Trans. Nonferrous Met. Soc. China 27(2017) 1417-1422
Effect of SnO2 intermediate layer on performance of Ti/SnO2/MnO2 electrode during electrolytic-manganese process
Xin CHEN, Hua-jun GUO, Shu-liang LUO, Zhi-xing WANG, Xin-hai LI
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 1 March 2016; accepted 25 August 2016
Abstract:
SnO2 intermediate layers were coated on the titanium (Ti) substrate by thermal decomposition. Scanning electron microscope (SEM) and X-ray diffraction (XRD) results show that uniform SnO2 intermediate layers with rutile crystal structure were successfully achieved. According to the results of linear sweep voltammetry (LSV), oxygen evolution potential (OEP) of the Ti/SnO2/MnO2 electrodes decreases with increasing SnO2 content, indicating that the electro-catalytic oxidation activity of the electrode increases. Accelerated service life tests results demonstrate that SnO2 intermediate layer can improve the service life of the Ti/SnO2/MnO2 electrode. As the content of SnO2 intermediate layer increases, the cell voltage and the energy consumption decrease apparently.
Key words:
tin dioxide intermediate layer; oxygen evolution potential; accelerated service life; cell voltage, electrolytic-manganese;
1 Introduction
Reducing energy consumption has been unremitting pursuit of electrolytic-manganese industry and many attempts have been carried out. Generally, reducing cell voltage and increasing current density are two effective ways to solve the problem. With increasing the current density, the current efficiency increases firstly and decreases thereafter [1]. The optimum current density is usually determined based on the deposit quality and electrodeposition time. Furthermore, the more important factor is that energy consumption closely relates to electrode materials, especially anode materials. Graphite, lead-silver alloy and titanium-coated electrode were generally used as anode materials in practical electrolytic-manganese process [2]. Graphite electrode, which shows low mechanical strength as well as short service life owing to severe oxidation, was used as anode at first. Moreover, iron mixed in graphite anode will be co-electrodeposited with manganese on cathode, decreasing the purity of manganese product and current efficiency. Lead-silver alloy electrode is a substitute for graphite electrode contributed to its stability. Unfortunately, high energy consumption is one of the obvious shortcomings [3]. In addition, parts of lead would dissolve in the electrolyte solution, resulting in lead impurity in manganese product. Although titanium plate replaced lead-silver alloy electrode as anode due to its less energy consumption and corrosion resistance, the electrolytic manganese process cannot proceed regularly because of its serious passivation. The serious passivation leads to high cell voltage and limits its further application [4]. So, titanium substrate is usually covered by one or more metallic oxide layers, which is a pragmatic approach to prevent substrate from passivating [5-7]. SnO2 is widely used as coating to modify titanium electrode [8,9] because of its high conductivity [10], strong combining ability, high electrocatalytic activity and oxygen evolution potential.
In this work, the main purpose is to study the effect of the SnO2 intermediate layer on performance of Ti/SnO2/MnO2 electrode during the electrolytic manganese process. SnO2 layer as an intermediate layer is mainly to improve the activity and service life of the electrode. Ti/SnO2/MnO2 electrodes with different contents of SnO2 intermediate layer were prepared by thermal decomposition. The surface morphology, crystal structure and electrochemistry property were fully investigated. Meanwhile, the optimum content of SnO2 intermediate layer was also determined.
2 Experimental
2.1 Electrode preparation
Ti substrate was firstly rubbed with fine sandpaper and degreased in 5% sodium hydroxide for 1 h, then etched in 10% oxalic acid for 1 h at 100 °C. Finally, it was rinsed with deionized water and finally dried at 60 °C.
The details of the electrodes preparation could be found in Ref. [11]. The precursor solution consisted of citric acid (CA) and ethylene glycol (EG) in molar ratio of 1:4.5, which reacted at 60 °C for 1 h. After that, SnCl4·5H2O was added into precursor solution and kept at 90 °C for 1 h. Precursor solutions with five different molar ratios were prepared, where CA:EG:SnCl4·5H2O was 0, 1:4.5:0.1, 1:4.5:0.21, 1:4.5:0.33, 1:4.5:0.45, respectively. Ti substrate was painted with precursor solution by brush, followed by drying at 130 °C for 10 min and heat-treating at 500 °C for 20 min. This process was repeated 10 times and the Ti/SnO2 electrodes were annealed at 500 °C for 1 h at the last time. After that, the Ti/SnO2 electrode was painted with 50% Mn(NO3)2 solution by brush and dried at 130 °C for 10 min followed by heat-treating at 500 °C for 20 min. This process was repeated 2 times and the Ti/SnO2/MnO2 electrodes were annealed at 500 °C for 1 h at the last time. Finally, Ti/SnO2/MnO2 electrodes were obtained.
2.2 Characterization and electrochemical measure- ments
The morphology of electrodes was observed by SEM (Sirion 200). The crystal structure of SnO2 intermediate layer was determined by XRD (Cu Kα radiation, Rint-2000, Rigaku).
All electrochemical measurements were performed at 40 °C in a conventional three-electrode cell using an electrochemical workstation (CHI600A, Chenhua, China). A large area 304 stainless steel plate was selected as the auxiliary electrode. The saturated calomel electrode (SCE) was used as the reference electrode. The interval distance between Ti/SnO2/MnO2 electrodes and auxiliary electrode always was 5 cm. The LSV curves were recorded from 0 to 2 V at a sweep rate of 2 mV/s.
2.3 Accelerated life test
Accelerated electrolysis was carried out with 1 mol/L H2SO4 solution as electrolyte, which was employed under galvanostatic electrolysis at a current density of 1 A/cm2. The cell temperature was 40 °C. The 304 stainless steel plate was used as auxiliary electrode. The interval distance between Ti/SnO2/MnO2 electrode and auxiliary electrode was 1 cm. The cell voltage values were recorded in every 1 min. Electrode was defined as deactivated when the cell voltage was beyond 10 V.
2.4 Electrolysis
The electrolysis was conducted in a diaphragm cell with 300 mL electrolyte, which was placed in a thermostatic water bath to maintain the reaction temperature at 40 °C. The experimental facility for electrodeposition is presented in Fig. 1. Ti/SnO2/MnO2 electrode was employed as anode. A 304 stainless steel plate with the same dimension was used as cathode. Electrolyte solution consisted of 52.24 g/L MnSO4·H2O, 110 g/L (NH4)2SO4 and 0.04 g/L SeO2, and dissolved in deionized water. The pH value of the electrolyte solution was adjusted to 7.20 by adding dilute ammonium hydroxide. The current efficiency (η) was calculated based on the mass of manganese and manganese oxide on electrodes and the following equation:
 (1)
(1)
where m is the mass of deposits, q is electrochemical equivalent (g/(A·h)), I is the electrolysis current (A), t is the electrolysis time (h).
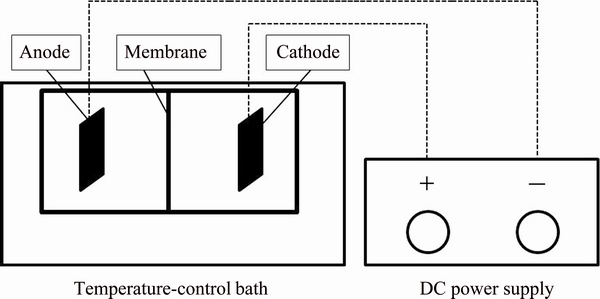
Fig. 1 Schematic diagram of experimental facility for electrodeposited manganese
3 Results and discussion
3.1 Morphology and structure of SnO2 intermediate layers
Figure 2 shows the SEM images of the SnO2 intermediate layers prepared from precursor solution at different mole ratios of CA:EG:SnCl4·5H2O. Uniform SnO2 intermediate layers are observed on the surface of electrodes. When CA:EG:SnCl4·5H2O mole ratio is low, the surface of SnO2 intermediate layer is smooth and no cracked mud is observed. However, the electrodes surface becomes more rough and a few of tiny holes appear as the CA:EG:SnCl4·5H2O mole ratio increases. Rough SnO2 intermediate layers surface and tiny holes usually result in large specific surface, which could provide more active sites for electro-catalytic oxidation [12]. Furthermore, rough SnO2 intermediate layer is in favor of combining with MnO2 layer.
The mass of SnO2 intermediate layers per unit area (ρ) is presented in Table 1. In addition, the MnO2 layer is about 8 mg/mm2. The mass of SnO2 intermediate layers per unit area increases with increasing content of SnCl4·5H2O in the precursor solution. However, it is a little out of proportion with SnCl4·5H2O amount in the precursor solution due to part of SnO2 peeling off electrode during preparation. In addition, the mass of MnO2 layers is ten times that of SnO2 intermediate layer on the electrode. The reason is that MnO2 layer decomposed from manganese nitrite is loose and porous, which is in favor of adsorbing manganese nitrite.
Table 1 Mass of SnO2 intermediate layer per unit area for electrode

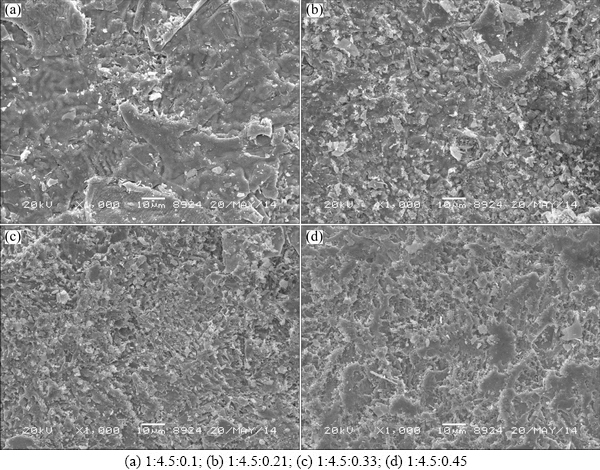
Fig. 2 SEM images of SnO2 intermediate layer decomposed at different mole ratios of CA:EG:SnCl4·5H2O in precursor solution

Fig. 3 XRD patterns of SnO2 intermediate layer decomposed at different mole ratios of CA:EG:SnCl4·5H2O in precursor solution
XRD patterns of the SnO2 intermediate layers decomposed from precursor solution with different mole ratios of CA:EG:SnCl4·5H2O are shown in Fig. 3. The peaks at 2θ=35.09°, 38.43°, 40.18°, 53.02° and 70.68° correspond to metallic titanium (JCPDS, No.65-3362). Clearly, several peaks at 2θ=26.61°, 33.89°, 51.78° 54.77°, 61.89° and 64.77° (JCPDS, No.72-1147) can be assigned well to the characteristic peaks of SnO2 cassiterite with a rutile type structure, and these peaks become more intense with increasing the concentration of SnCl4·5H2O in the precursor solution. On the contrary, the characteristic peaks of metallic titanium become weaker. The results indicate that the SnO2 intermediate layers become thicker and more compact so that they can prevent substrates from deactivating. No diffraction peak of TiO2 was detected, so it effectively suggested that the titanium substrate was not oxidized in the process of preparation. According to the Scherrer equation, the crystalline size of SnO2 intermediate layer with different mole ratios of CA:EG:SnCl4·5H2O were calculated based on the peak at 2θ=27.44° and their results are shown in Table 2. When SnCl4·5H2O content is the lowest (x=0.1), the crystalline size of SnO2 (11.4 nm) is much smaller than those of other samples. A high mole content of SnCl4·5H2O can facilitate the growth of grain.
Table 2 Crystalline size of SnO2 layer

3.2 Linear sweep voltammetry test
SnO2 is an alternative electrode material for oxygen evolution [13]. For further exploring, the effects of SnO2 content on the Ti/SnO2/MnO2 electrode, the linear sweep voltammetry was carried out to measure OEPs of the fresh Ti/SnO2/MnO2 electrodes [12]. The reaction current rises obviously on LSV curve when the potential increases above a certain value, which is marked as the oxygen evolution potential. The obviously increased reaction current corresponds to the oxygen evolution in solution. As shown in Fig. 4, the oxygen evolution current at high potential (for example, 1.75V) increases dramatically with increasing SnO2 content in the Ti/SnO2/MnO2 electrode, and the OEPs for the Ti/SnO2/MnO2 electrode are 1.27, 1.32, 1.35, 1.39 and 1.44 V (vs SCE) respectively. Therefore, increasing the content of SnCl4·5H2O leads to an decrease of OEP for the electrode. This is caused by difference of the surface morphology as well as oxygen vacancies of SnO2 crystal lattice [14,15]. Low OEP means that electrode owns good electron-catalytic oxidation performance and oxygen is easy to evolve. In other words, the higher the content of SnCl4·5H2O in precursor solution is, the better the electro-catalytic oxidation performance of Ti/SnO2/ MnO2 electrode has.

Fig. 4 Linear sweep voltammetry curves of Ti/SnO2/MnO2 electrodes
3.3 Accelerated life test
The electrode stability is another important parameter which determines whether the electrode could be applied in industrial scale finally [16]. A good electrode can often work effectively for a few years. The actual service life time is mainly influenced by the current density, temperature and pH of the electrolyte. Because a low current density is applied in practice, the actual service life time is usually long and difficult to evaluate. To reduce the test time, accelerated life test has been applied to evaluating electrode actual life. An empirical relationship between the accelerated service life (t1) and the actual service life (t2) was proposed, which could assess the actual service life of Ti/SnO2/ MnO2 electrodes at different current densities [17].
 (2)
(2)
where n is a constant often equal to 2, J1 is the actual current density and J2 is the accelerated current density. The accelerated life test results and the cell voltage varied with time for Ti/SnO2/MnO2 electrodes are shown in Fig. 5. The accelerated service lives of Ti/SnO2/MnO2 electrodes are 0, 0, 3, 8 and 7 min, respectively. The accelerated service life increases obviously with increasing SnO2 content. The cell voltage increases slowly when SnO2 content is high. Specially, the cell voltages keep constant and the accelerated service life is the longest when the molar ratio of CA:EG:SnCl4·5H2O is 1:4.5:0.33. However, the cell voltage decreases when the molar ratio of CA:EG:SnCl4·5H2O increases to 1:4.5:0.45. It can be ascribed to the exfoliation of SnO2 intermediate layer from the electrode during preparation. The reasons for the electrode deactivation are as follows. On one hand, the electrode surface is corroded and washed by electrolyte solution coupled with gas bubbles. On the other hand, the more important reason is that electrolyte reacts with Ti substrate and forms a layer of passive film after permeating SnO2 intermediate layer.
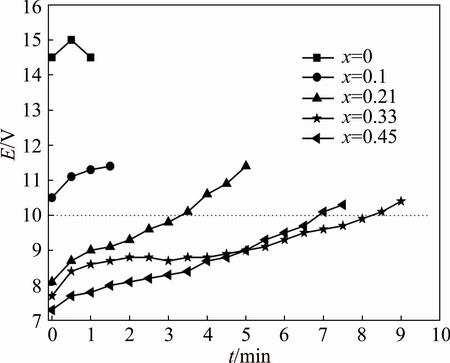
Fig. 5 Accelerated service life test on Ti/SnO2/MnO2 electrodes
3.4 Electrolysis
Figure 6 shows the cell voltage change of Ti/SnO2/MnO2 electrodes with different contents of SnO2 intermediate layer in the process of electrolysis. Clearly, the cell voltage gradually decreases when the content of SnO2 intermediate layers increases. The result is consistent with the trend of accelerated life test. Because the SnO2 intermediate can effectively prevent the electrolyte solution from penetrating it. In addition, the cell voltages always go up at first and then remain constant. It could be attributed to that TiO2 film formed on the Ti substrate [18]. As a consequence, the appropriate content of SnO2 intermediate layer can protect the Ti substrate from passivation. Additionally, current efficiency on the anode and cathode was calculated and listed in Table 3. The results show that all the cathodic current efficiency is more than 80%, and the mass of MnO2 on Ti/SnO2/MnO2 electrodes was almost similar. This suggests that the content of SnO2 intermediate layer has little influence on anodic efficiency. After electrolysis, electrolyte solutions at anodic cell are colorless and no residue exists. MnO2 can be regarded as raw material for lithium ion battery after further processing.

Fig. 6 Cell voltage of Ti/SnO2/MnO2electrodes
3.5 Energy consumption
The economic feasibility of Ti/SnO2/MnO2 electrodes as anode in electrolytic manganese industry was evaluated through the energy consumption. Energy consumption (W) can be calculated using the following equation:
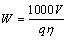 (3)
(3)
where V is the cell voltage (V). Energy consumption of Ti/SnO2/MnO2 electrodes is indicated in Fig. 7.

Fig. 7 Energy consumption of Ti/SnO2/MnO2 electrodes
It could be observed apparently that energy consumption is much higher than others if the content of SnCl4×H2O in the precursor solution is low. Energy consumption is almost similar when the amount of SnCl4·5H2O changes from 0.21 to 0.45. Generally, energy consumption is about 6 MW·h/t in industry [19]. The results show that Ti/SnO2/MnO2 electrodes with appropriate content of SnO2 intermediate layer can be used as anode in electrolytic manganese to save energy.
4 Conclusions
Ti/SnO2/MnO2 electrodes with different contents of SnO2 were successfully synthesized by thermal decomposition. Oxygen evolution potentials (OEPs) of the Ti/SnO2/MnO2 electrodes decreases with increasing SnO2 content. OEPs for Ti/SnO2/MnO2 electrodes made from different precursor solutions (CA:EG:SnCl4·5H2O= 1:4.5:x, x=0, 0.10, 0.21, 0.33, 0.45) were 1.44, 1.39, 1.35, 1.32 and 1.27 V, respectively. SnO2 intermediate layer could prevent the electrode from forming the passive film, and then decreases energy consumption and extends the service life. Based on these experimental results, an optimum molar ratio of CA:EG:SnCl4·5H2O can be determined as 1:4.5:0.33.
References
[1] LU J, DREISINGER D,  T. Manganese electrodeposition—A literature review [J]. Hydrometallurgy, 2014, 141: 105-116.
T. Manganese electrodeposition—A literature review [J]. Hydrometallurgy, 2014, 141: 105-116.
[2] SCHMACHTEL S, TOIMINEN M, KONTTURI K,  O, BARKER M H. New oxygen evolution anodes for metal electrowinning: MnO2 composite electrodes [J]. Journal of Applied Electrochemistry, 2009, 39(10): 1835-1848.
O, BARKER M H. New oxygen evolution anodes for metal electrowinning: MnO2 composite electrodes [J]. Journal of Applied Electrochemistry, 2009, 39(10): 1835-1848.
[3] ZHANG W, TU C Q, CHEN Y F, LI W Y, HOULACHI G. Cyclic voltammetric studies of the behavior of lead-silver anodes in zinc electrolytes [J]. Journal of Materials Engineering and Performance, 2013, 22(6): 1672-1679.
[4] HEPEL M, KUMARIHAMY I, ZHONG C J. Nanoporous TiO2-supported bimetallic catalysts for methanol oxidation in acidic media [J]. Electrochemistry Communications, 2006, 8(9): 1439-1444.
[5] ZHUO Q, DENG S, YANG B, HUANG J, YU G. Efficient electrochemical oxidation of perfluorooctanoate using a Ti/SnO2-Sb-Bi anode [J]. Environmental Science & Technology, 2011, 45(7): 2973-2979.
[6] ZHANG Wei, ROBICHAUD M, GHALI E, HOULACHI G. Electrochemical behavior of mesh and plate oxide coated anodes during zinc electrowinning [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(2): 589-598.
[7] SANTOS I D, GABRIEL S B, AFONSO J C, DUTRA A J B. Preparation and characterization of Ti/SnO2-Sb electrode by Pechini's method for phenol oxidation [J]. Materials Research, 2011, 14(3): 408-416.
[8] PANIZZA M, CERISOLA G. Direct and mediated anodic oxidation of organic pollutants [J]. Chemical Reviews, 2009, 109(12): 6541-6569.
[9]  C A, BRILLAS E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review [J]. Applied Catalysis B: Environmental, 2009, 87(3): 105-145.
C A, BRILLAS E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review [J]. Applied Catalysis B: Environmental, 2009, 87(3): 105-145.
[10] ZHAO Guo-hua, CUI Xiao, LIU Mei-chuan, LI Pei-qiang, ZHANG Yong-gang, CAO Tong-cheng, LI Hong-xu, LEI Yan-zhu, LEI Liu, LI Dong-ming. Electrochemical degradation of refractory pollutant using a novel microstructrured TiO2 nanotubes/Sb-doped SnO2 electrode [J]. Environmental Science & Technology, 2009, 43(5): 1480-1486.
[11] WANG Y, GU B, XU W. Electro-catalytic degradation of phenol on several metal-oxide anodes [J]. Journal of Hazardous Materials, 2009, 162(2): 1159-1164.
[12] ZHANG Li-chao, XU Li, HE Jing, ZHANG Jie-jing. Preparation of Ti/SnO2-Sb electrodes modified by carbon nanotube for anodic oxidation of dye wastewater and combination with nanofiltration [J]. Electrochimica Acta, 2014, 117: 192-201.
[13]  R, STUCKI S, CARCER B. Electrochemical waste water treatment using high overvoltage anodes. Part I: Physical and electrochemical properties of SnO2 anodes [J]. Journal of Applied Electrochemistry, 1991, 21(1): 14-20.
R, STUCKI S, CARCER B. Electrochemical waste water treatment using high overvoltage anodes. Part I: Physical and electrochemical properties of SnO2 anodes [J]. Journal of Applied Electrochemistry, 1991, 21(1): 14-20.
[14] OLIVEIRA F H, OSUGI M E, PASCHOAL F M M, PROFETI D, OLIVI P. Electrochemical oxidation of an acid dye by active chlorine generated using Ti/Sn(1-x)IrxO2 electrodes [J]. Journal of Applied Electrochemistry, 2007, 37(5): 583-592.
[15] CUI Y H, FENG Y J, LIU Z Q. Influence of rare earths doping on the structure and electro-catalytic performance of Ti/Sb-SnO2 electrodes [J]. Electrochimica Acta, 2009, 54(21): 4903-4909.
[16] CORREA-LOZANO B, COMNINELLIS C, de BATTISTI A. Service life of Ti/SnO2-Sb2O5 anodes [J]. Journal of Applied Electrochemistry, 1997, 27(8): 970-974.
[17] CHEN X, CHEN G. Stable Ti/RuO2–Sb2O5–SnO2 electrodes for O2 evolution [J]. Electrochimica Acta, 2005, 50(20): 4155-4159.
[18] HU J M, MENG H M, ZHANG J Q, CAO C N. Degradation mechanism of long service life Ti/IrO2-Ta2O5 oxide anodes in sulphuric acid [J]. Corrosion Science, 2002, 44(8): 1655-1668.
[19] HAGELSTEIN K. Globally sustainable manganese metal production and use [J]. Journal of environmental management, 2009, 90(12): 3736-3740.
Ti/SnO2/MnO2电极的SnO2中间层对锰电解过程的影响
陈 鑫,郭华军,罗树亮,王志兴,李新海
中南大学 冶金与环境学院,长沙 410083
摘 要:通过热分解法在钛板表面沉积SnO2中间层。SEM和XRD的结果显示得到了均匀的金红石型SnO2中间层。线性扫描结果表明,随着SnO2中间层含量的增加,氧气析出电势下降,说明电极的电催化活性提高。强化寿命测试结果表明,SnO2中间层可以提高电极的使用寿命。不同电极的槽电压随着SnO2含量的增加逐渐降低,电解过程的能耗也随着SnO2含量的增加而减少。
关键词:SnO2中间层;锰电解;析氧电位;强化电解;槽电压,电解锰
(Edited by Xiang-qun LI)
Foundation item: Project (51574287) supported by the National Natural Science Foundation of China; Project supported by the Collaborative Innovation Center of Manganese-Zinc-Vanadium Industrial Technology
Corresponding author: Hua-jun GUO; Tel: +86-731-88836633; E-mail: hjguo_csu@163.com
DOI: 10.1016/S1003-6326(17)60163-8
Abstract: SnO2 intermediate layers were coated on the titanium (Ti) substrate by thermal decomposition. Scanning electron microscope (SEM) and X-ray diffraction (XRD) results show that uniform SnO2 intermediate layers with rutile crystal structure were successfully achieved. According to the results of linear sweep voltammetry (LSV), oxygen evolution potential (OEP) of the Ti/SnO2/MnO2 electrodes decreases with increasing SnO2 content, indicating that the electro-catalytic oxidation activity of the electrode increases. Accelerated service life tests results demonstrate that SnO2 intermediate layer can improve the service life of the Ti/SnO2/MnO2 electrode. As the content of SnO2 intermediate layer increases, the cell voltage and the energy consumption decrease apparently.


