
Electroplating zinc transition layer for electroless nickel plating on AM60 magnesium alloys
WANG Xiao-min(王晓民)1, ZHOU Wan-qiu (周婉秋)1,2, HAN En-Hou(韩恩厚)2
1. College of Chemistry and Life Science, Shenyang Normal University, Shenyang 110034, China;
2. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
Received 28 July 2006; accepted 15 September 2006
Abstract:
Electroplating zinc coating as transition layer of electroless nickel plating on AM60 magnesium alloys was investigated. The zinc film can be deposited in a pyrophosphate bath at 50-60 ℃ under current density of 0.5-1.5 A/dm2. A new fore treatment technology was applied by acid cleaning with a solution containing molybdate and phosphorous acid, by alkaline cleaning in a bath containing molybdate and sodium hydroxide. The subsequent electroless plating was carried out in nickel sulfate bath. The SEM observation shows that the deposition is uniform and compact. The polarization curve measurements show that the corrosion potential of the zinc plating in 3.5% NaCl is about -1.3 V(vs SCE) which is noble than that of magnesium substrate. The zinc electroplating can be applied as the pretreatment process for electroless nickel plating on magnesium alloys.
Key words:
magnesium alloy; AM60; electroplating zinc; electroless nickel plating; corrosion resistance;
1 Introduction
Magnesium is valuable in a number of application including aerospace, automobile and computer parts due to its desirable properties of light mass combining of high strength-mass ratio, excellent damping performance and good electromagnetic shielding characteristic[1]. However, a challenge in magnesium application is their relatively high chemical activity which results in severe corrosion in service[2]. Electroless nickel plating is often used to alter the surface properties of a work piece to improve its corrosion resistance and electrical conductivity. A number of works focused on the direct electroless nickel plating in basic nickel carbonate bath with chromate acid pickling and HF activation[3-5]. However, the electrochemical potential of nickel is much noble than that of magnesium alloys, galvanic corrosion is easy to take place between the porous Ni-P film and metal substrate when the nickel plating is deposited directly on the substrate[6].
In order to decrease the galvanic corrosion between Ni-P film and metal substrate, the traditional transition layer is deposited on substrate by zinc immersion followed by cyanide copper plating process[7]. However, the cyanide copper plating is poisonous to the environment, and the thickness of zinc immersion layer is limited by the replacement reaction of zinc immersion treatment. The zinc immersion is especially difficult because intermetallic species such as Mg17Al12 precipitating on the grain boundary, which results in a non-uniform surface potential across the substrate and thus forms a loose deposition layer. In this study, a compact and adequate adhesion zinc transition layer is formed on AM60 magnesium alloys by electroplating method, the film thickness can be adjusted by controlling deposition time and cathode current density. The followed electroless nickel plating is conducted in nickel sulfate bath which is easy to control and has low cost than that of direct electroless nickel plating in basic nickel carbonate bath. The corrosion resistance and layer performance were investigated in this paper.
2 Experimental
The substrate material used in this study was AM60 magnesium alloy, the microstructure of AM60 alloy was α phase with discontinuous β phase (Mg17Al12) on the grain boundary. The chemical compositions (mass fraction, %) were given as follows: Al 5.6-6.4, Mg 0.26-0.6, Si <0.05, Cu <0.008, Ni <0.001, Fe <0.004, and balance Mg. The specimens were machined with size of 30mm ×20mm × 5mm and polished with emery papers up to 1000 grit to ensure similar surface roughness.
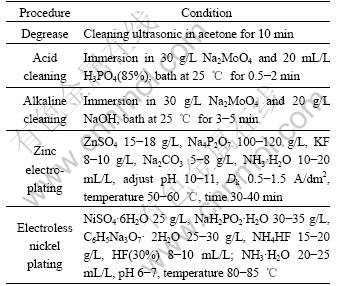
The process of electroless nickel plating was as follows: degrease→acid cleaning→alkaline cleaning→ zinc electroplating→electroless nickel plating. Distilled water resin was carried out between each step. The procedure is listed in Table 1.
Table 1 Procedure of electroless nickel plating
Electrochemical measurements were carried out using EG&G Model 273 potentiostat, platinum was used as a counter electrode, a saturated calomel electrode was used as reference electrode. Specimen was used as working electrode, the working area exposed to the electrolyte was 1 cm2. The polarization curves were measured at a scan rate of 0.5 mV/s.
The morphology was observed using ESEM (Model XL30 FEG), and composition analysis was conducted by the electron probe microanalysis system (EDX) attached to ESEM. The microstructure was tested by XRD (Model Philip PW1700).
3 Results and discussion
3.1 Composition of zinc electroplating bath
ZnSO4 is the main salt in the electroplating zinc bath, which supplies zinc ions. The content of zinc sulphate influences the quality of the electroplating zinc coating. When the concentration is higher than 20 g/L, the coating surface shows coarse appearance. When the concentration of zinc sulphate is too low, for example less than 12 g/L, the deposition rate of zinc layer is slow. Na4P2O7 is the main complexing agent. When it is added into zinc sulphate bath, the follow reaction occurs:
Zn2++2P2O74-=[Zn(P2O74-)2]6- (1)
The complex compound can enhance the cathodic polarization and make the zinc deposition grain finer. The complex compound can also improve the dispersed ability of electrolyte solution and thus provides higher coverage of zinc coating on metal substrate. Some free P2O74- ions are necessary, free P2O74- ions can increase the stability of the complex compound and prevent precipitation from deposition. KF acts as conducting salt in the bath. H3PO4 will be produced in the process of electroplating, which increases the viscosity of bath and reduces the electrolyte conductivity. Considering the protective effect of fluoride for magnesium, KF was selected to improve the conductivity. High KF content can promote the bath conductivity and increase the current efficiency, however, bath dispersed ability will be decreased and results in non-uniform distribution of coating. Low KF content will decrease the current efficiency. The adaptive dosage of KF is 5-15 g/L. Na2CO3 serves as the corrosion inhibitor, HUO et al[3] indicated that Na2CO3 can protect magnesium substrate from severe corrosion when the sulphate exists, the appropriate concentration for this bath is about 5-8 g/L according to the experimental result.
3.2 Morphology and microstructure of coatings
The obtained electroplate zinc coating shows light gray color. The appearance is compact and uniform as shown in Fig.1, which provides a good base for the subsequent electroless nickel plating. The chemical composition is given in Fig.2, which confirms that the electroplate zinc is consisted with pure zinc. The XRD pattern indicates that the zinc coating shows crystal structure shown in Fig.3, the peak of magnesium is from substrate. The surface morphology of electroless nickel plating with zinc transition layer is illustrated in Fig.4. The surface is uniform, and the low porosity can be observed on the film surface.
3.3 Polarization curve
The polarization curves of various samples were measured in 3.5% NaCl and the results are illustrated in Fig.5. The fitting results of polarization curves are shown in Table 2. This indicates that the corrosion potential of AM60 alloy substrate is -1.51 V (vs SCE), the corrosion potential of zinc coating shifts positively at a value about -1.30 V (vs SCE), which is higher than that of AM60 magnesium substrate. There is no anodic passive region in zinc coating polarization curve. The polarization curves of electroless nickel plating with zinc transition layer show evident passive characteristic in anode region, the corrosion potential is about -0.47 V (vs SCE). When the porous Ni-P film is exposed in environment containing chloride, the galvanic cell will be formed between the transition zinc layer and Ni-P film, the substrate magnesium will be protected from galvanic corrosion. The galvanic corrosion tendency between zinc layer and magnesium alloy is weak due to the little potential difference between AM60 magnesium substrate and zinc layer. The Zn/Ni-P coating can enhance the thermodynamic stability and protect the magnesium alloy from corrosion.
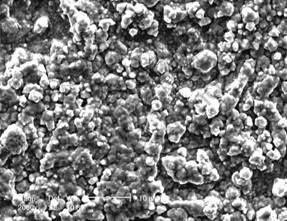
Fig.1 Morphology of electroplating zinc coating
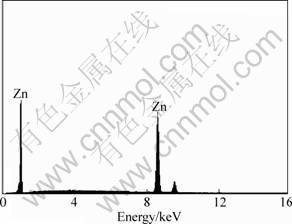
Fig.2 EDX analysis of zinc coating
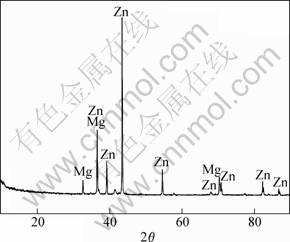
Fig.3 XRD pattern of zinc coating
Table 2 Electrochemical parameters of coatings

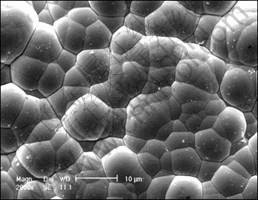
Fig.4 Morphology of electroless nickel plating on zinc coating
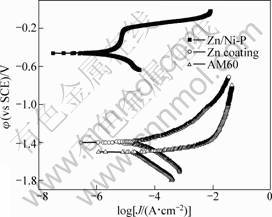
Fig.5 Polarization curves of coatings in 3.5% NaCl
4 Conclusions
Zinc film is deposited on AM60 magnesium alloys by electroplating as a transition layer of electroless nickel plating from pyrophosphate bath. The zinc film is composed of pure zinc with a crystal structure. The thick and compact zinc film provides good base for subsequent electroless nickel plating, and protects magnesium alloys from galvanic corrosion.
References
[1] ZENG Rong-chang, KE Wei, XU Yong-bo, HAN En-hou, ZHU Zi-rong. Recent development and application of magnesium alloys[J]. Acta Metallurgica Sinica, 2001, 37 (7): 673-685.
[2] SONG Guang-ling, ATRENS A. Corrosion mechanisms of magnesium alloys[J]. Advanced Engineering Materials, 1999, 1: 11-33.
[3] HUO Hong-wei, LI Ying, WANG Fu-hui. Electroless nickel plating on AZ91D magnesium alloys[J]. Journal of Chinese Society for Corrosion and Protection, 2002, 22(1): 14-17.(in Chinese)
[4] SHARMA A K, SURESH M R, BHOJRAJ H, NARAYANAMURTHY H, SAHU R P. Electroless nickel plating on magnesium alloys[J]. Metal Finishing, 1998, 96(3): 10-18.
[5] LIU Xin-kuan, XIANG Yang-hui, HU Wen-bin, et al. Ageing of electroless nickel bath of magnesium alloys[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(4): 1046-1050.(in Chinese)
[6] LI Jian-zhong, TIAN Yan-wen, HUANG Zhen-qi, ZHANG Xin. Studies of the porosity in electroless nickel deposits on magnesium alloys[J]. Applied Surface Science, 2006, 252: 2839-2846.
[7] LI Ying, YU Gang, LIU Yue-long, YE Li-yuan, GUO Xiao-hua, LEI Xi-ping. Electroless nickel plating technology of AZ91D magnesium alloy in nickel sulfate bath[J]. Material Protection, 2003, 36(10): 30-32.
(Edited by LI Xiang-qun)
Foundation item: Project (202113191) supported by the Science Fund of Education Office of Liaoning Province, China; Project supported by the Director Fund of Experimental Centre of Shenyang Normal University, China
Corresponding author: ZHOU Wan-qiu; Tel: +86-024-23893115; E-mail: wqzhou@imr.ac.cn


