Trans. Nonferrous Met. Soc. China 28(2018) 1377-1385
Dielectric characteristics and nonlinear properties of ZnO-polypyrrole composites
Fariba TABRIZI1, Mojtaba PARHIZKAR1, Hassan BIDADI1, Mohammad GHAFOURI2,3
1. Faculty of Physics, University of Tabriz, Tabriz, Iran;
2. Department of Basic Science, Shabestar Branch, Islamic Azad University, Shabestar, Iran;
3. Faculty of Physics, Islamic Azad University of Shabestar, Shabestar, Iran
Received 27 May 2017; accepted 28 September 2017
Abstract:
The nonlinear properties and frequency characteristics of ZnO-polypyrrole composites were investigated at 200 Hz-5 MHz frequency interval with different zinc oxide contents. Samples were prepared using hot press method at 130 °C. Results show an optimum point for breakdown voltage at ZnO content of 70%. Breakdown voltage decreases from 590 to 380 V and after that tends to increase from 450 to 740 V due to the absence of polypyrrole at grain boundaries. No matter how breakdown voltage behaves, nonlinear coefficient increases from 4.2 to 9 by increasing ZnO content because of the increase in acceptor-like states at grain boundaries by increasing ZnO content. The electrical parameters such as dielectric constant, dielectric loss and series resistance of samples show a strong dependence on frequency especially below 1 kHz. These parameters fall off by increasing frequency up to 1 kHz, which is related to charge transportation through the Schottky barrier at grain boundaries. The high dielectric constant of samples below 1kHz is related to the Maxwell-Wagner polarization at grain boundaries. The presence of different anomalies at different frequency intervals is related to interfacial polarization because of different structures of grains and intergranular layer with a huge difference in conductivity.
Key words:
dielectric characteristics; electrophysical properties; composite varistor; frequency dependence;
1 Introduction
ZnO-based ceramics with a few metal oxide additives such as Bi2O3, CoO, MnO and TiO2, are known for their high nonlinear properties against surges and over voltages [1]. This is because: 1) they can do protection several times without destruction, and 2) they are highly capable of absorbing surge energy [2-4]. The nonlinearity of a varistor is related to its microstructure and formation of potential barriers (Schottky barriers) at grain boundaries (GBs) [5-11]. The presence of additives increases the density of trap states at the GBs which could help to block charge carriers movements and results in producing Schottky barrier [12-15].
Although ZnO-based varistors have lots of advantages, there are some disadvantages for them such as: 1) complicate microstructure, 2) high concentration of additives, 3) low permittivity (it is not suitable to make low-voltage varistors), and 4) low stability against degradation [16-20]. These issues caused that scientists look for other materials such as SnO2. But, another common difficulty of ceramic varistors is their high sintering temperature and heat treatment (950-1150 °C). One solution to these kinds of problems is changing primary materials. This stems from the fact that the I-V (I is the electric current, V is the applied voltage) characteristics of a varistor depend on its initial mixture. To this end, composite varistors have also been developed recently. Simple microstructure, low breakdown voltage possibilities (~40 V), low preparation temperature (130 °C), and good nonlinear coefficient (~15), make composite varistors an acceptable candidate for a new generation of technologies [21-25].
In general, a composite varistor is made of an inorganic material (e.g., Si), and an organic one (e.g., polyaniline). Although conduction mechanism and microstructure of composite varistors are completely different from ceramic ones, intergranular layer plays an important role in both of them [21]. In composite varistors, the intergranular layer is a mixture of conducting polymer and some thermoplastic polymers.
The conducting polymer is responsible for nonlinear behavior because it acts as a bridge between grains and helps charge carriers to tunnel between grains [21,25].
It should be noted that the protecting devices are used not only in DC circuits, but also in alternating circuits. So, dielectric properties of compounds as a function of frequency, are not only important in designing electronic circuits, but also in protecting devices. To study the frequency dependence and microstructure of varistors in more details, it is better to study their electronic equivalent circuit. The equivalent circuit of a varistor is composed of several resistors and capacitors, and because of the frequency dependence of capacitive reactance (Xc=1/(cω), Xc is the copacitive reactance, c is the capacitance, ω is the angular frequency), by applying alternative electric fields with different frequencies, a varistor may show different behaviors that could affect the microstructure and electrophysical properties of the varistor [26]. So, the investigation of frequency dependence of a varistor is very important. The response of a material to an applied alternative electric field is determined by the complex dielectric constant ε which is given by
ε=ε′-iε″ (1)
where ε′ is the real part and ε″ is imaginary part of dielectric constant. Three sources have been suggested for dielectric constant: 1) electronic dielectric constant which is related to the displacement of charge distribution, by displacing charge distribution with respect to the nuclei, electronic polarization occurs; 2) ionic dielectric constant which is related to the ions displacement that is accompanied with charge distribution; 3) dipole or orientation polarization which is related to the existence of electric dipoles. Electronic polarization occurs in all atoms and ions and all kinds of dielectrics. One of the important specifics of this kind of polarization is that when an external electric field is applied, the polarization occurs during a very small period of time (~10-15 s, the ultraviolet region). On the other side, the ionic polarization requires a longer period with respect to electronic polarization (10-13-10-12 s). Finally, the dipole polarization must be understood as the introduction of an electric field. The important point about this kind of polarization is that it could dissipate electric energy, i.e., transforming it into heat in dielectrics.
Some materials and compounds also show a high dielectric constant. The presence of a high dielectric constant is due to the interfacial or Maxwell-Wagner polarization at GBs [27-29]. The interfacial polarization arises especially if a system is composed of two different phases differing from each other in dielectric constant and conductivity [30]. This polarization could have three sources: spatial inhomogeneity, contact effect, and internal barrier layer capacitor [27,28,31]. The latter could be the main factor because of the presence of defects and inhomogeneity in the microstructure of a varistor and could cause the dielectric constant to fall off by increasing frequency and showing Debye-like relaxation property [28,31-33].
In this paper, the results concerning functional and frequency dependence of dielectric parameters of ZnO- polypyrrole (PPY)-high density polyethylene (HDPE) composites as well as their nonlinear I-V characteristics are presented. It should be mentioned that although there are many published articles with regarded to dielectric properties of the materials (ZnO, HDPE, PPY) used individually (single phase), it hasn’t been reported data concerning their mixed (composite) phases in Refs. [28,34,35].
2 Experimental
2.1 Initial materials
The first step for manufacturing a sample is the preparation of its initial mixture: zinc oxide (ZnO, Merck Chemicals), polypyrrole (PPY, Merck Chemicals) and high-density polyethylene (HDPE, Tabriz Petrochemical Company, Iran). To prepare ZnO powder, it was sintered at 700 °C for 2 h and ground for 1 h. Due to the non-stoichiometric characteristic of ZnO, heat treatment is necessary to improve its electrical and structural properties [36,37]. After that, the powder was screened with a sieve (US sieve size 200) to hand in ZnO particles less than 74 μm. PPY was synthesized by chemical oxidative polymerization [38]. To do this, 1.35 g/mL FeCl3 of distilled pyrrole was dissolved in 50 mL of distilled water and stirred for 30 min. The synthesis was performed at 5-7 °C. Then, distilled pyrrole was added dropwise into the solution. The precipitated PPY was collected by filtration after 3 h and dried at 70 °C for 24 h. The final PPY is in doped form and has high electric conductivity. So, it was de-doped by dehydrogenating it. To do this, PPY was mixed in 1 mol/L ammonia solution for 2 h and dried at 80 °C for 24 h. In order to have a homogenous powder, it was ground a few times during the drying process. Finally, it was screened similarly to ZnO powder. The particle size of the applied HDPE in this mixture was less than 74 μm too.
2.2 Preparation of discs
Specified amount of ZnO-PPY-HDPE was mixed using magnetic balls for 4 h according to Table 1. Due to the average molar mass of polymers, initial substances were picked according to their mass fractions. All samples were prepared by hot press at 130 °C. At this temperature, HDPE is pasty and covers all other particles and increases the mechanical stability of the sample. At this temperature, HDPE is aqueous and effuses outside of the die. The applied pressure was 60 MPa. All discs had 10 mm in diameter and (140±10) μm in thickness. Samples were also checked by optical microscope for their quality confirmation before measurements.
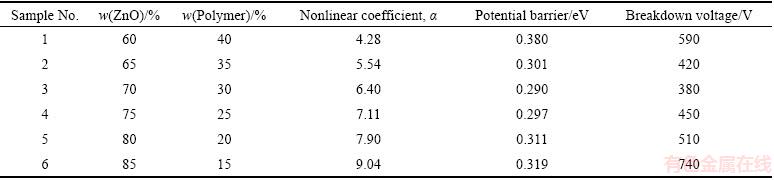
Table 1 Summarized information about samples
2.3 Measurements
Two copper electrodes with 6 mm in diameter were used in order to measure the I-V characteristics of samples. The accuracy of ammeter was 10 nA. The frequency dependence of dielectric parameters of samples including relative dielectric constant and series resistance was investigated by using KC-605 LCR meter in the range of 200 Hz-5 MHz. It was connected to a computer using rs232 cable. The scanning electron microscopy (SEM) micrographs (MIRA3 TESCAN) were used in order to study the microstructure of the samples.
3 Results and discussion
3.1 I-V characteristics
The I-V characteristics of samples were shown in Fig. 1. As this figure shows, by increasing ZnO content in the mixture up to 70%, the breakdown voltage reduces and leakage current increases. By increasing ZnO content beyond 70%, the process is reversed, i.e., breakdown voltage increases and leakage current decreases. It is clear that there is a minimum value for breakdown voltage (proportional to the potential barrier height in Fig. 2). This behavior is related to the microstructure of the varistor composed of several micro-varistors.
Grain-intergranular-grain structure defines a micro- varistor and the number of these micro-varistors between two electrodes defines total breakdown voltage according to the equation Vb=VGB/d. In this equation, Vb is the breakdown field, VGB is the barrier voltage and d is the grain size of samples. In general, two mechanisms may influence the breakdown voltage in a micro-varistor: spacing between the grains, and the presence of PPY and its effect on tunneling [25].
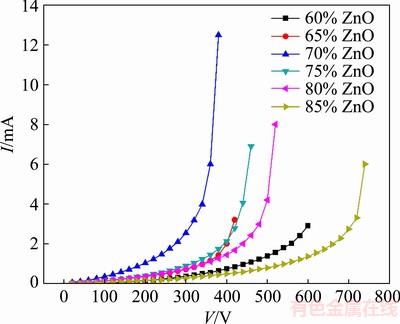
Fig. 1 I-V characteristics of samples with different ZnO contents
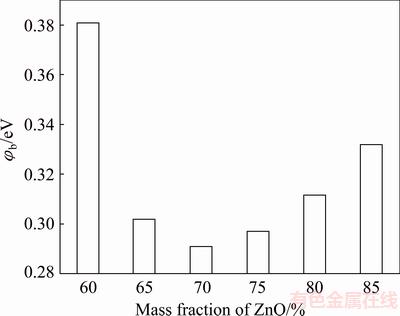
Fig. 2 Variation of potential barrier height φb (proportional to breakdown voltage) vs ZnO content in mixture
As it is known, at a given voltage, tunneling will not start until the spacing between grains reaches the required distance. When this is done, tunneling effect will begin. This is achieved by increasing ZnO content in the mixture. So, increasing ZnO content is equivalent to reducing the space between grains as well as reducing breakdown voltage or potential barrier height (as it will be calculated later, Table 1). But, most importantly, the presence of PPY particles between grains completely affects the tunneling as well. Although increasing ZnO content makes grains closer to each other, it is equivalent to decreasing PPY content at GBs [25]. Decreasing PPY content (which is equal to increase the effective spacing between grains) is more effective than that of increasing the ZnO content and results in higher breakdown voltage and potential barrier height (Table 1). So, increasing ZnO content more than 70% increases breakdown voltage. All of these explanations are recognizable in SEM images (Fig. 3). According to this figure, at low ZnO content, the polymer matrix is completely clear and recognizable (Figs. 3(a-c)). Nevertheless, by increasing ZnO content, the lack of polymer content is clear and the space between ZnO particles is empty and recognizable (Figs. 3(d-f)).
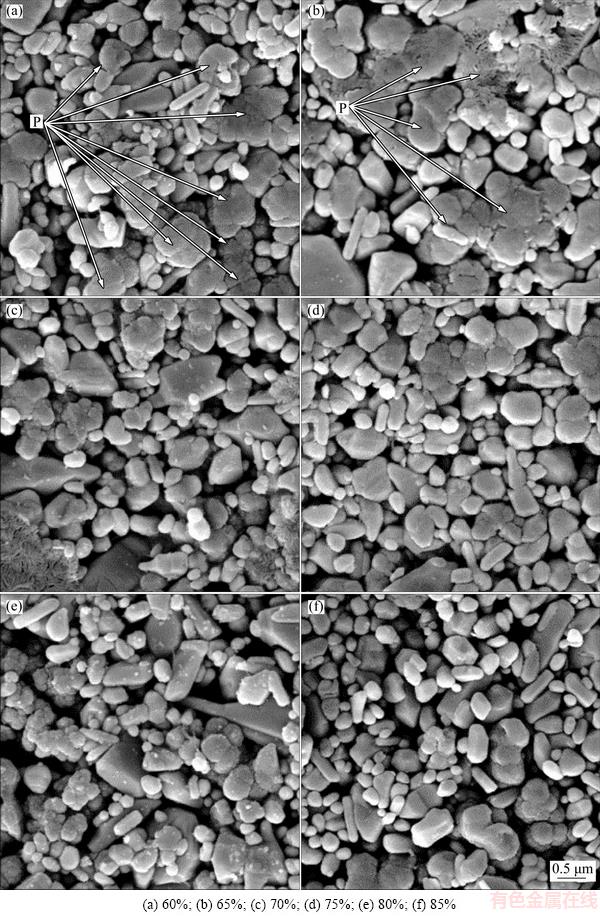
Fig. 3 SEM images of samples with different ZnO contents (P: Polymer)
The electrical conduction has two sources: electrical field (Pool-Frenkel emission) and temperature (Schottky emission). In Schottky type emission, an electron could tunnel through potential barrier height which is formed at the GBs with the cooperation of electric field and temperature. This results in the changes in energy band close to the interface. The related equation is as follows [39]:
I=AT2exp[(βV1/2-φb)/(KBT)] (2)
where I is the electric current, V is the electric potential difference, φb is the potential barrier height, KB is the Boltzmann constant; T is the temperature; A and β are constants.
By plotting ln I-V1/2 curve (Fig. 4) and calculating interception of the curve at a constant temperature, the potential barrier height will be calculated. The results are summarized in Table 1 which shows that the potential barrier height is completely in conformity with breakdown voltage.
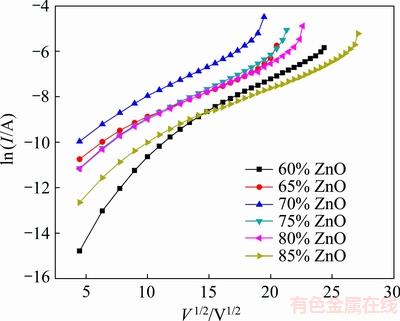
Fig. 4 ln I-V1/2 curves to calculate potential barrier height using Piannaro’s model
3.2 Nonlinearity
The I-V characteristic of a varistor is given by
I=KVα (3)
where V is the applied voltage, I is the electric current flowing through the sample, K is a constant and α is nonlinear coefficient which is the most important parameter indicating the quality of a varistor (α=1 for Ohm law and α=∞ for an ideal varistor). By plotting ln I-ln V curve, the slope of the curve will give α in the breakdown region (Fig. 5).
The obtained results are summarized in Table 1. As this table shows, no matter how breakdown voltage behaves, nonlinear coefficient increases by increasing ZnO content in the mixture. As it was mentioned before, the origin of nonlinear behavior is the presence of PPY at GBs which is related to the interaction between grains and GBs. Two conductive grains and a thin insulator layer between them create a potential barrier called Schottky barrier. According to the conduction mechanism on varistors, there are different models, e.g., inter-granular layer, additives segregation and disordered thin layer that could successfully describe this barrier. The origin of this barrier is based on the existence of acceptor-like states at grain boundaries. These states trap electrons and applying an electric field makes electrons tunnel between grains. So, the more the ZnO content is, the more the trapped electrons are, resulting in higher nonlinear coefficient [2,40-42].
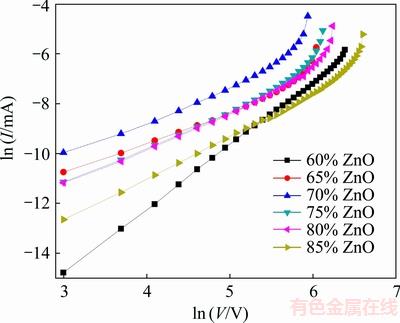
Fig. 5 ln I-ln V curves to calculate nonlinear coefficient of samples
The presence of PPY and HDPE supports this effect because they increase trapped electrons at GBs. For composite varistors, the formation of the Schottky barrier is as follows. PPY in its de-doped form has high resistivity. But, it could be easily doped by partial reduction or oxidation with electrons. By joining inter-granular layer (i.e., PPY) to ZnO grains which are n-type semiconductors, the acceptor states at the boundary could trap electrons provided by ZnO which results in the formation of the potential or Schottky barrier. To study this, an experiment was done. At first, the I-V characteristics of both HDPE and PPY were considered in a single phase. They both exhibited linear property which is shown in the inset of Fig. 6. But mixing them with ZnO gave rise to completely different results. ZnO+HDPE still exhibited linear behavior, while ZnO+PPY exhibited nonlinear property but at relatively high leakage current.
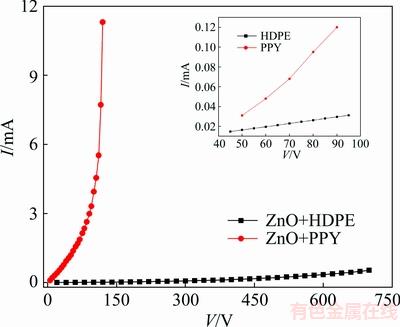
Fig. 6 I-V characteristics of HDPE and PPY in single phase (inset) and mixed with ZnO
The origin of these different behaviors is concealed inside the difference between chemical bonds of HDPE and PPY. Chemical bonds of HDPE are strong sigma bonds and mixing it with other materials does not change its properties. But PPY is one of the organic polymers and its well-known property is that it could be partially oxidized with an electron acceptor (p-doped) or reduced with electron donors (n-doped) by dopants [43]. Here, the dopant is n-type ZnO. So, combining PPY with ZnO increases its conductivity and this process is responsible for the nonlinear behavior of the mixture. Because of high leakage current in this mixture, some insulators such as HDPE (a thermoplastic polymer) are required to reduce leakage current and increase mechanical stability.
3.3 Frequency dependent characteristics
3.3.1 Relative dielectric constant-frequency (k-f) characteristic
The relative dielectric constant (RDC)-frequency characteristics (equivalently c-f, c=kεoA/d, εo is the vacuum permitivity, A is the area of the plates, d is the distance between the two plates) of samples are shown in Fig. 7. Three points are considerable in this figure.
1) Changing RDC by changing frequency. By increasing frequency, the RDC (or capacitance) of the samples decreases. Actually, this curve may be divided into two main regions: below and beyond 1 kHz. The high RDC below 1 kHz is due to the charge transportation through the double Schottky barrier which exists at GBs [35]. As it was discussed earlier, this is known as the Maxwell-Wagner effect and related to high series barrier capacitance at GBs [44]. The Maxwell-Wagner effect is a result of charge accumulation at the interface of two materials with different charge carrier relaxation time. Charge accumulation itself is a result of current flow through interface. These kinds of charges could be effective traps for the injected electrons from ZnO grains under high electric potential. This results in the increase in electrical resistivity and dielectric life time [45]. On the other hand, the basic idea is just so simple: the conductivity of the sample is reduced by a thin insulating layer at GBs which blocks charge transportation between two grains and enhances the static dielectric constant.
2) Changing RDC by changing ZnO content. As shown in Fig. 7, by increasing ZnO content in the mixture, RDC is reduced. This is explained by the fact that at low ZnO content, it can be considered that the sample is composed of three phases which affect the RDC of the sample. Several formulas have been suggested for calculating static RDC of a mixture which has been derived from the basis of both theoretical and experimental data. Among them, Lichtenecker-Rother or logarithmic law of mixing formula is considerable [46].
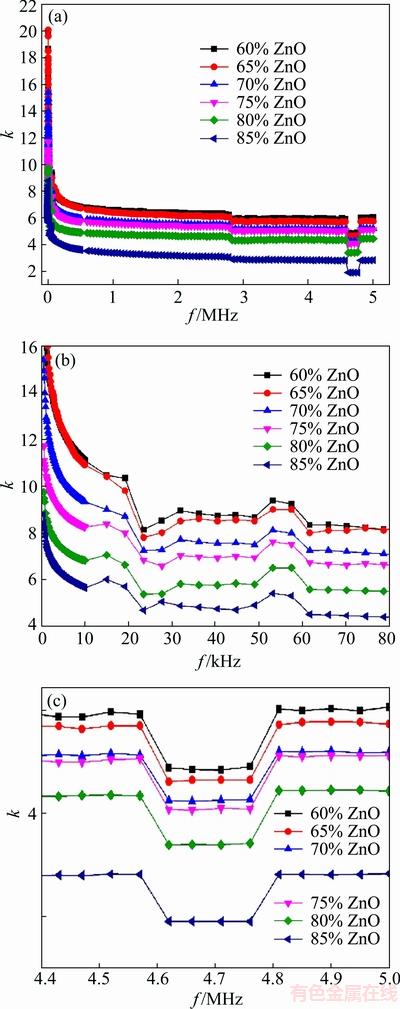
Fig. 7 Total behavior of RDC with frequency in general (a) and exact form (b, c)
According to this law, RDC of a mixture is a logarithmic summation of the three RDCs related to three phases of ZnO, PPY and HDPE. But, by increasing ZnO content, the sample relatively tends to single phase state and the total RDC will be less than that of the sample with low ZnO content.
3) Nonlinearity at frequency characteristics. As shown in Fig. 7, the RDC varies by increasing frequency in four regions: a) at 23 kHz, the capacitance possesses its minimum value; b) at 50-60 kHz, RDC tends to increase; c) at around 2800 kHz, there is an abrupt decrease in RDC; and d) at 4600-4750 kHz, it also falls off. As it discussed before, there are three sources for polarization (electronic, ionic and orientational polarization). But there is another kind of polarization called interfacial polarization for systems which are composed of two phases. The electronic and ionic polarization occurs at high frequencies (ultraviolet and infrared region), so these are not the case here. At low frequencies (~kHz), the interfacial or space charge polarization occurs. Especially in varistors, grains and intergranular phases have completely different dielectric constant and conductivity (ε1, σ1 and ε2, σ2) and the condition for interfacial polarization (ε1σ2 ≠ ε2σ1) was established. In this condition, charge carries could accumulate at the interface between phases [30]. But at higher frequencies (~MHz), the orientational polarization occurs and RDC reduces [46]. Finally, RDC drops off as each kind of polarization becomes unable to keep up with the switching electric field.
3.3.2 Series resistivity and dielectric loss
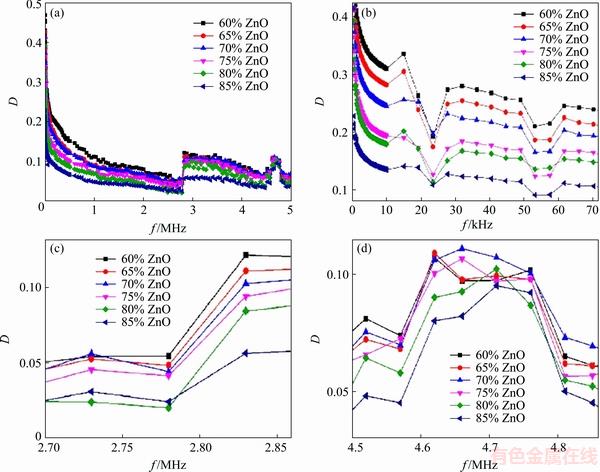
Fig. 8 Total behavior of dielectric loss with frequency in general (a) and exact forms (b-d)
One of the important parameters in defining a real capacitor is its series resistor (Rs). It is related to the loss factor via D=tan δ=Rs/Xc=Rscω (Fig. 8), where D is the dissipation factor, δ is the loss angle, c is the capacitance and ω is the applied frequency. Dielectric loss is described as changing electromagnetic energy into heat (loss) which is an inherent characteristic of materials. There are two kinds of dissipations: conduction loss because of the charge flow (electric current in conductors) through materials which can cause energy dissipation, and dielectric loss due to the fact that the motion of a charge in an electromagnetic field can cause energy dissipation. Here, conduction loss does not exist due to the applied electromagnetic field on samples. Dielectric loss, especially at resonance frequencies, is high. Owing to these frequencies, the interaction between dipole moments and the electromagnetic field is strong and this interaction will produce heat which causes energy dissipation. In these situations, the dielectric constant of materials will decrease. These anomalies are presented in Figs. 8(b-d), showing that by increasing ZnO content, dielectric loss decreases. This is because the dielectric loss for materials with a high dielectric constant is high which can be confirmed by looking at Fig. 7. This figure shows that by increasing ZnO content dielectric constant decreases (as we discussed earlier).
4 Conclusions
All investigated parameters concerning dielectric properties fall off up to 1 kHz. This is due to the limitation of charge transportation through the double Schottky barrier which exists at GBs. Beyond that, there are some anomalies at different frequencies which indicate the polarization or non-polarization state of the samples. The ZnO-PPY-HDPE composites show good nonlinear properties which can be used as a surge protector. Although breakdown voltage has a minimum value at 70% ZnO, nonlinear coefficient increases by increasing ZnO content in the mixture.
References
[1] CLARKE D R. Varistor ceramics [J]. American Ceramic Society, 1999, 82: 485-502.
[2] MATSUOKA M. Nonohmic properties of zinc oxide ceramics [J]. Japanese Journal of Applied Physics, 1971, 10: 736-746.
[3] GUPTA T K. Application of zinc oxide varistors [J]. Journal of the American Ceramic Society, 1990, 73: 1817-1840.
[4] EMTAGE P R. The physics of zinc oxide varistors [J]. Journal of Applied Physics, 1977, 48: 4372-4384.
[5] PIANARO S, BUENO P R, LONGO E, VARELA J A. Microstructure and electric properties of a SnO2 based varistor [J]. Ceramics International, 1999, 25: 1-6.
[6] BERNIK S, MACEK S, AI B. Microstructural and electrical characteristics of Y2O3-doped ZnO–Bi2O3-based varistor ceramics [J]. Journal of the European Ceramic Society, 2001, 21: 1875-1878.
[7]  Z, BERNIK S, GOES M,
Z, BERNIK S, GOES M,  G. ZnO varistors from intensively milled powders [J]. Journal of the European Ceramic Society, 2007, 27: 3897-3900.
G. ZnO varistors from intensively milled powders [J]. Journal of the European Ceramic Society, 2007, 27: 3897-3900.
[8] METZ R, DELALU H, VIGNALOU J, ACHARD N, ELKHATIB M. Electrical properties of varistors in relation to their true bismuth composition after sintering [J]. Materials Chemistry and Physics, 2000, 63: 157-162.
[9] LI J L, CHEN G H, YUAN C L. Microstructure and electrical properties of rare earth doped ZnO-based varistor ceramics [J]. Ceramics International, 2013, 39: 2231-2237.
[10] DONG X, SHI X F, CHENG X N, JUAN Y, FAN Y E, YUAN H M, SHI L Y. Microstructure and electrical properties of Lu2O3-doped ZnO-Bi2O3-based varistor ceramics [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 2303-2308.
[11] XU D, SHI L, WU Z, ZHONG Q, WU X. Microstructure and electrical properties of ZnO-Bi2O3-based varistor ceramics by different sintering processes [J]. Journal of the European Ceramic Society, 2009, 29: 1789-1794.
[12] GUPTA T, MILLER A. Improved stability of the ZnO varistor via donor and acceptor doping at the grain boundary [J]. Journal of Materials Research, 1988, 3: 745-754.
[13] GUPTA T K, CARLSON W G. A grain-boundary defect model for instability/stability of a ZnO varistor [J]. Journal of Materials Science, 1985, 20: 3487-3500.
[14] MIYOSHI T, YAMAZAKI T, TAKAHASHI K, MAEDA K. Effects of dopants on the characteristics of ZnO varistors [J]. American Ceramics Society Bulletin, 1980, 735: 335-335.
[15] CARLSON W, GUPTA T. Improved varistor nonlinearity via donor impurity doping [J]. Journal of Applied Physics, 1982, 53: 5746-5753.
[16] RAMIREZ M, BASSI W, BUENO P R, LONGO E, VARELA J A. Comparative degradation of ZnO and SnO2-based polycrystalline non-ohmic devices by current pulse stress [J]. Journal of Physics D: Applied Physics, 2008, 41: 122002.
[17]  M A, BASSI W, PARRA R, BUENO P R, LONGO E, VARELA J A. Comparative electrical behavior at low and high current of SnO2- and ZnO-based varistors [J]. Journal of the American Ceramic Society, 2008, 91: 2402-2404.
M A, BASSI W, PARRA R, BUENO P R, LONGO E, VARELA J A. Comparative electrical behavior at low and high current of SnO2- and ZnO-based varistors [J]. Journal of the American Ceramic Society, 2008, 91: 2402-2404.
[18] WANG Y, ZHANG G, LIU P, XU Y, ZENG Y, JIANG S. The clamp characteristics and DC aging behavior of ZnO-based varistor ceramics doped with Na2CO3 [J]. Ceramics International, 2016, 42: 2106-2114.
[19] GUPTA T K, CARLSON W, HOWER P. Current instability phenomena in ZnO varistors under a continuous AC stress [J]. Journal of Applied Physics, 1981, 52: 4104-4111.
[20] LI C, WANG J, SU W, CHEN H, WANG Y, ZHUANG D. Effect of sinter temperature on the electrical properties of TiO2-based capacitor–varistors [J]. Materials Letters, 2003, 57: 1400-1405.
[21] GHAFOURI M, PARHIZKAR M, BIDADI H, AREF S M, OLAD A. Effect of Si content on electrophysical properties of Si-polymer composite varistors [J]. Materials Chemistry and Physics, 2014, 147: 1117-1122.
[22] BIDADI H, AREF S M, GHAFOURI M, PARHIZKAR M, OLAD A. Effect of changing Gallium arsenide content on Gallium arsenide–polymer composite varistors [J]. Journal of Physics and Chemistry of Solids, 2013, 74: 1169-1173.
[23] GHAFOURI M, PARHIZKAR M, AREF S M, OLAD A, BIDADI H. Effect of temperature on the electrophysical properties of Si–polymer composite varistors [J]. Microelectronics Reliability, 2014, 54: 965-971.
[24] BIDADI H, OLAD A, PARHIZKAR M, AREF S M, GHAFOURI M. Nonlinear properties of ZnO-polymer composites prepared by solution-casting method [J]. Vacuum, 2013, 87: 50-54.
[25] MASHKOURI S, GHAFOURI M, ARSALANI N, BIDADI H, MOSTAFAVI H. Mechanochemical green synthesis of exfoliated graphite at room temperature and investigation of its nonlinear properties based zinc oxide composite varistors [J]. Journal of Materials Science: Materials in Electronics, 2017, 28: 4839-4846.
[26] EDA K. Conduction mechanism of non-ohmic zinc oxide ceramics [J]. Journal of Applied Physics, 1978, 49: 2964-2972.
[27] COHEN M H, NEATON J, HE L, VANDERBILT D. Extrinsic models for the dielectric response of CaCu3Ti4O12 [J]. Journal of Applied physics, 2003, 94: 3299-3306.
[28] SINCLAIR D C, ADAMS T B, MORRISON F D, WEST A R. CaCu3Ti4O12: One-step internal barrier layer capacitor [J]. Applied Physics Letters, 2002, 80: 2153-2155.
[29] LUNKENHEIMER P, FICHTL R, EBBINGHAUS S, LOIDL A. Nonintrinsic origin of the colossal dielectric constants in CaCu3Ti4O12 [J]. Physical review B, 2004, 70: 172102-172104.
[30] SMYTH C P. Dielectric behavior and structure [M]. Michigan: McGraw-Hill, 1955.
[31] RAMIREZ A, SUBRAMANIAN M, GARDEL M, BLUMBERG G, LI D, VOGT T, SHAPIRO S. Giant dielectric constant response in a copper-titanate [J]. Solid State Communications, 2000, 115: 217-220.
[32] HOMES C, VOGT T, SHAPIRO S, WAKIMOTO S, RAMIREZ A. Optical response of high-dielectric-constant perovskite-related oxide [J]. Science, 2001, 293: 673-676.
[33] SUBRAMANIAN M, LI D, DUAN N, REISNER B, SLEIGHT A. High dielectric constant in ACu3Ti4O12 and ACu3Ti3FeO12 phases [J]. Journal of Solid State Chemistry, 2000, 151: 323-325.
[34] LI X, HUANG Y, XU L, LIU L, WANG Y, CAO X, MENG C, WANG Z. Effect of powder size on the microstructure and dielectric properties of ZnO ceramics [J]. Materials Research Bulletin, 2015, 68: 87-91.
[35] CHENG C, HU J, HE J. Characterization of dielectric behavior in ZnO electroceramic: Superior grain boundary, inferior grain boundary and grain [J]. Materials Letters, 2014, 132: 240-242.
[36] LIM J, YEOH C K, THE P L, ARIF W, CHIK A. The effect of sintering temperature to the properties of zinc oxide [J]. Advanced Materials Research, 2013, 795: 419-423.
[37] WANG M, WANG J, CHEN W, CUI Y, WANG L. Effect of preheating and annealing temperatures on quality characteristics of ZnO thin film prepared by sol–gel method [J]. Materials Chemistry and Physics, 2006, 97: 219-225.
[38] BREZOI D V. Polypyrrole films prepared by chemical oxidation of pyrrole in aqueous FeCl3 solution [J]. Journal of Science and Arts, 2010, 1: 53-58.
[39] SZE S M S, NG K K. Physics of semiconductor devices [M]. New Jersey: John Wiley & Sons, 2006.
[40] MAGNUSSON K, WIKLUND S. Interface formation of Bi on ceramic ZnO: A simple model varistor grain boundary [J]. Journal of Applied Physics, 1994, 76: 7405-7409.
[41] GAMBINO J, KINGERY W, PIKE G, PHILIPP H, LEVINSON L. Grain boundary electronic states in some simple ZnO varistors [J]. Journal of Applied Physics, 1987, 61: 2571-2574.
[42] HOWER P, GUPTA T. A barrier model for ZnO varistors [J]. Journal of Applied Physics, 1979, 50: 4847-4855.
[43] DAI L. Intelligent macromolecules for smart devices: From materials synthesis to device applications [M]. New York: Springer, 2004.
[44]  A, KREMER F. Broadband dielectric spectroscopy [M]. New York: Springer, 2003.
A, KREMER F. Broadband dielectric spectroscopy [M]. New York: Springer, 2003.
[45] TSENG J K, TANG S, ZHOU Z, MACKEY M, CARR J M, MU R, FLANDIN L, SCHUELE D E, BAER E, ZHU L. Interfacial polarization and layer thickness effect on electrical insulation in multilayered polysulfone/poly(vinylidene fluoride) films [J]. Polymer, 2014, 55: 8-14.
[46] TAREEV B M. Physics of dielectric materials [M]. Moscow: Mir Publishers, 1975.
氧化锌-聚吡咯复合材料的介电性能和非线性特性
Fariba TABRIZI1, Mojtaba PARHIZKAR1, Hassan BIDADI1, Mohammad GHAFOURI2,3
1. Faculty of Physics, University of Tabriz, Tabriz, Iran;
2. Department of Basic Science, Shabestar Branch, Islamic Azad University, Shabestar, Iran;
3. Faculty of Physics, Islamic Azad University of Shabestar, Shabestar, Iran
摘 要:研究不同氧化锌(ZnO)含量的氧化锌-聚吡咯复合材料在200 Hz~5 MHz频率区间的非线性性能和频率特性。用热压法在130 °C下制备样品。结果表明,当ZnO含量为70%时,击穿电压最小。击穿电压先从590 V降低到380 V,后从450 V增加到740 V,这是由于在晶界处没有聚吡咯。与击穿电压的变化不同,非线性系数随氧化锌含量的增加从4.2增加到9,这是由于随氧化锌含量的增加,晶界处的受主能级增加。电学参数如介电常数、介电损耗和样品的串联电阻对频率的依赖性很强,特别是在1 kHz以下,这些参数随频率增加而下降,这与晶界上通过肖特基势垒的电荷传输有关。样品在1 kHz以下的高介电常数与晶界处的麦克斯韦-瓦格纳(Maxwell-Wagner)极化有关。在不同频率间隔区间存在不同的常数异常变化的现象,这与界面极化有关,是由于晶粒和晶界层结构的不同,导电性差异较大。
关键词:介电特性;电物理性能;复合压敏电阻;频率依赖性
(Edited by Wei-ping CHEN)
Corresponding author: Mohammad GHAFOURI; E-mail: ghafouri.sar@gmail.com
DOI: 10.1016/S1003-6326(18)64776-4
Abstract: The nonlinear properties and frequency characteristics of ZnO-polypyrrole composites were investigated at 200 Hz-5 MHz frequency interval with different zinc oxide contents. Samples were prepared using hot press method at 130 °C. Results show an optimum point for breakdown voltage at ZnO content of 70%. Breakdown voltage decreases from 590 to 380 V and after that tends to increase from 450 to 740 V due to the absence of polypyrrole at grain boundaries. No matter how breakdown voltage behaves, nonlinear coefficient increases from 4.2 to 9 by increasing ZnO content because of the increase in acceptor-like states at grain boundaries by increasing ZnO content. The electrical parameters such as dielectric constant, dielectric loss and series resistance of samples show a strong dependence on frequency especially below 1 kHz. These parameters fall off by increasing frequency up to 1 kHz, which is related to charge transportation through the Schottky barrier at grain boundaries. The high dielectric constant of samples below 1kHz is related to the Maxwell-Wagner polarization at grain boundaries. The presence of different anomalies at different frequency intervals is related to interfacial polarization because of different structures of grains and intergranular layer with a huge difference in conductivity.
[1] CLARKE D R. Varistor ceramics [J]. American Ceramic Society, 1999, 82: 485-502.
[30] SMYTH C P. Dielectric behavior and structure [M]. Michigan: McGraw-Hill, 1955.
[39] SZE S M S, NG K K. Physics of semiconductor devices [M]. New Jersey: John Wiley & Sons, 2006.
[46] TAREEV B M. Physics of dielectric materials [M]. Moscow: Mir Publishers, 1975.


