Trans. Nonferrous Met. Soc. China 27(2017) 1404-1410
Leaching of blended copper slag in microwave oven
M. Deniz TURAN1, Z. Abidin SARI1, Jan D. MILLER2
1. Department of Metallurgical and Materials Engineering, University of Firat, 23279 Elazig, Turkey;
2. Department of Metallurgical Engineering, University of Utah, 84112 Salt Lake City, UT, United States
Received 4 May 2016; accepted 10 September 2016
Abstract:
Leaching of blended slag (BS) was investigated in a microwave oven using hydrogen peroxide and acetic acid. The BS was a mixture of converter and flash furnace slag containing 51% Fe2O3, 3.8% CuO, and 3.2% ZnO. The important variables that influence the metal extraction yield were leaching time, liquid-solid ratio, H2O2 and CH3COOH concentrations. The preferred leaching conditions were as follows: CH3COOH concentration 4 mol/L; H2O2 concentration 4 mol/L; microwave power 900 W; leaching time 30 min; liquid-solid ratio 25 mL/g BS; leaching temperature 100 °C. Under these conditions, the metal extractions of 95% Cu, 1.6% Fe, and 30% Zn were obtained. The results were compared with the traditional leaching results. It is evident that microwave heating causes a reduction in the leaching time. Also, the extraction yield results indicate that selective leaching of BS can be achieved under the preferred conditions. The dissolution kinetic of BS in hydrogen peroxide with acetic acid is controlled by a shrinking unreacted core model equation. The apparent activation energy and reaction order were found to be 16.64 kJ/mol and 1.09, respectively.
Key words:
slag; copper; microwave; selective leaching;
1 Introduction
Copper is produced mostly from sulfide ores by pyrometallurgical techniques. Typical copper ores contain from 0.5% Cu (open pit mines) to 1%-2% Cu (underground mines). Chalcopyrite contains the least copper compared with other copper minerals [1,2]. The copper concentrate is typically fed to a smelter which includes melting-converting-refining-electrorefining stages. During the pyrometallurgical processing, various types of slags are generated containing significant amounts of some valuable metals such as Cu, Zn and Co. Slag containing valuable metals is generated during smelting and converting. The copper content of the slag is significant (average 4%) when the copper grade of the ore is considered.
Due to economical reasons, there has been growing interest in hydrometallurgical processes to recover the valuable metals from slag. In these studies, efforts have been mainly focused on the hydrometallurgical recovery processes. Recovery of valuable metals from slag can be classified as oxidative leaching [3-10], and roasting with various reagents [11-15]. The purpose of these research efforts is to offer an alternative hydrometallurgical method for slags because of selective leaching and low cost.
Although pyrometallurgical routes are available, the valuable metals from slags cannot be recovered economically. Also, it is difficult to recover valuable metals from these slags using froth flotation. The copper slag used in this study is a blended slag (BS) that is generated during flash smelting and converting. After cooling/solidification, these two slags (BS) are crushed, ground and treated by flotation. However, for reasons mentioned above, flotation recovery of valuable metals from BS is not effective with existing plant facilities. Therefore, treatment of the slag by a hydrometallurgical method involving microwave heating by hydrogen peroxide oxidation is considered.
Microwaves are electromagnetic waves. They are governed by the same physical laws as other waves (i.e., radio or radar waves), and therefore are reflected, transmitted or absorbed by materials. Microwaves cause molecular motion by migration of ionic species or rotation of dipolar species. The extent to which a material absorbs microwave energy is primarily determined by its conductivity. Materials with low conductivities, such as insulators, are effectively transparent to the incident waves and thus, do not store any of the energy in the form of heat [16]. However, the permittivities of most materials are related with several variables such as the moisture content of materials, the frequency of the applied electric field, the temperature and the density and the structure of materials [17].
Compared to conventional heating, microwave heating offers a number of advantages which supplies non-contact heating, energy transfer (no heat transfer), rapid heating, material selective heating, heating start from interior of the material body. Materials which are excellent absorbers of microwave energy are easily heated and are classed as dielectrics [18].
The influence of microwaves on various hydrometallurgical processing has been widely reported in Refs. [16,19-22], also for sulphide ores [23-26]. The main reasons for using microwave energy are to reduce processing cost, process time, rapid heating of materials, and use of a controllable clean energy source.
As an oxidant, hydrogen peroxide is a good oxidizing agent for leaching studies because its oxidation potential (1.77 V) is adequate for oxidizing almost all metal sulfide minerals. The oxidative action of hydrogen peroxide in acidic solution may be represented by [27]
H2O2+2H++2e 2H2O (1)
2H2O (1)
Furthermore, hydrogen peroxide can also behave as a reducing agent:
H2O2 O2+2H++2e (2)
O2+2H++2e (2)
Hydrogen peroxide is an unstable compound, whose decomposition can be catalyzed by certain factors such as the presence of acid, base, mineral surface or soluble ions. Due to rapid exothermic decomposition of hydrogen peroxide in solution, isothermal leaching conditions may disappear and the active oxygen may not be sufficiently used for oxidation of sulfide minerals in the leaching system. To avoid rapid decomposition of hydrogen peroxide, some stabilizers have been used such as glycol, phosphoric acid, oxalic acid, citric acid, and acetic acid in the leaching solution [28-33]. However, acetic acid is known to be a good complexing agent as well as a stabilizer for hydrogen peroxide and reduces the rate of decomposition.
The aim of this study is to investigate the leaching of blended slag (BS) with microwave energy in the presence of hydrogen peroxide and acetic acid.
2 Experimental
2.1 Materials
Blended slag (BS) was obtained from the Karadeniz Copper Plant, Samsun, Turkey. The BS used in these experiments was from converter and flash furnace slag. First, the BS was cooled in air by water. The cooled material was crushed, ground and fed to froth flotation to recover precious metals. Although it was intended to reduce the economic losses, the BS material thus prepared was floated with a low yield.
For the research program, the BS sample was crushed, ground, and sieved to <0.074 μm. Constant particle size was used in all experiments because it represented similar size in flotation plant. Subsequently, this sample was dried in a furnace, and it was stored in a closed vessel for later use.
2.2 Characterization of sample
Chemical analyses of the BS were carried out by AAS in clear supernatant that was obtained by the digestion process. Also, the results of chemical analysis were supported with XRF, to quantify the chemical composition of the oxidized metals. The results of the chemical analyses are shown in Table 1. BS contains various mineral phases as shown from the XRD analysis (Fig. 1). As seen in Fig. 1, there are mostly copper and iron-sulfur compounds in BS. The presence of these mineral phases indicates that non-oxidized minerals are collected in slag phase during copper production process. On the other hand, the presence of silicon is observed in some parts of SEM image in BS. SEM result of BS is shown in Fig. 2. Mapping and EDX analysis show that iron and silicon are dominant phases; however, these analyses did not provide information about the liberalization of metals. Particle size distribution measurement of the BS was carried out by using the laser scattering technique. According to particle size distribution analysis, d0.1, d0.5 and d0.9 values are 4.939, 32.056 and 77.161 μm, respectively (Table 2).
Table 1 Chemical analyses of BS (mass fraction, %)

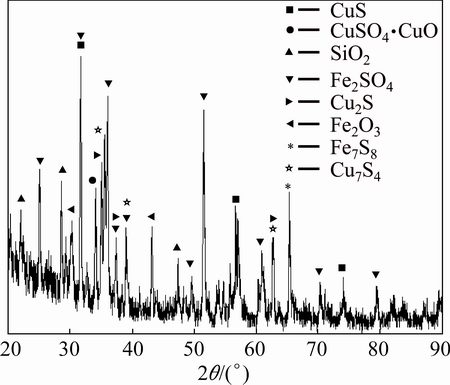
Fig. 1 XRD pattern of BS
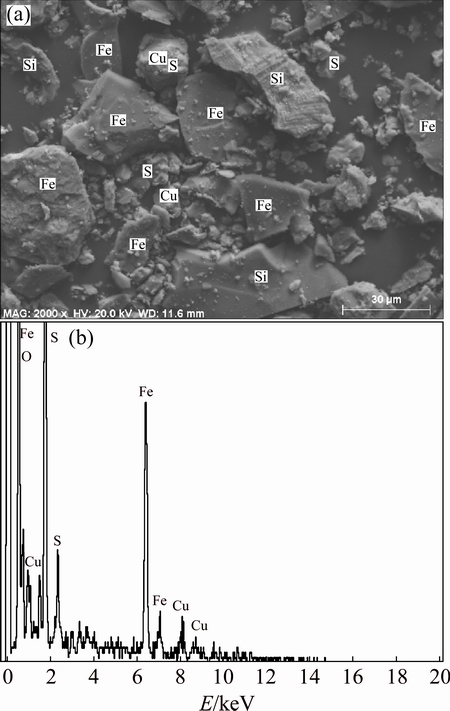
Fig. 2 SEM, mapping and EDX analysis of BS
Table 2 Particle size distribution of BS
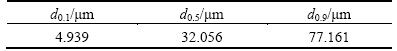
As seen from BS characterization studies, the dominant element is iron which plays an important role in choosing a selective hydrometallurgical leaching method. It is desired to select a selective leaching method that limits the release of iron to solution.
2.3 Leaching method
The microwave oven used in these experiments is a kitchen type having a capacity of 34 L and 900 W of microwave output power (LG, MP-9485S Model). The dissolution experiments in the microwave oven were carried out in 50 mL Teflon containers. The desired amount of BS was added to the Teflon container containing a mixture of hydrogen peroxide and acetic acid solution, followed by placement in the microwave oven. The effects of selected leaching variables were investigated, and except for the stirring speed- experiments, were performed under non stirring conditions. At the end of the desired leaching period, the Teflon containers were rapidly cooled and the contents were filtered.
Conventional leaching experiments were carried out in 0.3 L flasks under reflux conditions. These experiments were performed under atmospheric conditions by using a conventional magnetic multi stirrer (Velp Scientific MultiStirrer 15). As before, at the end of leaching period, the contents were cooled and filtered.
The filtered solutions were analyzed for copper, iron, and zinc using an AAS (Perkin Elmer-Analyst 400).
Hydrogen peroxide (35%, Merck 1.08600) and acetic acid (100%, Merck, 1.00063.2511) were used in the experiments. All chemicals were used as received, without any further purification. Double distilled water was used in all experiments.
3 Results and discussion
Based on the BS characterization studies, it can be said that copper in BS is present as sulfide phases having various stages of oxidation. The sulfide mineral phases of the BS suggest that an oxidant is necessary for dissolution.
Oxygen formed during the decomposition of hydrogen peroxide can be useful for oxidative leaching. Nevertheless, fast decomposition of hydrogen peroxide in the leaching studies is undesirable due to excessive reagent consumption. For these reasons, acetic acid was used as a stabilizer and for complexing the dissolved copper. When hydrogen peroxide and acetic acid are used together the expected dissolution reactions can be written as follows:
CuS+H2O2+2H+→2H2O+S0+Cu2+ (3)
Cu2++2CH3COO-→Cu(CH3COO)2 (4)
and total reaction:
CuS+H2O2+CH3COO-+H3O+→Cu(CH3COO)2+H2O+S0 (5)
Also reaction between acetic acid and hydrogen peroxide:
CH3COOH+H2O2→C2H4O3+H2O (6)
The effect of leaching temperature was examined in a solution containing 2 mol/L H2O2, 4 mol/L CH3COOH, 25 mL/g BS, 900 W microwave power, and 60 min of leaching time (Fig. 3). From Fig. 3, it is evident that iron dissolution was increased with an increase in leaching temperature, reaching a maximum of 9% at 160 °C while copper dissolution was around 75%. As known, the effect of leaching temperature is important in terms of revealing the leaching kinetics for hydrometallurgical researches. In this regard, the dissolution rates of various metals (Cu, Fe, Zn) in same sample particle have different dissolution kinetics with increasing the leaching temperature. These findings pointed that leaching kinetics might have complicated mechanism.
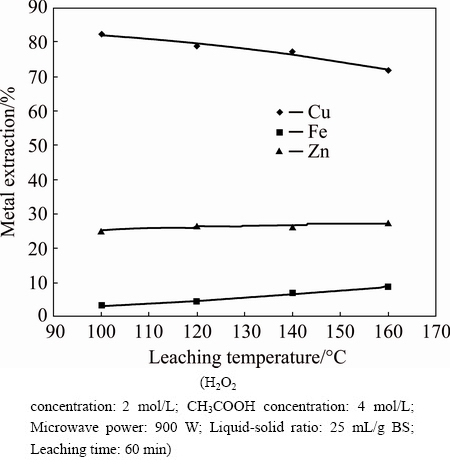
Fig. 3 Effect of leaching temperature on metal extraction
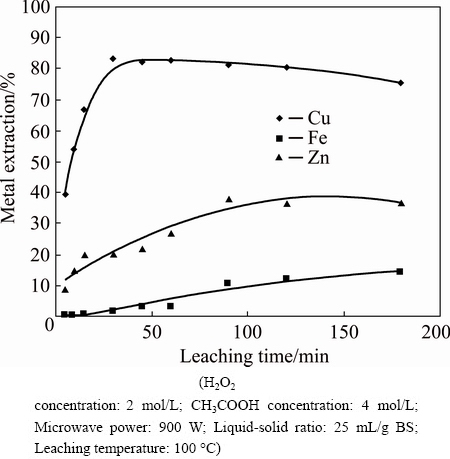
Fig. 4 Effect of leaching time on metal extraction
Figure 4 presents the effect of leaching time. Experiments were carried out in the range of 5-180 min at 100 °C. The results showed that, especially for copper dissolution, metal extraction yields increased with an increase in leaching time, then reached a plateau after 30 min. However, it seems that short leaching time is more appropriate in order to minimize iron dissolution.
The experimental data for various microwave output power levels are shown in Fig. 5. It can be concluded that varying the microwave power did not affect the metal extraction.
The effect of H2O2 concentration on metal extraction was investigated. From Fig. 6, it was observed that H2O2 was necessary for leaching. Only 9% of copper dissolution was achieved in the absence of H2O2; however, copper extraction was around 96% in 4 mol/L H2O2. Further, an increase in the H2O2 peroxide concentration caused a decrease of iron dissolution. This situation may be due to the stabilization of iron oxide under high oxygen potential.
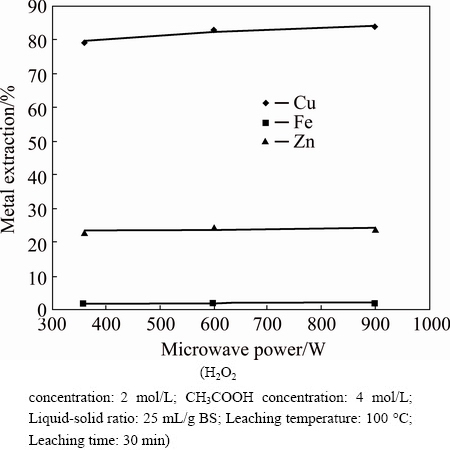
Fig. 5 Effect of microwave power on metal extraction
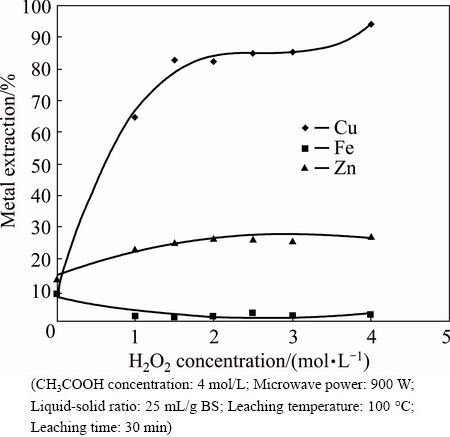
Fig. 6 Effect of H2O2 concentration on metal extraction
The effect of acetic acid concentration on metal extraction was investigated in the range of 0-10 mol/L CH3COOH. The results are shown in Fig. 7. All metal extraction yields increased with an increase in the acetic acid concentration. These results may be attributed to acetic acid having the ability to complex metal ions in the leaching solution according to Eq. (4). Although all of the copper in the BS was extracted at high acetic acid concentration, the selectivity of the leaching process decreased with increasing iron dissolution under these conditions.
The effect of liquid-solid ratios on metal extraction at conditions of 100 °C for 30 min, 4 mol/L H2O2, and 4 mol/L CH3COOH is shown in Fig. 8. Copper extraction of 100% was attained under high liquid-solid ratio conditions. Furthermore, to obtain high copper-rich pregnant solution, the liquid-solid ratio must be kept at lower values in the leaching process.
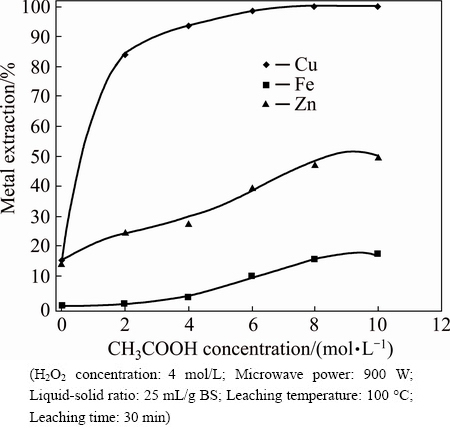
Fig. 7 Effect of acetic acid concentration on metal extraction
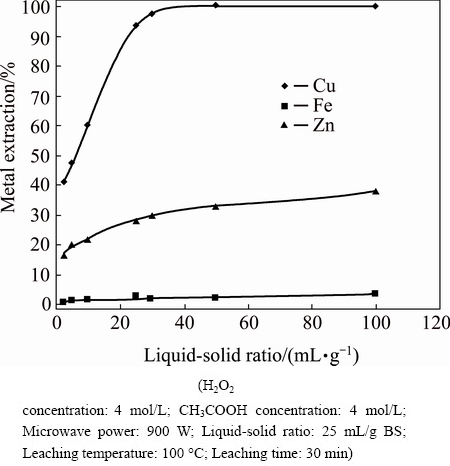
Fig. 8 Effect of liquid-solid ratio on metal extraction
As well known, microwave leaching includes higher temperature, energy transfer (not heat transfer), and different dissolution mechanism in leaching solutions compared with the conventional leaching systems. Several experiments were performed to give some ideas under the atmospheric leaching condition in which a magnetic stirrer was used for various leaching temperature experiments. The results showed that the maximum copper extraction was achieved at 65 °C for 120 min, and 400 r/min of stirring speed. When this result at 65 °C is compared with the results from microwave leaching at 100 °C, it can be said that almost the same extraction yield can be obtained for only 30 min of leaching time, under non-stirring conditions. However, stirring of the leaching solution is very important because it provides for mass transfer to promote the dissolution process. So, obtaining the high copper extraction without stirring is remarkable.
There are the most common two approach kinetic analyses of a solid-liquid multi-phase reaction system. These approaches are referred to as chemical rate- controlling step and diffusion controlling step. For the kinetic analysis in this study, the shrinking core models with chemical reaction and diffusion controlling models were evaluated. The results of kinetic evaluating results indicated that both the kinetic models could illustrate in a similar degree of the leaching mechanism. Therefore, a new variant of the shrinking unreacted core model [34,35], which is based on both the interface transfer and diffusion across of the solid-liquid reaction, was employed to describe the leaching process. The expression can be presented as follows:
(1/3)ln(1-x)+(1-x)-1/3-1=kt (7)
where x is the fraction reacted, k is the apparent rate constant, and t is the reaction time. According to Eq. (7), the plot of kinetic vs time is shown in Fig. 9.
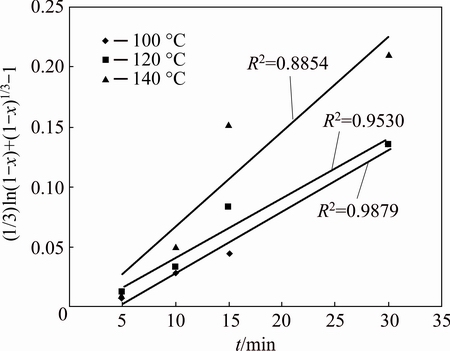
Fig. 9 Plot of (1/3)ln(1-x)+(1-x)-1/3-1 vs reaction time at different temperatures
The relationship between the rate constant k and temperature is given by the Arrhenius equation.
k=Ae-E/(RT) (8)
The apparent rate constants calculated from the slopes in Fig. 9 were used in the Arrhenius plot. The estimated activation energy from the slope was 16.64 kJ/mol. To demonstrate the reaction order with respect hydrogen peroxide, (1/3)ln(1-x)+(1-x)-1/3-1 vs t was plotted (Fig. 10). From Fig. 10, ln k values were calculated, and plotted vs ln[H2O2]. According to the slope of ln k-ln[H2O2], the reaction order is 1.09.
The results of XRD analysis of the leach residue are shown in Fig. 11. It was found that the dominant peaks in the pattern are iron and sulfur compounds, so that while most of the copper was extracted from BS, sulphide sulfur was oxidized to elemental sulfur according to Eq. (3). Because non-stirring condition was current through all experiments, sulfur precipitation on particle’s sundry places may occur that the leaching rate partially affected.
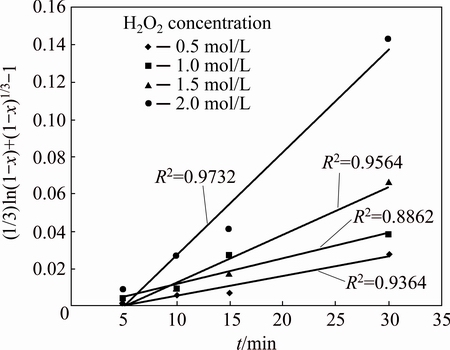
Fig. 10 Plot of (1/3)ln(1-x)+(1-x)-1/3-1 vs time t at different hydrogen peroxide concentration
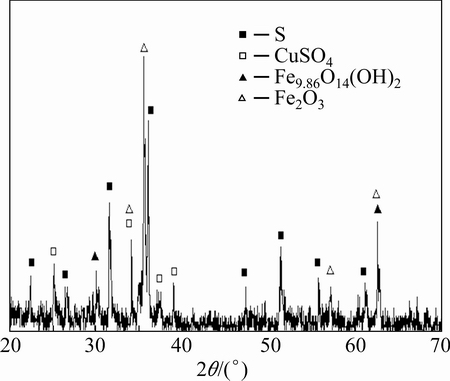
Fig. 11 XRD pattern of leach residue
4 Conclusions
Leaching of copper, iron and zinc from blended slag (BS) with hydrogen peroxide and acetic acid in a microwave was investigated. The effects of various leaching variables on metal extraction were studied. According to the results, the following variables are of significance: leaching time, hydrogen peroxide concentration, acetic acid concentration, and the liquid-solid ratio. It was determined that using both H2O2 and CH3COOH is mandatory for metal extraction from BS. While copper extraction was around only 10% in the absence of H2O2 or CH3COOH, all of the copper was extracted using 4 mol/L CH3COOH and 4 mol/L H2O2. Further, increasing the liquid-solid ratio was determined to have a significant influence on the copper extraction yield. Copper was extracted at 40% and 100% for liquid-solid ratios 5 and 50 mL/g BS, respectively. Nevertheless, it was found that under conditions of a high liquid-solid ratio, a concentrated copper solution cannot be obtained.
Results of microwave leaching and atmospheric leaching experiments were compared. Copper extraction was around 98% under atmospheric conditions at 65 °C for 120 min, and 400 r/min of stirring speed; however, all of the copper was extracted in the microwave oven for 30 min, non-stirring, and at a leaching temperature of 100 °C.
The dissolution kinetics of BS in hydrogen peroxide with acetic acid is controlled by this model equation given as (1/3)ln(1-x)+(1-x)-1/3-1=kt. The apparent activation energy and reaction order were found to be 16.64 kJ/mol and 1.09, respectively.
Finally, considering that the results were obtained in a microwave oven (short leaching time, non stirring), it has been shown that selective leaching is possible with stabilization of iron oxide in the leach residue.
Acknowledgments
The authors wish to express their thanks to metallurgical engineers Isa Savas and A. Vahap Dursun for their help in conducting the experiments.
References
[1] DAVENPORT W G, KING M, SCHLESINGER M, BISWAS A K. Extractive metallurgy of copper [M]. 4th ed. Pergamon, UK. 2002.
[2] ANTONIJEVIC M M, JANKOVIC Z D, DIMITRIJEVIC M D. Kinetics of chalcopyrite dissolution by hydrogen peroxide in sulphuric acid [J]. Hydrometallurgy, 2004, 71: 329-334.
[3]  A V. Effect of ultrasound on the dissolution of copper from copper converter slag by acid leaching [J]. Ultrasonics Sonochemistry, 2007, 14: 790-796.
A V. Effect of ultrasound on the dissolution of copper from copper converter slag by acid leaching [J]. Ultrasonics Sonochemistry, 2007, 14: 790-796.
[4] BOYRAZLI M,  Recovery of metals from copper converter slag by leaching with K2Cr2O7-H2SO4 [J]. Canadian Metallurgical Quarterly, 2006, 45: 145-152.
Recovery of metals from copper converter slag by leaching with K2Cr2O7-H2SO4 [J]. Canadian Metallurgical Quarterly, 2006, 45: 145-152.
[5] HARAHSHEH M, KINGMAN S, BRADSHAW S. Scale up possibilities for microwave leaching of chalcopyrite in ferric sulphate [J]. Mineral Processing, 2006, 80: 198-204.
[6] ZHANG Yang, MAN Rui-lin, NI Wang-dong, WANG Hui. Selective leaching of base metals from copper smelter slag [J]. Hydrometallurgy, 2010, 103: 25-29.
[7] BANZA A N, GOCK E, KONGOLO K. Base metals recovery from copper smelter slag by oxidising leaching and solvent exraction [J]. Hydrometallurgy, 2002, 67: 63-69.
[8] CARRANZA F, ROMERO R, MAZUELOS A, IGLESIAS N, FORCAT O. Biorecovery of copper from converter slags: Slags characterization and exploratory ferric leaching tests [J]. Hydrometallurgy, 2009, 97: 39-45.
[9] BAGHALHA M, PAPANGELAKIS V G, CURLOOK W. Factors affecting the leachability of Ni/Co/Cu slags at high temperature [J]. Hydrometallurgy, 2007, 85: 42-52.
[10] LI Y, PEREDERIY L, PAPANGELAKIS V G. Cleaning of waste smelter slags and recovery of valuable metals by pressure oxidative leaching [J]. Journal of Hazardous Materials, 2008, 152: 607-615.
[11]  F, BAILEY N T. Recovery of metals values from copper smelter slags by roasting with pyrite [J]. Hydrometallurgy, 1990, 25: 317-328.
F, BAILEY N T. Recovery of metals values from copper smelter slags by roasting with pyrite [J]. Hydrometallurgy, 1990, 25: 317-328.
[12] RUDNIK E, BURZYNSKA L, GUMOWSKA W. Hydrometallurgical recovery of copper and cobalt from reduction-roasted copper converter slag [J]. Minerals Engineering, 2009, 22: 88-95.
[13] SATAPATHY A K, DUTTA P, DEY D N, JENA P K. Recovery of copper from converter slag by segregation roasting [C]//Extraction Metallurgy ’85 Symposium by Institution of Mining and Metallurgy in London, 1985: 79-82. ISBN: 0900488824.
[14]  Metal recovery from copper converter slag by roasting with ferric sulphate [J]. Hydrometallurgy, 1997, 44: 261-267.
Metal recovery from copper converter slag by roasting with ferric sulphate [J]. Hydrometallurgy, 1997, 44: 261-267.
[15] ARSLAN A, ARSLAN F. Recovery of copper, cobalt, and zinc from copper smelter and converter slags [J]. Hydrometallurgy, 2002, 67: 1-7.
[16] XIA D K, PICKLES C A. Microwave caustic leaching of electric arc furnace dust [J]. Minerals Engineering, 1999, 13: 79-94.
[17] MA S J, ZHOU X W, SU X J, MO W, YANG J L, LIU P. A new practical method to determine the microwave energy absorption ability of materials [J]. Minerals Engineering, 2009, 22: 1154-1159.
[18] HAQUE K E. Microwave energy for mineral treatment processes—A brief review [J]. International Mineral Processing, 1999, 57: 1-24.
[19] HARAHSHEH M A, KINGMAN S, HANKINS N, SOMERFIELD C, BRADSHAW S, LOUW W. The influence of microwaves on the leaching kinetics of chalcopyrite [J]. Minerals Engineering, 2005, 18: 1259-1268.
[20] ZHAI X, WU Q, FU Y, MA L, FAN C, LI N. Leaching of nickel laterite ore assisted by microwave technique [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 77-81.
[21] JAFARIFAR A, DARYANAVARD M R, SHEIBANI S. Ultra fast microwave-assisted leaching for recovery of platinum from spent catalyst [J]. Hydrometallurgy, 2005, 78: 166-171.
[22] LOVAS M, MUROVA I, MOCKOVCIAKOVA A, ROWSON N, JAKABSKY S. Intensification of magnetic separation and leaching of Cu-ores by microwave radiation [J]. Separation and Purification Technology, 2003, 31: 291-299.
[23] OLUBAMBI P A, POTGIETER J H, HWANG J Y, NDLOVU S. Influence of microwave heating on the processing and dissolution behaviour of low-grade complex sulphide ores [J]. Hydrometallurgy, 2007, 89: 127-135.
[24] SCHMUHL R, SMIT J T, MARSH J H. The influence of microwave pre-treatment of the leach behaviour of disseminated sulphide ore [J]. Hydrometallurgy, 2011, 108: 157-164.
[25] HUANG M, PENG J, YANG J, WANG J. Microwave cavity perturbation technique for measuring the moisture content of sulphide minerals concentrates [J]. Minerals Engineering, 2007, 20: 92-94.
[26] OBUT A. Direct conversion of celestine to SrS by microwave heating [J]. Minerals Engineering, 2007, 20: 1320-1322.
[27] ANTONIJEVIC M M, DIMITRIJEVIC M, JANKOVIC Z. Leaching of pyrite with hydrogen peroxide in sulphuric acid [J]. Hydrometallurgy, 1997, 46: 71-83.
[28] MAHAJAN V, MISRA M, ZHONG K, FUERSTENAU M C. Enhanced leaching of copper from chalcopyrite in hydrogen peroxide-glycol system [J]. Minerals Engineering, 2007, 20: 670-674.
[29] PECINA T, FRANCO T, CASTILLO P, ORRANTIA E. Leaching of a zinc concentrate in H2SO4 solutions containing H2O2 and complexing agents [J]. Minerals Engineering, 2008, 21: 23-30.
[30] NAGIP S, INOUE K. Recovery of lead and zinc from fly ash generated from municipal incineration plants by means of acid and/or alkaline leaching [J]. Hydrometallurgy, 2000, 56: 269-292.
[31]  M. Dissolution kinetics of galena in acetic acid solutions with hydrogen peroxide [J]. Hydrometallurgy, 2007, 89: 189-195.
M. Dissolution kinetics of galena in acetic acid solutions with hydrogen peroxide [J]. Hydrometallurgy, 2007, 89: 189-195.
[32]  B, DEMIR F. Dissolution kinetics of natural magnesite in acetic acid solutions [J]. International Journal of Mineral Processing, 2005, 75: 91-99.
B, DEMIR F. Dissolution kinetics of natural magnesite in acetic acid solutions [J]. International Journal of Mineral Processing, 2005, 75: 91-99.
[33] GHARABAGHI M, NOAPARAST M, IRANNAJAD M. Selective leaching kinetics of low-grade calcareous phosphate ore in acetic acid [J]. Hydrometallurgy, 2009, 95: 341-345.
[34] DICKSON C F, HEAL G R. Solid-liquid diffusion controlled rate equations [J]. Thermochimica Acta, 1999, 340: 89-103.
[35] LIU Z X, YIN Z L, XIONG S F, CHEN Y G, CHEN Q Y. Leaching and kinetic modeling of calcareous bornite in ammonia ammonium sulfate solution with sodium persulfate [J]. Hydrometallurgy, 2014, 144: 86-90.
混合铜渣的微波浸出
M. Deniz TURAN1, Z. Abidin SARI1, Jan D. MILLER2
1. Department of Metallurgical and Materials Engineering, University of Firat, 23279 Elazig, Turkey;
2. Department of Metallurgical Engineering, University of Utah, 84112 Salt Lake City, UT, United States
摘 要:在微波炉中采用双氧水和乙酸溶液浸出由转炉和闪速炉渣组成的混合铜渣。该混合铜渣含51% Fe2O3、3.8% CuO、3.2% Zn。研究表明,对混合渣浸出率影响较大的因素有:浸出时间,液固比,双氧水浓度和乙酸浓度。在最优的浸出条件下:乙酸浓度 4 mol/L,双氧水浓度4 mol/L,微波功率900 W,浸出时间30 min,液固比25 mg/L,浸出温度 100 °C,铜、铁、锌的浸出率分别可达到95%、1.6%和30%。与传统的浸出工艺相比较,微波浸出可缩短浸出时间,同时,可选择性浸出渣中的金属元素。动力学研究表明,渣中金属元素的浸出可用一收缩未反应核模型来描述,浸出反应的表观活化能为16.64 kJ/mol,反应级数为1.09.
关键词:渣;铜;微波;选择性浸出
(Edited by Sai-qian YUAN)
Corresponding author: M. Deniz TURAN; Tel: +90-0424-2370000; Fax: +90-0424-2415526; E-mail: mdturan@firat.edu.tr
DOI: 10.1016/S1003-6326(17)60161-4
Abstract: Leaching of blended slag (BS) was investigated in a microwave oven using hydrogen peroxide and acetic acid. The BS was a mixture of converter and flash furnace slag containing 51% Fe2O3, 3.8% CuO, and 3.2% ZnO. The important variables that influence the metal extraction yield were leaching time, liquid-solid ratio, H2O2 and CH3COOH concentrations. The preferred leaching conditions were as follows: CH3COOH concentration 4 mol/L; H2O2 concentration 4 mol/L; microwave power 900 W; leaching time 30 min; liquid-solid ratio 25 mL/g BS; leaching temperature 100 °C. Under these conditions, the metal extractions of 95% Cu, 1.6% Fe, and 30% Zn were obtained. The results were compared with the traditional leaching results. It is evident that microwave heating causes a reduction in the leaching time. Also, the extraction yield results indicate that selective leaching of BS can be achieved under the preferred conditions. The dissolution kinetic of BS in hydrogen peroxide with acetic acid is controlled by a shrinking unreacted core model equation. The apparent activation energy and reaction order were found to be 16.64 kJ/mol and 1.09, respectively.


