
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1753-1759
Extraction of lithium from lepidolite using chlorination roasting-water leaching process
YAN Qun-xuan, LI Xin-hai, WANG Zhi-xing, WANG Jie-xi, GUO Hua-jun, HU Qi-yang, PENG Wen-jie, WU Xi-fei
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 11 July 2011; accepted 9 January 2012
Abstract:
Chlorination roasting followed by water leaching process was used to extract lithium from lepidolite. The microstructure of the lepidolite and roasted materials were characterized by X-ray diffraction (XRD). Various parameters including chlorination roasting temperature, time, type and amount of chlorinating agents were optimized. The conditional experiments indicate that the best mass ratio of lepidolite to NaCl to CaCl2 is 1:0.6:0.4 during the roasting process. The extraction of lithium reaches peak value of 92.86% at 880 ℃, potassium, rubidium, and cesium 88.49%, 93.60% and 93.01%, respectively. The XRD result indicates that the major phases of the product after roasting lepidolite with mixture of chlorinating agents (CaCl2 and NaCl) are SiO2, CaF2, KCl, CaSiO3, CaAl2Si2O8, NaCl and NaAlSi3O8.
Key words:
lepidolite; lithium; chlorination roasting; water leaching;
1 Introduction
Lithium is the lightest known metal and has been widely utilized for many commercial lithium products due to its fascinating electrochemical reactivity as well as other unique properties. Lithium compounds and minerals have attracted much attention for their applications in ceramics, glass, aluminum, lubrication industries, and pharmaceuticals [1,2]. It was reported that the global consumed amount of lithium related products for batteries has increased by more than 20% per year during the past several years, which shows a great demand for lithium [3].
Most lithium products are currently from brine’s sources in the world. But, in China, most lithium products are still from lithium minerals. Compared with brine, extracting lithium from spodumene, lepidolite, and other solid minerals is not cost effective [4]. Nevertheless, the tremendous growth in demand over the coming century for lithium batteries used in power hybrid and fully electric automobiles has raised great concern about the future availability of lithium. For this reason, the current production levels should be further improved to meet the increasing demand for lithium and lithium salts. Therefore, those solid lithium minerals could be considered sub-economic resources if the price of lithium continues to rise [5,6].
Various methods have been developed to obtain lithium from lithium ores [7-10]. Presently, the most important process for extraction of lithium from lithium alumino-silicates is the lime method [11]. However, it is well known that too many limestone and high energy are consumed. The drawbacks of above process limit it’s further applications. Chloride metallurgy is emerging as an alternative process, which has been proved to be more efficient and cheaper for the extraction and used to refine precious, base, and refractory metals [12-15]. Chlorination process with hydrogen chloride as a reactant gave high yield of lithium [16]. However, such process is complicated and requires highly corrosion- resistant equipment.
To save operating cost and enhance lithium recovery from the ores, herein, an improved chlorination method is introduced. It demonstrates that a considerable product yield for lithium was obtained when a mixture of NaCl and CaCl2 was used as chlorinating agent. Moreover, some determination methods, such as thermo gravimetry (TG), X-ray diffraction (XRD), inductively coupled plasma atomic emission spectroscopy (ICP) and atomic absorption spectrometry (AAS) are employed to study the mechanism.
2 Experimental
The used lepidolite in this study was from Jiangxi province, China. The lepidolite was first crushed in a jaw crusher, then ground in a ball mill and sieved to sizes lower than 150 μm. The chemical composition analysis results of the ore are recorded in Table 1. The X-ray diffraction (XRD) pattern of the raw ore is shown in Fig. 1. The main minerals are lepidolite K(Li,Al)3(Si,Al)4O10(F,OH)2, albite (Na(AlSi3O8)) and quartz (SiO2).
Table 1 Chemical composition of raw ore (mass fraction, %)
![]()
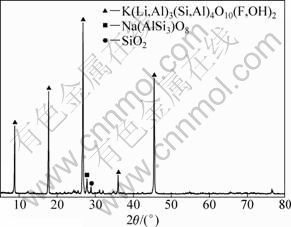
Fig. 1 XRD pattern of lepidolite
The chlorination roasting stage was conducted by placing the lepidolite mixed with calcium chloride and/or sodium chloride (AR grade) in an electrically heated tube furnace at a certain temperature. The chlorination roasted samples were then leached with water at 60 ℃. The ratio of solid-to-liquid was 1:2.5 and leaching time was 30 min. The liquid and solid phases were separated by filtration and the residue was washed thoroughly with distilled water several times. Figure 2 presents the flow sheet of the experimental procedure. The leach residue dried overnight in an oven at 120 ℃. The dried residue was dissolved with a mixed acid solution (V(HF):V(HNO3):V(HClO4)=2:2:1) and heated to near dryness to obtain solid salt, which was then dissolved with HCl (1% in mass fraction). A mass balance was performed for each experiment based on the total content of Li presenting in the feed, the leached solution, the residue and the volatilization of lithium during chlorination roasting. Based on the mass balance, the yield of lithium extraction was calculated based on Eqs. (1), (2) and (3).
L=100Vlρl/(m0c0) (1)
R=100mrcr/(m0c0) (2)
v=100(1-L-R) (3)
where L is the lithium leaching efficiency by water; R is the residual ratio of lithium in leached residue; v is the volatilization ratio of lithium during chlorination roasting; m0 is the mass of lepidolite; c0 is the initial content of lithium in the lepidolite; V1 is the volume of the leach liquor; ρ1 is the concentration of lithium in leach liquor; mr is the mass of leached residue; cr is the content of lithium in leach residue.
For all experiments, atomic absorption spectrometry was used to determine the content of Li, K employing standard procedures. In regard of the other metal elements in leach liquor, inductively coupled plasma atomic emission spectroscopy was employed.
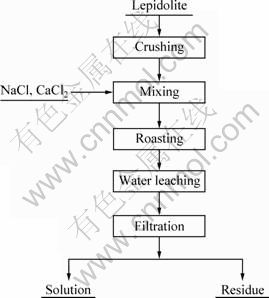
Fig. 2 Flow sheet for chlorination roasting-leaching process
3 Results and discussion
3.1 Effect of chlorination roasting temperature
The lepidolite was mixed with CaCl2 on a mass ratio of 1:1, and then chlorination roasted to form the solid soluble state. DTA-TG curves of the ore mixed with calcium chloride after chlorination roasting at atmospheric pressure are shown in Fig. 3. Three endothermic peaks at about 128 ℃, 697.68 ℃ and 782 ℃ are observed in DTA curve. The endothermic peak at 128 ℃ is due to the release of adsorbed water of calcium chloride, and the corresponding mass loss is 8.37%. The endothermic peaks at 700 ℃ and 782 ℃ are due to the ore starting to react with calcium chloride and the melting point of the calcium chloride, respectively. A sharp mass loss from the sample is observed between 697.68 and 1000 ℃, mainly due to the dehydroxylation in the lepidolite (KLiAlSi3O10(OH)F) and the volatilization of alkaline chloride [17]. TG and DTA curves indicate that chlorination roasting temperature is an important factor in determining the metal leaching efficiency.
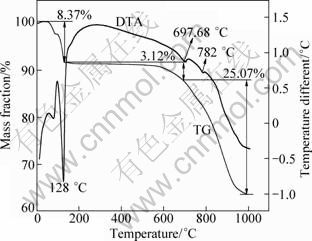
Fig. 3 TG–DTA curves of lepidolite mixed with CaCl2 (m(ore):m(CaCl2)=1:1)
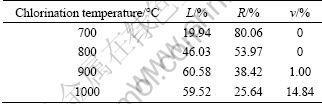
The effect of chlorination roasting temperature from 700 to 1000 ℃ on metal extraction was investigated. The related results are shown in Table 2. It shows that the lithium leaching efficiency increases rapidly from a low value at 700 ℃ to the maximum at 900 ℃. At higher temperatures, the volatilization of lithium chloride increases. Considering that recovery of lithium from leach liquor is more convenient than from tail gas in the industrial production, the balance of the lithium leaching efficiency and the volatilization of lithium during chlorination roasting is very important. It is desired to increase the lithium leaching efficiency and decrease the volatilization of lithium during chlorination roasting. Therefore, further experiments were carried out at 900 ℃.
Table 2 Effect of chlorination temperature on lithium extraction (m(ore):m(CaCl2)=1:1, roasting time of 30 min)
3.2 Effect of chlorination roasting time
Coupled with the mass ratio of lepidolite to calcium sodium of 1, chlorination roasting temperature of 900 ℃, a series of experiments were carried out for 5-90 min to study the effect of chlorination roasting time on metal extractions. Leaching results are shown in Table 3. As observed, the lithium leaching efficiencies at 5 and 30 min were 21.85% and 60.58% respectively. When the chlorination roasting time was over 30 min, there was no significant increasing for the lithium leaching efficiency due to the volatilization of lithium chloride. Therefore, further experiments were carried out for 30 min.
Table 3 Effect of chlorination roasting time on lithium extraction

3.3 Effect of mass ratio of lepidolite to chlorinating agent
Chlorination roasting test of the ore mixed with sodium chloride was investigated from room temperature to 1000℃ under atmospheric pressure using TG-DTA. The results are shown in Fig. 4. Two endothermic peaks are observed at about 773 ℃ and 893 ℃ in DTA curve due to the ore starting to react with sodium chloride and the melting point of the lepidolite, respectively. A sharp mass loss from the sample is observed between 773 ℃ and 935 ℃ mainly due to the dehydroxylation in the lepidolite (KLiAlSi3O10(OH)F) and the volatilization of alkaline chloride [17].
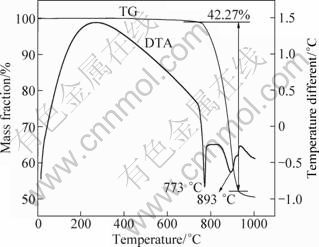
Fig. 4 TG-DTA curves of lepidolite mixed with NaCl (m(ore):m(NaCl)=1:1)
A series of chlorination roasting experiments were conducted at different mass ratios of lepidolite to calcium chloride or sodium chloride with the other parameters as constant. XRD patterns and leaching results of samples are shown in Fig. 5 and Table 4, respectively. When the calcium chloride and lepidolite with the mass ratio of 1:2, the main products contain LiAlSi2O6, SiO2, CaF2, KCl, CaSiO3 and CaAl2Si2O8, and the lithium leaching efficiency is 46.68%. When the mass ratio of calcium chloride and lepidolite increases to 1:1, the LiAlSi2O6 phase disappears and the lithium leaching efficiency is up to 60.58%. When the sodium chloride and lepidolite with the mass ratios of 1:1 and 2:1, the both products consist of NaCl, KCl, NaAlSi3O8 and SiO2, and the corresponding lithium leaching efficiencies is 51.15% and 61.66%, respectively. The results above suggest that CaCl2 is more efficiency than NaCl to extract lithium from lepidolite.
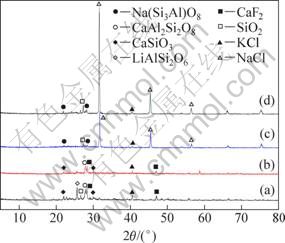
Fig. 5 XRD patterns of chlorination roasted samples leached at 900 ℃ for 30 min: (a) m(ore):m(CaCl2)=1:2; (b) m(ore): m(CaCl2)=1:1; (c) m(ore):m(NaCl)=1:1; (d) m(ore):m(NaCl)= 1:2
Table 4 Effect of mass ratio of lepidolite to chlorinating agent on lithium leaching efficiency
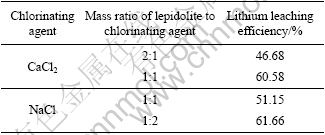
Figure 6 depicts the XRD patterns of leached residues. Compared with chlorination roasting sample, the KCl and NaCl phases disappear while other phases do not change.
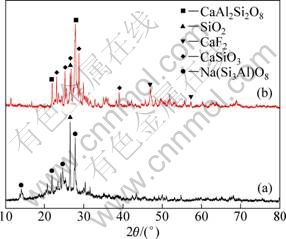
Fig. 6 XRD patterns of leached residues: (a) m(ore):m(NaCl)= 1:1; (b) m(ore):m(CaCl2)=1:1
In this work, a mixed chlorinating agent of NaCl and CaCl2 was used during the chlorination roasting process to optimize the extraction of lithium. The chlorination roasting temperature and time were 900 ℃ and 30 min, respectively. Figure 7 shows the chlorination roasting test of the ore mixed with sodium chloride and calcium chloride using DTA-TG curves. The mixed chlorinating agent started to melt at temperature higher than 500.99 ℃ and a mass loss of about 23.11% was obtained at 900 ℃. The melting temperature of calcium chloride and sodium chloride are 772 ℃ and 801 ℃, respectively. The mixture chlorinating agent melting temperature is 500 ℃ which is lower than either calcium chloride or sodium chloride alone.
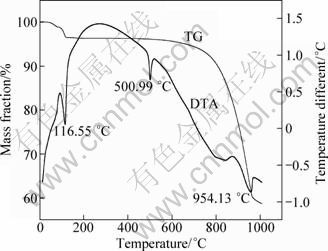
Fig. 7 TG-DTA curves of lepidolite mixed with CaCl2 and NaCl (m(ore):m(NaCl):m(CaCl2)=1:0.5:0.5)
XRD patterns and leaching results of samples are shown in Fig. 8 and Table 5, respectively. It can be observed that the chlorination roasting products are composed of SiO2, CaF2, KCl, CaSiO3, CaAl2Si2O8, NaCl and NaAlSi3O8, and the lithium leaching efficiency increases rapidly with increasing the addition of sodium chloride which is the partial substitution of calcium chloride. When the mass ratio of lepidolite, sodium chloride and calcium chloride is 1:0.4:0.6, the lithium leaching efficiency reaches 89.19%. When the lepidolite, sodium chloride and calcium chloride are in the mass ratios of 1:0.4:0.6, 1:0.5:0.5 and 1:0.6:0.4, lithium leaching efficiency keeps almost a steady value. However, the leaching efficiency decreases rapidly with increasing the mass ratio of sodium chloride further. Because the cost of calcium chloride is higher than that of sodium chloride in the market, the mass ratio of 1:0.6:0.4 of lepidolite, sodium chloride and calcium chloride should be the best one in the industrial production.
For the calcium chloride and/or sodium chloride roasts with lepidolite, the reactions for lithium extraction may be proposed as follows (R=Li, K, Rb, Cs):
![]() (4)
(4)
![]() (5)
(5)
![]() (6)
(6)
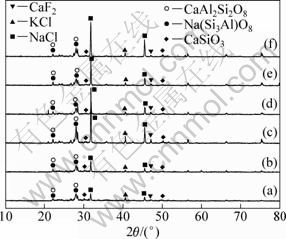
Fig. 8 XRD patterns of chlorination roasted samples of lepidolite, sodium chloride and calcium chloride leached at 900 ℃ for 30 min: (a) m(ore):m(NaCl):m(CaCl2)=1:0.2:0.8; (b) m(ore):m(NaCl):m(CaCl2)=1:0.3:0.7; (c) m(ore):m(NaCl): m(CaCl2)=1:0.4:0.6; (d) m(ore):m(NaCl):m(CaCl2)=1:0.5:0.5; (e) m(ore):m(NaCl):m(CaCl2)=1:0.6:0.4; (f) m(ore):m(NaCl): m(CaCl2)= 1:0.8:0.2
Table 5 Effect of mass ratio of lepidolite to chlorinating agent on lithium leaching efficiency
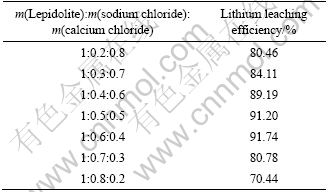
The contents of SiO2 and Al2O3 in initial lepidolite are 50.78% and 26.93%, respectively. So, the molar ratio of SiO2 to Al2O3 in lepidolite is 3.4. The chemical stoichiometric ratios of SiO2 and Al2O3 of Eqs. (4)-(6) indicate that calcium chloride is more efficiency than sodium chloride to decompose lepidolite.
Apparently, chlorination roasting with a mixture of calcium chloride and sodium chloride gave a better lithium extraction yield than either chlorinating agent alone. The melting point of the mixture of calcium chloride and sodium chloride is lower than either chlorinating agent alone, which increases the fluidity of chloride melt and decreases the viscosity of liquid phase [18]. This allows a sufficiently diffusion of chlorinating agent to surface of lepidolite. So, the mixture of calcium chloride and sodium chloride could facilitate the lithium extraction.
In order to optimize the chlorination roasting temperature and time conditions for lithium leaching efficiency, a series of experiments were performed by maintaining lepidolite, sodium chloride, calcium chloride in the mass ratio of 1:0.6:0.4. The lithium leaching results of samples are shown in Fig. 9. It shows increasing trend when the chlorination roasting time is increased from 15 to 30 min. However, the lithium leaching efficiency decreases when the chlorination roasting time is up to 35 min. The chlorination roasting temperature also affects the lithium leaching efficiency significantly. The lithium leaching efficiency increases with increasing the chlorination roasting temperature but drops beyond 880 ℃. It should be noted that a maximum lithium leaching efficiency of 92.09% is obtained at 880 ℃ in about 30 min. The residual ratio of lithium in leached residue and the volatilization ratio of lithium during chlorination roasting are shown in Fig. 10. Too long chlorination roasting time and higher chlorination roasting temperature are unfavourable for the lithium leaching efficiency due to volatilization of lithium chloride.
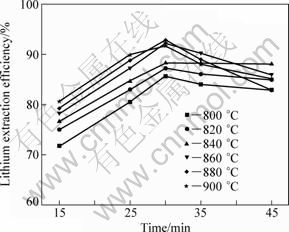
Fig. 9 Effect of chlorination roasting temperature and time on lithium leaching efficiency
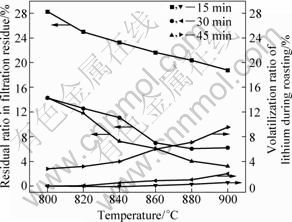
Fig. 10 Effect of chlorination roasting temperature and time on residual ratio of lithium in leached residue and volatilization ratio of lithium during chlorination roasting
3.4 Effect of leaching time and temperature
Maintaining the mass ratio of lepidolite to NaCl to CaCl2 at 1:0.6:0.4, the chlorination roasting time of 30 min, chlorination roasting temperature of 880 ℃, solid-to-liquid ratio of 1:2.5, the effect of leaching time and temperature on lithium leaching efficiency were investigated. The results are shown in Fig. 11. It can be noted that the lithium leaching efficiency increases from 10 to 50 min at 30 ℃. At 60 and 90 ℃, the lithium leaching efficiency increases from 10 to 30 min, and reaches a maximum at 30 min. With further increasing leaching time, lithium leaching efficiency almost keeps a steady value.
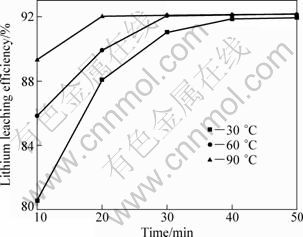
Fig. 11 Effect of leaching time and temperature on lithium leaching efficiency
3.5 Effect of solid-to-liquid ratio in leaching process
The effect of solid-to-liquid ratio ranging from 0.2 to 1 on the leaching efficiency was performed, by maintaining mass ratio of lepidolite to NaCl to CaCl2 at 1:0.6:0.4, chlorination roasting time 30 min, chlorination roasting temperature 880 ℃, leaching time 30 min and leaching temperature 60 ℃. Figure 12 shows the effect of solid-to-liquid ratio in the leaching process. When the S/L ratios are 1:5 and 1:2.5, lithium extraction efficiencies reaches 92.15% and 92.09%, respectively. The lithium extraction efficiency shows a slight decrease when the S/L ratio further increases. The S/L ratio of 1:2.5 is the most favourable economical situation.
3.6 Composition of leaching solution
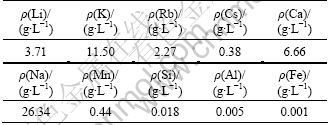
Table 6 lists the chemical composition of the leaching solution under the optimum condition: chlorination roasting time 30 min, chlorination roasting temperature 880 ℃ , mass ratio of lepidolite to NaCl to CaCl2 1:0.6:0.4, solid-to-liquid ratio 1:2.5, leaching temperature and time 60 ℃ and 30 min. The concentrations of major elements Li, K, Rb, Cs, Ca and Na are 3.71, 11.50, 0.38, 6.66 and 26.34 g/L, respectively. It is well known that the potassium, sodium, rubidium, and cesium are very important by-products. The corresponding leaching efficiencies here are 88.49%, 55.80%, 93.60% and 93.01%, respectively (The Na mass balance was based on the total content of Na present in the ore, addition of NaCl and leaching solution). Na can be recycled as NaCl in the chlorination roasting process. There are a few impurity elements of Al and Si in leaching liquor, which is quite helpful for the purification of lithium, and is more realistic for large-scale application. The concentration of Ca is a little high.
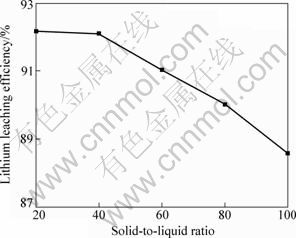
Fig. 12 Effect of solid-to-liquid ratio in leaching process
Table 6 Chemical composition of leaching solution
The extraction of Li from the solution can be proceed by any known method, notably by the addition of lime water, which will remove any aluminum and any heavy metals by precipitation, followed by the addition of Na2CO3 to remove the remaining lime, then by filtration and concentration of the filtrate. From this solution the addition of alkali carbonate precipitates lithium carbonate [19].
4 Conclusions
An improved process including the chlorination roasting followed by water leaching was employed to extract lithium from lepidolite. The results indicate that chlorination roasting temperature, time and mass ratio of chlorinating agent (CaCl2 and NaCl) affects the lithium leaching efficiency significantly. The lithium leaching efficiency of 92.86% could be reached according to owing optimized process: chlorination roasting time 30 min, chlorination roasting temperature 880 ℃, and mass ratio of lepidolite to NaCl to CaCl2 1:0.6:0.4. There are a few impurity elements of Al and Si in leaching liquor, which is quite helpful for the purification of lithium, and more realistic for full scale application.
References
[1] EBENSPERGER A, MAXWELL P, MOSCOSO C. The lithium industry: Its recent evolution and future prospects [J]. Resources Policy, 2005, 30(3): 218-231.
[2] FERRO R, PIPOLI R. The future of lithium. Mining intelligence series [EB/OL]. http://www.salareslithium.com/i/pdf/Media/Lithium_ 2010_BNA.pdf.
[3] WANG H, HUANG K, LIU S. Electrochemical property of NH4V3O8·0.2H2O flakes prepared by surfactant assisted hydrothermal method [J]. Journal of Power Sources, 2011, 196(2): 788-792.
[4] HRLVACI C, MORDOGAN C H, COLAK M. Presence and distribution of lithium in borate deposits and some recent lake waters of west-central turkey [J]. International Geology Review, 2004, 46(2): 177-190.
[5] ROCA A, CRUELLS M. Study of chloridizing volatilization roasting of cinnabar as a basis for a process to obtain mercuric and mercurous chlorides [J]. Metallurgical and Materials Transactions B, 1990, 21(2): 259-268.
[6] JANDOVA J, DVORAK H N. Processing of zinnwaldite waste to obtain Li2CO3 [J]. Hydrometallurgy, 2010, 103(1-4): 12-18.
[7] WANG Jian-ling, WANG Ji-min, ZHU Jian-chun, ZHOU Jin-yun, GU Heng. Extracting lithium carbonate by sulphate process from lepidolite [J]. Journal of Guangdong Non-ferrous Metals of China, 1994, 4(2): 107-112. (in Chinese)
[8] B?Y?KBUR? A, MASLIOGLU D, UGUR BILICI M S, K?KSAL G. Extraction of lithium from boron clays by using natural and waste materials and statistical modelling to achieve cost reduction [J]. Minerals Engineering, 2006, 19(5): 515-517.
[9] HAUS B F R. New concepts for lithium minerals processing[J]. Minerals Engineering, 2010, 23(8): 659-661.
[10] SUN You-run. Approach to improve the recovery of Li2O by Li mica-limestone sintering process [J]. Rare Metals and Cemented Carbides of China, 2000(143): 22-26. (in Chinese)
[11] LIN Gao-kui. Improvement on sintering of Jiangxi Li-mica and Limestone [J]. Rare Metals and Cemented Carbides of China, 1999(137): 46-48. (in Chinese)
[12] OJEDA M W, PERINO E, RUIZ M D C. Gold extraction by chlorination using a pyrometallurgical process [J]. Minerals Engineering, 2009, 22(4): 409-411.
[13] JENA P K, BARBOSA F, VASCONCELOS I C. Studies on the kinetics of slurry chlorination of a sphalerite concentrate by chlorine gas [J]. Hydrometallurgy, 1999, 52(2): 111-122.
[14] BROCCHI E A, MOURA F J. Chlorination methods applied to recover refractory metals from tin slags [J]. Minerals Engineering, 2008, 21(2): 150-156.
[15] HU Tian-xi, YU Jian-guo. Experimental study on decomposition of K-feldspar with CaCl2 and NaCl for extraction of potassium [J]. The Chinese Journal of Process Engineering, 2010, 10(4): 701-705. (in Chinese)
[16] L?F G O G, LEWIS W K. Lithium chloride from lepidolite [J]. Industrial & Engineering Chemistry, 1942, 34(2): 209-216.
[17] KOMETANI T Y. Effect of temperature on volatilization of alkali salts during dry ashing of tetrafluoroethylene fluorocarbon resin [J]. Analytical Chemistry, 1966, 38(11): 1596-1598.
[18] FRANK H, JANET H. The potter's dictionary of materials and techniques [M]. 15th ed. Philadelphia: University of Pennsylvania Press, 2004.
[19] BOTTEN R P, DELGRANNGE J P, VESINET L, STEINMETZ A. Method of recovering lithium from lepidolite. US Patent, 3189407 [P]. 1965.
氯化焙烧-水浸法从锂云母矿提锂
颜群轩,李新海,王志兴,王接喜,郭华军,胡启扬,彭文杰,伍习飞
中南大学 冶金科学与工程学院,长沙 410083
摘 要:采用氯化焙烧-水浸法处理锂云母矿,并对氯化处理温度、时间、氯化剂的类型及用量进行研究。条件优化实验表明,在锂云母、氯化钠、氯化钠的质量比为1:0.6:0.4,氯化处理温度为880 ℃,氯化处理时间为30 min时,锂的提取率可达92.86%,钾、铷、铯的提取率分别为88.49%、93.60%和93.01%,。 采用XRD对锂云母原矿及焙烧后物料的物相进行分析。XRD结果分析表明,当将锂云母和混合氯化剂一起焙烧(氯化钙及氯化钠)时,所得物相为SiO2、CaF2、KCl、CaSiO3、CaAl2Si2O8、NaCl和NaAlSi3O8。
关键词:锂云母;锂;氯化焙烧;水浸
(Edited by YANG Hua)
Corresponding author: LI Xin-hai; Tel: +86-731-88836633; E-mail: xhli@mail.csu.edu.com
DOI: 10.1016/S1003-6326(11)61383-6
Abstract: Chlorination roasting followed by water leaching process was used to extract lithium from lepidolite. The microstructure of the lepidolite and roasted materials were characterized by X-ray diffraction (XRD). Various parameters including chlorination roasting temperature, time, type and amount of chlorinating agents were optimized. The conditional experiments indicate that the best mass ratio of lepidolite to NaCl to CaCl2 is 1:0.6:0.4 during the roasting process. The extraction of lithium reaches peak value of 92.86% at 880 ℃, potassium, rubidium, and cesium 88.49%, 93.60% and 93.01%, respectively. The XRD result indicates that the major phases of the product after roasting lepidolite with mixture of chlorinating agents (CaCl2 and NaCl) are SiO2, CaF2, KCl, CaSiO3, CaAl2Si2O8, NaCl and NaAlSi3O8.


