J. Cent. South Univ. (2017) 24: 1959-1967
DOI: https://doi.org/10.1007/s11771-017-3604-8
Process mineralogy and characteristic associations of iron and phosphorus-based minerals on oolitic hematite
LUO Li-qun(罗立群)1, ZHANG Han-quan(张汉泉)2
1. School of Resources and Environmental Engineering, Wuhan University of Technology, Wuhan 430070, China;
2. School of Resources and Civil Engineering, Wuhan Institute of Technology, Wuhan 430205, China
 Central South University Press and Springer-Verlag GmbH Germany 2017
Central South University Press and Springer-Verlag GmbH Germany 2017
Abstract:
The chemical compositions, mineralogical characteristics, as well as dissemination of iron- and phosphorus-based minerals were studied for the E’xi oolitic hematite from western Hubei Province in China by using chemical analysis, optical microscope, electron probe micro-analyzer (EPMA) and energy dispersive spectroscopy (EDS). It is found that this kind of oolitic hematite ore contains 47.71% TFe, 10.96% SiO2, as well as 0.874% P, with hematite as the dominant Fe-bearing minerals, and quartz, chamosite, illite and cellophane as main gangue minerals. The microscope examination showed that the ore has an oolitic structure, with some ooids principally formed by a series of concentric layers of hematite collophanite around nucleus that is hematite in the association with collophanite. Based on the EPMA and EDS analysis, it can be known that some ooids are primarily composed of hematite and collophanite. The separation can be achieved through fine grinding for those collophanite laminae with a higher P content. However, the dissemination of two minerals at the interface will result in the difficulty in effective separation. Besides, some ooids are made of chamosite with some nucleus formed of quartz, which is principally finely disseminated with hematite. In view of the close association and dissemination of iron- and phosphorus-based minerals in the ooids, it is found that the process of stage-grindings and stage-separations can be adopted to effectively increase the iron recovery and decrease the P content in the concentrate to some extent.
Key words:
1 Introduction
The depletion of high-grade, low-impurity iron ore resources with the development of modern civilization has turned the research focus to the exploitation and utilization of complex refractory lean ore resources [1]. Oolitic hematite ore is abundant in resources but regarded as refractory ore due to the iron oxide associated with gangue minerals in a concentric banded formation [2, 3]. The oolitic hematite ore is widely distributed around the world, such as in Dilband Mine in Pakistan, northwest area of Sardinia in Italy and Wade Fatima area in Saudi Arabia, as well as two kinds of typical oolitic hematite distributed respectively in the north and south of China [1, 4, 5]. China has abundant reserves of high-phosphorous iron ore with recoverable deposits of 7.4 billion tons, accounting for 1/9 of total iron ore and 30% of hematite.
Lots of researches have been conducted aiming at the beneficiation of oolitic hematite ore, leading to various separation indexes [2, 6-11], which is mainly attributed to several facts: several gangue minerals contained in the ore, complicated associations and structures among minerals, high cost brought by limited processing technique and equipment. Furthermore, the less knowledge about the process mineralogy of the ore and association of iron- and phosphorus-bearing minerals, results in the obtained concentrate with high content of impurity phosphorus, which is difficult to be removed [5, 12-14]. Thus, such E’xi oolitic hematite ore was taken for investigation in this work, including studies on the physical properties of ores by chemical analysis and optical microscope, the ore characteristics and dissemination of iron- and phosphorus-bearing minerals through EPMA and EDS [15], which can provide a detailed mineralogy data for the future beneficiation of this kind of oolitic hematite.
2 Experimental
2.1 Samples and its characteristics
The tested sample was some sort of oolitic hematite from western Hubei Province, collected by using groove sampling method from the underground tunnel, with particle size in the range of 100-0 mm (part of 150 mm).The sample was crushed by double–roll crusher to less than 2.0 mm after coarse crushing, fine-crushing and division. The multi-element analysis of the sample and phase analysis of iron and phosphorus are shown in Table 1 to Table 3. The particle-size analysis is shown in Table 4.
Table 1 Multi-element analysis result of ore sample (mass fraction, %)
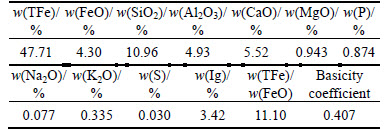
Table 2 Phase analysis of Fe in ore sample (mass fraction)
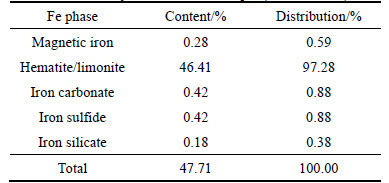
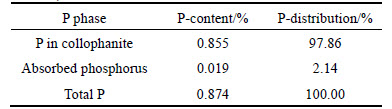
Table 3 Phase analysis of phosphorus in ore sample (mass fraction)
Based on analyzing data in Table 1 to Table 4, it can be known as follows.
1) The tested sample assayed 47.71% TFe on average, containing 10.96% SiO2, 4.93% Al2O3 and 5.52% CaO, respectively, with the content of harmful phosphorus up to 0.874%, and S content relatively low at 0.030%.
2) The ratio of TFe/FeO for the sample is 11.10, lower than 2.33 (ratio for pure magnetite). It is found that the ore is weakly magnetic, and the Fe-bearing minerals in it predominantly occur as hematite and limonite with the content up to 46.41%, accounting for 97.28% of the TFe content, while the content of magnetic iron just around 0.28%. Besides, the content of iron carbonate, iron sulfide and iron silicate is too low to be considered in the beneficiation.
3) The phosphorus content in the ore is 0.874%. The phase analysis showed the phosphorus predominantly occurs as collophanite (with content at 0.82%, accounting for 97.86% of the total phosphorus), only trace in the form of adsorbed phosphorus. Thus the collophanite is the main target to be removed in the separation process.
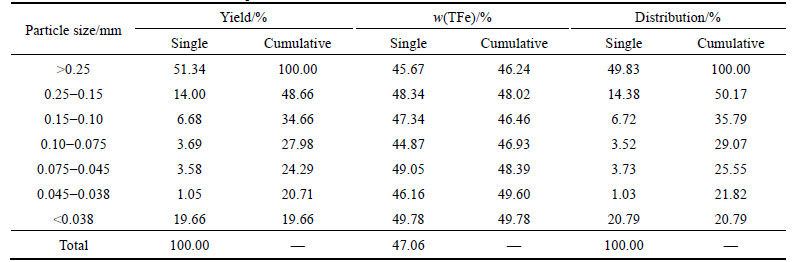
4) It is also found that ore with a higher hardness is hard to be broken, with coarse fraction and fine fraction accounting for 51.34% and 19.66%, respectively. Furthermore, the uniform distribution of iron in different size fraction results in little difference in iron grade within different size fraction, and the highest iron grade of 49.78% was observed for the fine fraction, indicating the fine-dissemination in the ore unfavorable for beneficiation.
2.2 Experimental methods
For being representative, ores at the size fraction of <2.0 mm were selected for microscopic examination, which was mixed with epoxy resin to prepare a polished thin section. Based on the observation under polarizer bias-reflecting microscope (Nikon Lv100pol), the dissemination size of different minerals was analyzed. Then, the oolitic structure and compositions of ore sample were studied using XRD, EPMA and EDS energy spectrum microanalysis, so as to explore the dissemination size and association structure of hematite with collophanite, as well as get to know chemical compositions and their occurrences in the “microzones” in the hematite ooids [16]. The instruments used for examination include electronic probe microscopic analyzer (EPMA) plus energy disperse spectroscopy (Mode: JXA-8230/INCAX-ACT) produced by JEOL Ltd. The test data were used to draw XRD and EDS curve charts by software of MDI Jade and Origin.
Table 4 Results of sieve tests of ore sample
3 Results and discussion
3.1 Main mineral compositions and corresponding contents
The ore sample appears to be bright red by naked eyes and feels to be smooth. Figure 1 shows the XRD patterns of high phosphorus oolitic hematite ore sample. And minerals in the ore include hematite, chlorite, calcite, quartz and dolomite.
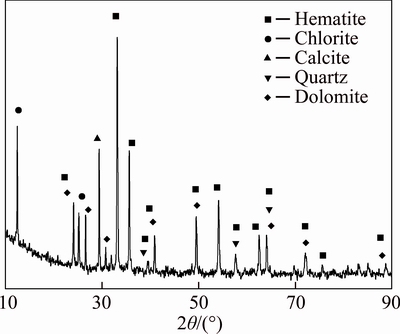
Fig. 1 X-ray diffraction pattern for high phosphorus oolitic hematite ore sample
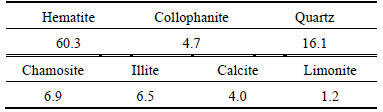
The multi-element analysis, optical microscope observation and XRD patterns show that hematite is the dominant mineral in the ore (60.3%) followed by quartz (16.1%), while the content of collophanite as a harmful impurity is 4.7%. The mineral compositions of ore and corresponding contents are shown in Table 5.
Table 5 Mineral compositions and corresponding contents of ore sample (mass fraction, %)
3.2 Morphological characteristics of ooids in ore
The microscopic structure of ooids under optical microscope is shown in Fig. 2. Ore sample mainly consists of ooids in the shape of roundness, ellipse and cobble. The bright part in the ooids is iron minerals, with gangue minerals in the dark part. And the ooids of 50-350 μm in size are formed by concentric layers of iron mineral and gangue minerals. The oolite nucleus being made of either gangue or iron minerals varies in size, shape and compositions.
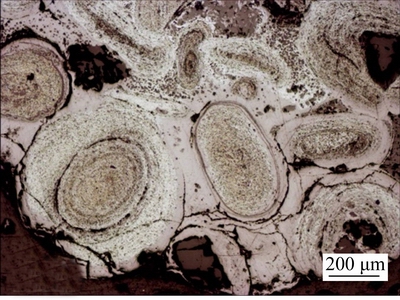
Fig. 2 Morphology of ooids in oolitic hematite of ore samples (reflected light)
The internal structure of ooids observed with SEM is shown in Fig. 3. A typical ooid is composed of several concentric layers of iron minerals and gangue, with layer space varying from 20 μm to 110 μm. SEM results showed different number of layer with the different layer space inside the ooid, and more layers, less in layer space.
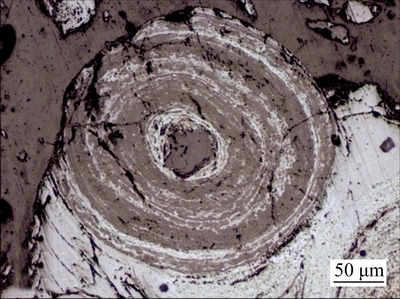
Fig. 3 Internal structure of a typical ooid (reflected light)
The special morphological characteristics of iron and gangue minerals in the ooids bring difficulty to the beneficiation of oolitic hematite. The ore must be subjected to a fine-grinding process for the liberation of the iron mineral. However, it is very hard to get satisfied results for finely-ground iron minerals by flotation or magnetic separation. Thus, it is quite important to develop some new grinding technique and beneficiation process specifically for such fine particles based on the structural characteristics of oolitic hematite.
3.3 Main occurrences
3.3.1 Hematite & limonite
Iron in the ore occurs primarily as hematite, a target mineral for iron recovery. Hematite is observed to be very finely grained or cryptocrystalline under the optical microscope, appearing bright in reflected light, but dark in transmitted light. The distribution of hematite (limonite) in association with collophanite in the ore is shown in Fig. 4. It can be found that hematite is predominantly locked in concentric layered gangue minerals to form ooids, with a few in the form of monomer among the ooids. Besides, the hematite in ooids is distributed between layers with dissemination size from 0.001 to 0.03 mm. Meanwhile, a trace of hematite occurs individually or associated with collophanite, forming oolite nucleus.
Limonite, as another principal iron-bearing mineral in the ore, appears in an irregular shape and with low content. The limonite is mainly distributed in ooids or exists in collophanite as martitization due to a metasomatic process with pyrites (shown in Fig. 4), with particle size from 0.01 to 0.03 mm.
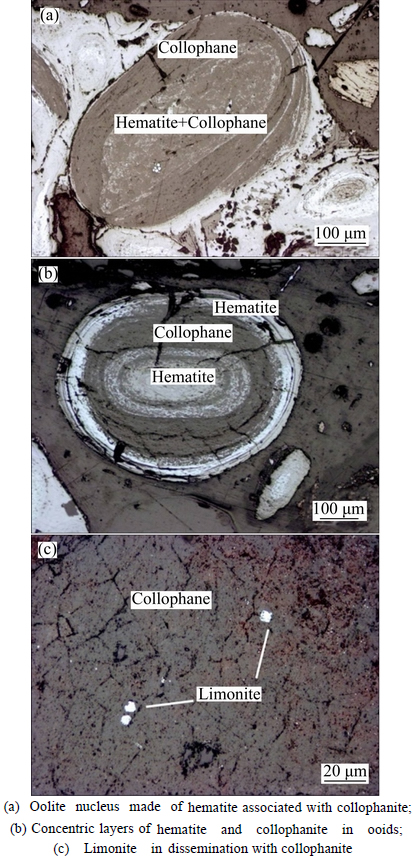
Fig. 4 Distribution of hematite (limonite) associated with collophanite in ore samples:
3.3.2 Collophanite
The phosphorus, as a harmful impurity, primarily occurs as collophanite with P content accounting for 97% of total phosphorus in the ore. The collophanite is distributed along oolitic concentric bands as compacted allotriomorphic granular, or as irregular conglomerate (shown in Fig. 5). The cemented oolitic hematite varies in size from 0.01 to 1.5 mm. Figure 4 shows a complicated association between collophanite and hematite. The bulk collophanite is easy to be separated from hematite through coarse crushing, while the collophanite being finely-disseminated with hematite in concentric layers usually needs to be subjected to fine-grinding process for liberation, which is unfavorable for beneficiation [17].
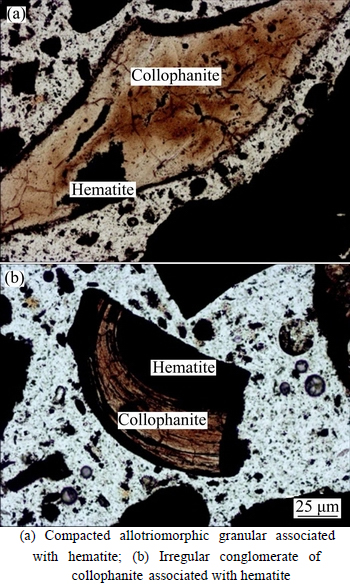
Fig. 5 Irregular conglomerate of collophanite distributed in sample:
3.3.3 Quartz
As one of the main gangue minerals in the ore, quartz mainly contains SiO2 and is distributed among ooids individually or in association with feldspar (shown as Fig. 6(a)), or acted as oolite nucleus (shown as Fig. 6(b)). However, quartz is not observed in the concentric layers of ooids, which is beneficial for removal of quartz, and the grain size of disseminated quartz is between 0.02 and 0.05 mm.
3.3.4 Chamosite
Chlorite is a kind of Fe-bearing silicate, also called chamosite due to its occurrence as oolitic conglomerate, difficult for separation. As shown in Figs. 7(a)-(c), chamosite (around 6-8% in the ore), is sporadically distributed in the nucleus of oolitic hematite in the form of allotriomorphic granular with particle size of 0.03-0.15 mm.
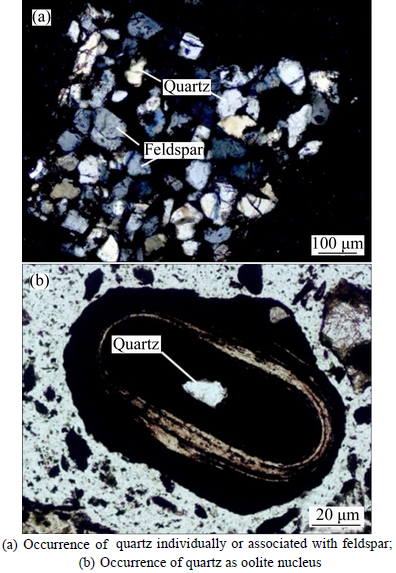
Fig. 6 Occurrence of quartz in ore sample:
3.4 Association of hematite and collophanite in ooids
3.4.1 Electronic probe micro analysis (EPMA)
The microscopic structure shows that ooids consist of iron and gangue minerals in concentric layers. Composition analysis with EMPA and EDS was conducted for the different “microzones” in the oolite nucleus to investigate the association of hematite and collophanite in the ooids [18]. Typical backscattered electron image of ooids and SEM images of area-scanning for elements are shown in Fig. 8.
It can be seen from Fig. 8 that several minerals are concentrated in ooids, and hematite associated with collophanite in concentric layers with quartz as the oolite nucleus. Hematite was observed in a finely-grained association with collophanite with no remarkable boundaries in the outer concentric band, but with obvious boundaries in the inner band, indicating a possible self-concentration effect during the ooid growth. The collophanite layer with minimal space of a few microns is disseminated between hematite and quartz, the outer layer of quartz nucleus, being difficult to be separated. The oolite nucleus is made of quartz with particle size around 100 μm, is preferably separated by the process of stage grinding-stage separation.
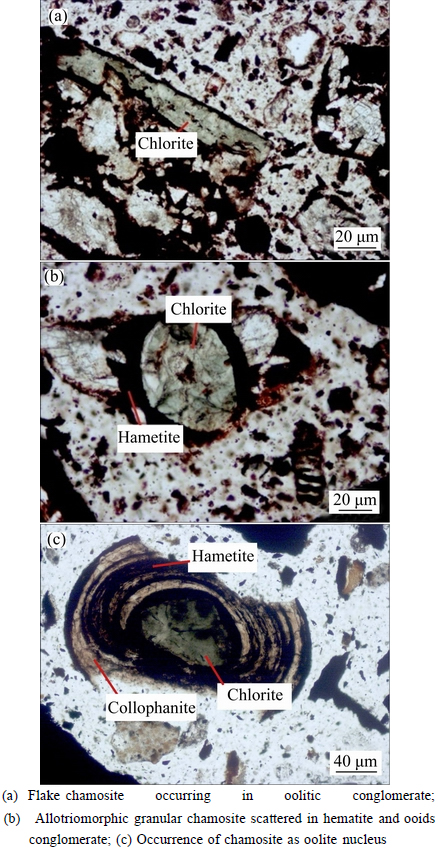
Fig. 7 Distribution of chlorite in ore sample:
3.4.2 EDS analysis
In order to investigate the main elemental compositions and corresponding contents in “microzones” of the ooids, EDS was used to conduct energy spectrum analysis for the microzones of iron[19], phosphorus and quartz. The positions in ooid microzones for the EDS analysis are shown in Fig. 9 (the top left conner of the mark point) and the elemental analysis results can be seen from Table 6. Based on positions of different elements, the ooid is classified into “Fe microzone”, “P microzone” and “Si microzone” etc. The EDS energy spectrum analysis results are shown in Fig. 10.
It can be seen from Table 6 that the chemical compositions and the corresponding contents varied in different microzones, and there is some certain enrichment of various minerals. The microzone of hematite shows a much higher Fe content than others, varying from the lowest value of 46.82% to the highest 65.30%. However, it has lower contents of P and Si, that is consistent with the EDS spectrum analysis result shown in Fig. 10(a). The microzone of collophanite shows a higher content of P than others, with average value over 26%, but with low contents of Fe and Si. Figure 10(b) shows a moderately high peak value for contents of P and Ca, but no peaks for Fe and Si, indicating a moderately high content of collophanite in this microzone. The spectrum shows a positive correlation between F, Ca contents and P content, which means that the main component of collophanite is fluorapatite (Ca5(PO4)3F). The analysis result also shows that microzone of quartz primarily contains Si with content up to 46.59%, almost same as the theoretical content of Si in pure SiO2 (46.67%). Figure 10(c) shows peaks just for Si and O contents, indicating this microzone contains high-purity quartz.
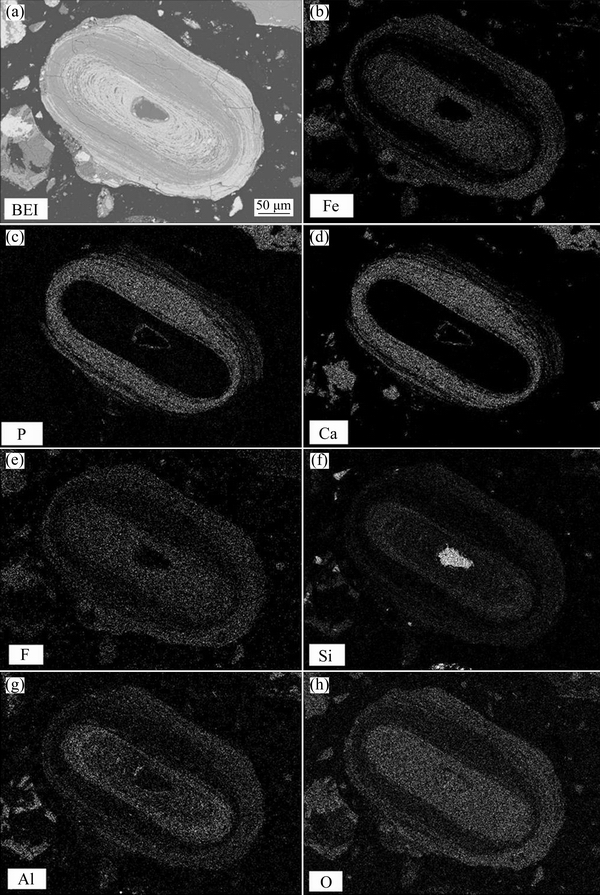
Fig. 8 Backscattered electron images (a) of ooids and area-scanning of Fe, P, Ca, F, Si, Al, O elements (b-h)
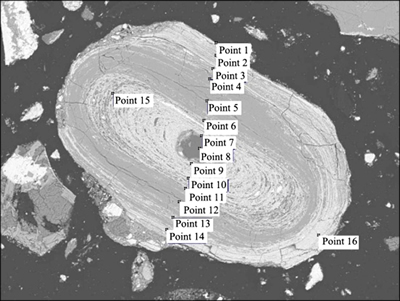
Fig. 9 Positions in microzones of oolite for EDS spectrum analysis
Table 6 Results of EDS spectrum analysis for compositions of ooids
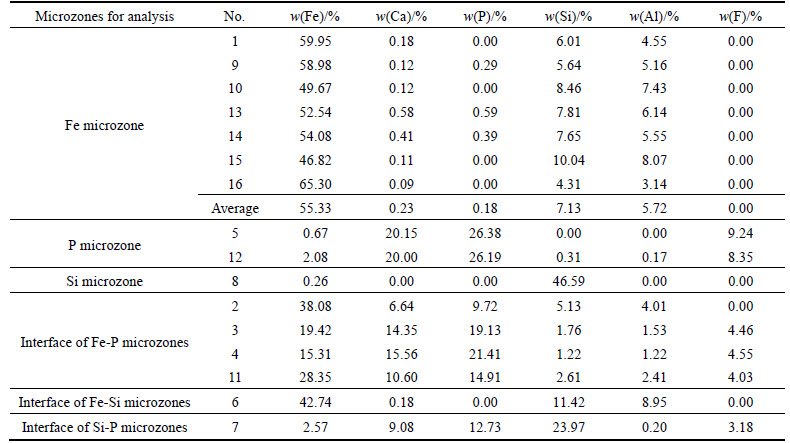
Based on the results in Table 6 and Fig. 10, interface between different mineral layers in ooids can be simply classified as Fe-P microzone interface, Fe-Si microzone interface and Si-P microzone interface. The collophanite mainly existed in the middle part of ooid and is disseminated with hematite in concentric layers. The Fe-P microzone interface is the interface of collophanite and hematite or the association section of the two components. The removal of phosphorus in the separation process is largely depended on the dissemination characteristics of Fe-P interface [20]. It can be seen from Fig. 9, that positions 2 and 3 are Fe-P association microzone, while positions 4 and 11 are the interface with a higher content of Fe and P. The hematite and collophanite are in a fine-grained association, the EDS energy spectrum analysis of interface is shown in Fig. 10(d). It can be known from Fig. 8 and Table 6, most of Si in the ooids, besides forming the nucleus, are associated with fine-grained Fe, with trace associated with P, but no concentration in the form of individual lamellar mineral. The EDS spectrum image of Fe-Si interface in Fig. 10(e) shows a simple relationship between P and Si. Only when the oolite nucleus is formed of quartz, Si may be contacted with P directly, namely (collophanite), which can be seen from the EDS energy spectrum image of Si-P interface in Fig. 10(f). From the peaks of Si and Al showed in Fig. 10(a) and element analysis results in Table 6, Si content at 4.31%-10.04% and Al content at 3.14%-8.07% indicate that the gangue in the Fe-microzone occurs principally in the form of iron-bearing aluminosilicates, such as chamosite, while other Si occurs either in the form of chamosite or in a finely-grained dissemination with hematite in concentric layers, which can be seen from Fig. 10(a) and Fig. 9. Consequently, an expected separation may not be realized by magnetic separation. However, if reverse flotation process is adopted to remove Si and Al for reducing impurities, a great amount of iron will be lost, which is another factor making for the difficult separation.
According to analysis results detecting iron micro area by EDS in Table 6, oolitic hematite containing iron micro average iron is up to 55.33% and harmful impurity phosphorus of 0.18%, which is the theoretical or optimal grades in concentrate by high intensity magnetic separation. If oolitic hematite completely had been transformed all artificial magnetite by magnetization roasting process, iron content will improve to 57.23% (impurities decomposition is negligible). It was concluded that the reduction degree increases with the increase of residence time for long distance diffusion in magnetic roasting-magnetic separation, while the removal rates of silica, phosphorus and alumina increase with the decreases of particle size in reverse flotation technology.
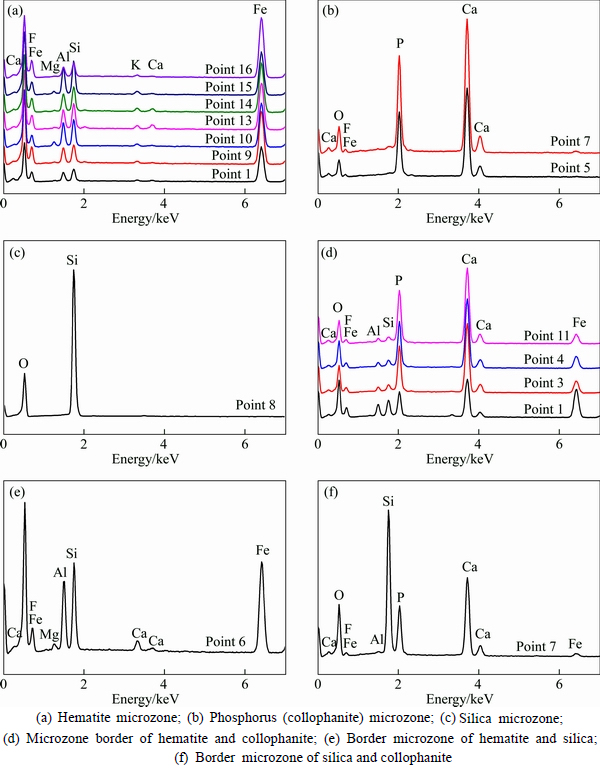
Fig. 10 EDS spectrum image of different zones in oolite:
4 Conclusions
1) This iron ore, classified as a typical acid oolitic hematite with high phosphorus and low sulphur, contains 47.71% TFe, 0.874% P and 10.96% SiO2, and has the dominant minerals composed of hematite (limonite), quartz, chamnosite, collophanite, etc.
2) The ore mainly consists of a series of concentric layer of iron-bearing ooids that vary in size from 50 to 350 μm. Some ooids are primarily composed of hematite and collophanite, with quartz finely disseminated with hematite. The oolite nucleus is usually filled with hematite, collophanite and chamnosite, etc., and a few quartz grains.
3) The EPMA and EDS analyses results showed that the chemical compositions vary greatly in different positions of Fe-P microzone interface, Fe-Si microzone interface and Si-P microzone interface in ooids, with iron mineral in the same concentric layer being of higher purity. Oolitic hematite containing iron micro average iron is up to 55.33% and harmful impurity phosphorus of 0.18%, which is the theoretical grades in concentrate by high intensity magnetic separation. The phosphorus in ooids occurs predominantly as individual collophanite, a few finely disseminated with hematite. Most of collophanite can be liberated by fine-grinding process, but the collophanite at the interface of Fe-P microzones could hardly be removed by the conventional beneficiation process.
References
[1] ZHANG Han-quan. Mineralogical properties and tendency on utilizatiing methods of oolitic hematite [J]. China Metallurgy, 2013, 23(11): 6-10. (in Chinese)
[2] LUO Li-qun, CHEN Min, YAN Hao-tian, CUI Shuang-shuang, ZHANG Yan-juan. Magnetic reduction roasting and magnetic separation of oolitic iron ore [J]. The Chinese Journal of Process Engineering, 2014, 14(4): 593-598. (in Chinese)
[3] SUN Yong-sheng, HAN Yue-xin, GAO Peng, ZHOU Man-geng. Study on process mineralogy of a high phosphorus oolitic hematite ore [J]. Journal of Northeastern University: Natural Science, 2013, 34(12): 1773-1777. (in Chinese)
[4] SONG Shao-xian, EMESTO F C, ZHANG Yi-min, ALEJANDRO L V. Morphological and mineralogical characterizations of oolitic iron ore in the exi region, China [J]. International Journal of Minerals Metallurgy and Materials, 2013(2): 113-118.
[5] BAIOUMY H M. Iron–phosphorus relationship in the iron and phosphorite ores of egypt [J]. Chemie der Erde - Geochemistry, 2007, 67(3): 229-239.
[6] YU Wen, SUN Ti-chang, CUI Qiang. Can sodium sulfate be used as an additive for the reduction roasting of high-phosphorus oolitic hematite ore? [J]. International Journal of Mineral Processing, 2014, 133: 119-122.
[7] XU Cheng-yan, SUN Ti-chang, KOU Jue, LI Yong-li, MO Xiao-lan, TANG Li-gang. Mechanism of phosphorus removal in beneficiation of high phosphorous oolitic hematite by direct reduction roasting with dephosphorization agent [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(11): 2806-2812.
[8] LIN Qing, ZHONG Le-le, GONG Wen-qi, WANG En-wen, LU Yu, XIA Dao-hong. Experimental study on magnetic separation of oolitic hematite ore [J]. Advanced Materials Research, 2014, 834: 374-377.
[9] GUO Chao, WAN Gui, FU Jian-gang, CHEN Kai-da. Recovery a refractory oolitic hematite by magnetization roasting and magnetic separation [J]. Advanced Materials Research, 2012, 361: 305-310.
[10] TANG Hui-qing, GUO Zhan-cheng, ZHAO Zhi-long. Phosphorus removal of high phosphorus iron ore by gas-based reduction and melt separation [J]. Journal of Iron and Steel Research (International),2010, 9: 1-6.
[11] HAN H, DUAN D, WANG X, CHEN S. Innovative method for separating phosphorus and iron from high-phosphorus oolitic hematite by iron nugget process [J]. Metallurgical and Materials Transactions B, 2014, 45(5): 1634-1643.
[12] OLUBAMBI P A, NDLOVU S, POTGIETER J H, BORODE J O. Mineralogical characterization of ishiagu (nigeria) complex sulphide ore [J]. International Journal of Mineral Processing, 2008, 87(3, 4): 83-89.
[13] SANTOS L D, BRANDAO P R G. Morphological varieties of goethite in iron ores from minas gerais, brazil [J]. Minerals Engineering, 2003, 16(11, Supplement 1): 1285-1289.
[14] PETIT-DOMINGUEZ M D, RUNDIO M I, GALAN-SAULNIER A, GARCIA-GIMENEZ R. Usefulness of geological, mineralogical, chemical and chemometric analytical techniques in exploitation and profitability studies of iron mines and their associated elements [J]. Journal of Geochemical Exploration, 2008, 98(3): 116-128.
[15] LUO Li-qun, LI Jin-liang, CAO Jia-hong. Process mineralogy and factors affecting mineral processing for copper-nickel ore in Hami [J]. The Chinese Journal of Nonferrous Metals, 2014, 24(7): 1846-1855. (in Chinese)
[16] OMRAN M, FABRITIUS T, ELMAHBY M A, ABDEL-KHALEK A N, EL-AREF M, ELMANAWI E A. XPS and FTIR spectroscopic study on microwave treated high phosphorus iron ore [J]. Applied Surface Science, 2015, 345: 127-140.
[17] GRGIC D, GIRAUD A, AUVRAY C. Impact of chemical weathering on micro/macro-mechanical properties of oolithic iron ore [J]. International Journal of Rock Mechanics and Mining Sciences, 2013, 64: 236-245.
[18] HOPE G A, WOODS R, MUNCE C G. Raman microprobe mineral identification [J]. Minerals Engineering, 2001, 14(12): 1565-1577.
[19] DONSKOI E, SUTHERS S P, FRADD S B, YOUNG J M, CAMPBELL J J, RAYNLYN T D, CLOUT J M F. Utilization of optical image analysis and automatic texture classification for iron ore particle characterisation [J]. Minerals Engineering, 2007, 20(5): 461-471.
[20] LI Guang-hui, ZHANG Shu-hui, RAO Ming-jun, ZHANG Yuan-bo, JIANG Tao. Effects of sodium salts on reduction roasting and Fe–P separation of high-phosphorus oolitic hematite ore [J]. International Journal of Mineral Processing, 2013, 124: 26-34.
(Edited by HE Yun-bin)
Cite this article as:
LUO Li-qun, ZHANG Han-quan. Process mineralogy and characteristic associations of iron and phosphoros-based minerals on oolitic hematite [J]. Journal of Central South University, 2017, 24(9): 1959–1967.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-017-3604-8Foundation item: Project(51474161) supported by the National Natural Science Foundation of China
Received date: 2016-03-21; Accepted date: 2016-06-20
Corresponding author: ZHANG Han-quan, Professor, PhD; Tel: +86-27-87194821; E-mail:springt@139.com
Abstract: The chemical compositions, mineralogical characteristics, as well as dissemination of iron- and phosphorus-based minerals were studied for the E’xi oolitic hematite from western Hubei Province in China by using chemical analysis, optical microscope, electron probe micro-analyzer (EPMA) and energy dispersive spectroscopy (EDS). It is found that this kind of oolitic hematite ore contains 47.71% TFe, 10.96% SiO2, as well as 0.874% P, with hematite as the dominant Fe-bearing minerals, and quartz, chamosite, illite and cellophane as main gangue minerals. The microscope examination showed that the ore has an oolitic structure, with some ooids principally formed by a series of concentric layers of hematite collophanite around nucleus that is hematite in the association with collophanite. Based on the EPMA and EDS analysis, it can be known that some ooids are primarily composed of hematite and collophanite. The separation can be achieved through fine grinding for those collophanite laminae with a higher P content. However, the dissemination of two minerals at the interface will result in the difficulty in effective separation. Besides, some ooids are made of chamosite with some nucleus formed of quartz, which is principally finely disseminated with hematite. In view of the close association and dissemination of iron- and phosphorus-based minerals in the ooids, it is found that the process of stage-grindings and stage-separations can be adopted to effectively increase the iron recovery and decrease the P content in the concentrate to some extent.


