93.
[6] Pang J H L, Low T H, Xiong B S, et al. Thermal cycling aging effects on Sn–Ag–Cu solder joint microstructure,IMC and strength[J]. Thin Solid Films, 2004, 462/463: 370-375.
[7] Kim K S, Yu C H, Yang J M. Aging treatment characteristics of solder bump joint for high reliability optical module[J]. Thin Solid Films, 2004, 462/463: 402-407
[8] Amagai M, Watanabe M, Omiya M, et al. Mechanical characterization of Sn–Ag–based lead-free solders[J]. Microelectronics Reliability, 2002, 42(6): 951-966.
[9] Lee H T, Chen M H, Jao H M, et al. Influence of interfacial intermetallic compound on fracture behavior of solder joints[J]. Materials and Engineering A, 2003, A358(1/2): 134-141.
[10] Zeng K, Kivilahti J K. Use of multi-component phase diagrams for predicting phase evolution in solder/conductor systems[J]. J Electr Mater, 2001, 30(1), 35-44.
[11] Kerr M, Chawla N. Creep deformation behavior of Sn–3.5Ag solder/Cu couple at small length scales[J]. Acta Materialia, 2004, 52(15): 4527-4535.
[12] HE Min, CHEN Zhong, QI Guo-jun. Solid state interfacial reaction of Sn–37Pb and Sn–3.5Ag solders with Ni–P under bump metallization[J]. Acta Materialia, 2004, 52(7): 2047-2056.
[13] Choi W K, Kang S K, Sohn Y C, et al. Study of IMC morphologies and phase characteristics affected by the reactions of Ni and Cu metallurgies with Pb-Free solder joints[C]//Proc 53st Electron Comp Technol Conf. New Orleans: IEEE Inc, 2003: 1190-1196.
[14] Sharif A, Islam M N, Chan Y C. Interfacial reactions of BGA Sn–3.5%Ag–0.5%Cu and Sn–3.5%Ag solders during high-temperature aging with Ni/Cu metallization[J]. Materials Science and Engineering, 2004, 113B(3): 184-189.
[15] Li M, Zhang F, Chen W T, et al. Interfacial microstructure evolution between eutectic SnAgCu solder and Al/Ni(V)/Cu thin films[J]. J Mater Res, 2002, 17(7): 1612-1621.
[16] Alam M O, Chan Y C, Tu K N. Effect of reaction time and P-content on mechanical strength of the interfaces formed between eutectic Sn–Ag solder and Au/electroless Ni(P)/Cu bond pad[J]. J Appl Phys, 2003, 94(6): 4108-4115.
[17] Li G Y, Chen B L. Formation and growth kinetics of interfacial intermetallics in Pb-free solder joint[J]. IEEE Transactions on Components and Packaging Technologies, 2003, 26(3): 651-658.
[18] Kim K S, Huh S H, Suganuma K. Effects of intermetallic compounds on properties of Sn–Ag–Cu lead-free soldered joints[J]. Journal of Alloys and Compounds, 2003, 352(1): 226-236.
[19] Choi W J, Yeh E C C, Tu K N. Mean-time-to-failure study of flip chip solder joints on Cu/Ni(V)/Al thin film under-bump metallization[J]. Appl Phys, 2003, 94(9): 5665-5671.
[20] Alam M O, Chan Y C, Tu K N. Effect of 0.5wt.% Cu addition in the Sn-3.5%Ag solder on the interfacial reaction with Au/Ni metallization[J]. Chemistry of Materials, 2003, 15(6): 4340-4342.
[21] LI De-zhi, LIU Chang-qing, Conway P P. Characteristics of intermetallics and micromechanical properties during thermal ageing of Sn–Ag–Cu flip-chip solder interconnects[J]. Materials Science and Engineering A, 2005, A391(1/2): 95-103.
[22] Salam B, Ekere N N, Rajkumar D. Study of the interface microstructure of Sn-Ag-Cu lead-free solders and the effect of solder volume on intermetallic layer formation[C]//Proc 51st Electron Comp Technol Conf. Orlando: IEEE Inc, 2001: 471-477.
[23] Kim K S, Huh S H, Suganuma K. Effects of cooling speed on microstructure and tensile properties of Sn–Ag–Cu alloys[J]. Materials Science and Engineering A, 2002, A333(1): 106-114.
[24] Jang J W, Frear D R, Lee T Y, et al. Morphology of interfacial reaction between Pb-free solders and electroless Ni(P) under-bump-metallization[J]. J Appl Phys, 2000, 88: 6359-6363.
[25] MA Xin, WANG Feng-jiang, QIAN Yi-yu, et al. Development of Cu–Sn intermetallic compound at Pb-free solder-Cu joint interface[J]. Materials Letters, 2003, 57(22): 3361-3365.
[26] Hirose A, Yanagawa H, Ide E, et al. Joint strength and interfacial microstructure between Sn–Ag–Cu and Sn–Zn–Bi solders and Cu substrate[J]. Science and Technology of Advanced Materials, 2004, 5(1/2): 267-276.
[27] Lee H T, Chen M H. Influence of intermetallic compounds on the adhesive strength of solder joints[J]. Materials Science and Engineering A, 2002, A333(1/2): 24-34.
收稿日期:2006-06-08
基金项目:国家自然科学基金资助项目(50475043);国家教育部博士点基金资助项目(20040005012);北京市自然科学基金资助项目(2052006)
作者简介:李晓延(1962-),男,陕西礼泉人,教授,博士,从事焊接材料的研究
通讯作者:杨晓华,女,教授,博士;电话:0591-87892447-801(O);E-mail: xhyang@fzu.edu.cn


