两级UASB-A/O-SBR工艺深度处理晚期垃圾渗滤液
吴莉娜1, 2,宋燕杰1,刘牡1,张树军1,彭永臻1
(1. 北京工业大学 环境与能源工程学院 北京市水质科学与水环境恢复工程重点实验室,北京,100022;
2. 中国环境科学出版社,北京,100062)
摘要:应用“两级上流式厌氧污泥床(UASB)-缺氧/好氧(A/O)-序批式反应器(SBR)系统”深度处理实际晚期垃圾渗滤液,研究在A/O反应器中实现并维持稳定短程硝化的影响因素。该系统按如下模式运行:首先在一级UASB(UASB1)中实现反硝化,残余亚硝态氮和硝态氮利用剩余COD(化学需氧量)在二级UASB(UASB2)中通过反硝化被进一步去除,之后在A/O反应器的好氧区进行NH4+ -N的硝化,最后在SBR中去除硝化产生的亚硝态氮及硝态氮深度脱氮。试验结果表明:原渗滤液COD质量浓度仅3.00 g/L左右,氨氮质量浓度在2.00 g/L左右;系统进水采用将原渗滤液与生活污水1:1混合液,且投加相当于1.56 g/L COD的外碳源无水乙酸钠,将C和N质量浓度比由1.7提高到3.0;通过FA与FNA对NOB的联合抑制,在A/O反应器中实现了稳定的短程硝化,其中亚硝态氮积累率大与70%;产生的亚硝态氮和硝态氮在SBR中被彻底去除;最终出水氨氮质量浓度小于2 mg/L,氨氮的去除率为99%;最终出水总氮质量浓度为26 mg/L,系统总氮去除率接近98%。
关键词:
晚期垃圾渗滤液;生活污水;短程硝化;UASB;A/O;SBR;FA;FNA;
中图分类号:X703.1 文献标志码:A 文章编号:1672-7207(2011)08-2520-06
Advanced treatment of mature landfill leachate by
two-stage UASB-A/O-SBR process
WU Li-na1, 2, SONG Yan-jie1, LIU Mu1, ZHANG Shu-jun1, PENG Yong-zhen1
(1. Key Laboratory of Beijing for Water Quality Science and Water Environment Recovery Engineering,
College of Environmental and Energy Engineering, Beijing University of Technology, Beijing 100022, China;
2. China Environmental Science Press, Beijing 100062, China)
Abstract: A combined process of a two-stage up-flow anaerobic sludge blanket (UASB), an anoxic/aerobic (A/O) reactor and a sequencing batch reactor (SBR) was used to treat mature landfill leachate, and the influencing factors on short-cut nitrification in A/O reactor were also researched. The operational mode of the process was as follows: Denitrification took place in the first stage UASB (UASB1). The residual nitrite and nitrate of UASB1 by using the residual chemical oxygen demand (COD) was further removed in the second stage UASB (UASB2). The ammonia nitrogen was removed by short-cut nitrification in the aerobic zone of A/O reactor. The nitrite and nitrate produced from the nitrification were removed in SBR. The experiment results show that the COD and ammonia nitrogen mass concentrations of raw leachate are about 3.0 g/L and 2.0 g/L, respectively. The influent leachate is mixed by raw leachate as well as domestic wastewater in a ratio of 1. The external carbon source (sodium acetate anhydrous) is added in order to enhance the ρ(C)/ρ(N) ratio from 1.7 to 3.0. Through the cooperative inhibition of the free ammonia (FA) and free nitrite acid (FNA), the short-cut nitrification is achieved stably in the A/O reactor with more than 70% of the nitrite accumulation ratio. The nitrite and nitrate produced from the nitrification could be removed completely in SBR. The ammonia mass concentration of final effluent is less than 2 mg/L, and its removal efficiency is 99%. The total nitrogen mass concentration of final effluent is about 26 mg/L, and its removal efficiency is about 98%.
Key words: mature landfill leachate; domestic wastewater; short-cut nitrification; UASB; A/O; SBR; FA; FNA
虽然我国城市垃圾处理中90%以上是采用卫生填埋法,但垃圾卫生填埋以后,会产生相当数量的渗滤液[1]。由于垃圾渗滤液的水质会随填埋时间的延长而出现很大变化,成分越来越复杂,可生化性下降,氨氮含量上升,填埋一定时间后会出现C和N质量浓度比即ρ(C)/ρ(N)小于3的情况,造成营养比例的严重失调,不利于有机物降解和生物脱氮反应的进行[2-3]。通常填埋时间在5 a以上的填埋场产生的渗滤液属于晚期渗滤液[4],如广州市大田山、李坑2座垃圾填埋场的填埋时间超过10 a,产生的垃圾渗滤液属于晚期垃圾渗滤液,氨氮质量浓度相当高,碳氮比例严重失调,且含有大量难生物降解的有机物,可生化性低[5-6]。垃圾渗滤液的处理目前运用较多的是生物法[7]。采用生物技术去除有机污染物和氨氮,从而为解决垃圾渗滤液处理这个世界性难题开辟了新的技术途径。如SBR(Sequencing batch reactor,间歇式活性污泥法)工艺、UASB (up-flow anaerobic sludge blanket,上流式厌氧污泥床)工艺、Anammox(厌氧氨氧化)工艺、厌 氧-好氧工艺、人工湿地等[8-9]都是以生物法为主。然而晚期渗滤液中有机物浓度低且难降解,氨氮含量高,故如何有效去除高氨氮和反硝化时碳源缺乏是生物处理的重点和难点[10]。短程硝化反硝化生物脱氮和全程生物脱氮工艺相比,可节省25%的嚗气量和40%的碳源,缩短反应历程[11]。晚期垃圾渗滤液的C和N质量浓度比极低,通常需补充部分碳源才能充分反硝化。因此,短程生物脱氮技术尤其适用于处理含有高氨氮而又碳源缺乏的晚期垃圾渗滤液。Ismail等[12]虽然也采用厌氧-好氧工艺处理垃圾渗滤液,但其好氧反应器是通过全程硝化去除氨氮的,如能通过短程硝化去除氨氮,该工艺将更加经济有效。孙洪伟等[13]虽然也采用UASB-A/O系统深度去除渗滤液内高浓度有机物和氨氮,但其系统最终出水总氮(TN)质量浓度仍高达325.7 mg/L,TN去除率仅为84.6%。而国家2008年最新公布的生活垃圾填埋场污染控制标准中要求TN质量浓度不超过40 mg/L[14],故其工艺需进一步提高对总氮的去除能力。此外,国内外文献报道对短程硝化的研究试验用水大多采用模拟废水,用实际废水研究的很少[15],且对处理晚期垃圾渗滤液同时实现完全硝化和短程硝化并且氨氮和总氮的去除率都在90%以上的工艺还很少报道。本试验以实际的高氮晚期垃圾渗滤液废水为研究对象,采用“两级UASB–缺氧/好氧(A/O)–SBR深度脱氮系统”,在获得了晚期垃圾渗滤液高氨氮深度去除的前提下,考察在系统内实现并维持稳定的短程硝化的影响因素。
1 试验材料与方法
1.1 试验装置及水质
本试验采用两级UASB与A/O及SBR组合处理工艺,工艺流程见图1。系统进水从原水水箱与一部分A/O反应器出水混合进入到UASB1(一级UASB)中,其中,NOX--N(亚硝态氮与硝态氮)利用原水中的有机碳源进行反硝化。残余的NOX--N(亚硝态氮与硝态氮)在UASB2(二级UASB)中进一步反硝化。同时,二沉池的污泥回流到A/O工艺缺氧段,回流污泥中的NOX--N在此进行反硝化。有机物首先在UASB1和UASB2中被作为反硝化碳源所利用。剩余有机物在UASB2中通过产甲烷反应进一步降解,但是由于进水中可生物降解的有机物含量很低,在UASB2中产生的甲烷量很少。A/O工艺主要进行NH4+-N(氨氮)的硝化。二沉池出水进入SBR,在SBR中,主要是通过反硝化去除产生的亚硝态氮和硝态氮,从而提高总氮的去除率达到深度脱氮的目的。UASB1和UASB2的有效容积分别为4.25 L和8.25 L。A/O反应器材质为有机玻璃其有效容积为15 L,平均分成10个格室,第1格室为缺氧区,其余为好氧区。SBR有效容积8 L,每个周期处理水量3 L。
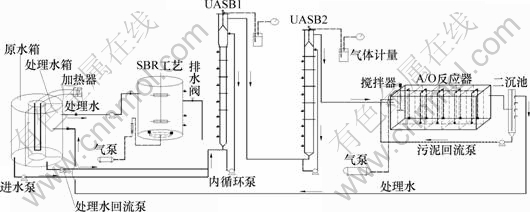
图1 两级UASB-A/O-SBR系统流程
Fig.1 Flow diagram of two-stage UASB-A/O-SBR system
试验用垃圾渗滤液取自已有10 a填埋史的北京六里屯垃圾填埋场,其产生的渗滤液C和N质量浓度比极低,属晚期渗滤液。具体水质(质量浓度)如表1所示,其pH为7.5~8.5。
表1 渗滤液水质指标
Table 1 Characteristics of leachate mg?L-1

1.2 试验运行模式
试验共进行60 d,流量为4.5 L/d。试验采用原渗滤液与生活污水按体积比1:1混合并投加相当于1.56 g/L COD(化学需氧量)的外碳源无水乙酸钠进入系统实现和维持稳定的短程硝化。A/O反应器出水回流至UASB1的回流比为200%,二沉池污泥回流比为200%。UASB1与UASB2的温度采用预热加保温的方式分别控制在32 ℃和35 ℃。由于试验时值冬季,故A/O反应器温度通过加热棒控制在30 ℃左右,SBR也通过加热棒将温度控制在30 ℃左右。在A/O反应器中,pH从第1格到第10格在8~7之间变动。试验期间在A/O反应器中只以自然流失和取样形式排泥,其污泥龄维持在30~40 d,污泥质量浓度一直维持在3.2~4.4 g/L。SBR按如下模式运行:瞬间进水→缺氧搅拌(外加碳源)反硝化→曝气去除多余有机物→静沉→排水。SRT(污泥龄)为30 d,污泥质量浓度维持在4~5 g/L。采用ORP(Oxidation reduction potential,氧化还原电位)、pH仪实时控制反硝化时间,沉淀时间为60 min,排水时间为4 min。两级UASB与A/O反应器设置取样点如下:系统进水(R),UASB1进水即系统进水与回流A/O反应器出水的混合液(U1),UASB1出水(U1e),UASB2出水(U2e),A/O反应器的缺氧区(A1),好氧区的不同格室(O2~O10)。SBR按时间设置取样点。
1.3 分析项目与测定方法
COD(化学需氧量)、 氨氮、亚硝酸盐氮、硝酸盐氮、碱度等常规水质指标均采用国家标准方法[16]。DO (Dissolved oxygen,溶解氧),ORP,pH和温度通过在线测量(WTW DO 330i,WTW ORP 340i,WTW pH 340i)。TON(总有机氮),TN,TOC(总有机碳),IC(无机碳)和TC(总碳)等采用TN/TOC分析仪(Multi N/C3000,德国耶拿)测定。
2 结果与讨论
2.1 采用两级UASB-A/O-SBR处理原渗滤液与生活污水混合液
所取渗滤液COD质量浓度仅有3 g/L左右,氨氮质量浓度为1.7~2.0 g/L,ρ(C)/ρ(N)为1.5~1.7。低ρ(C)/ρ(N)不仅增大了处理难度,而且对生物处理也有抑制作用。同时,由于缺乏有机碳源,故很难进行有效的反硝化。因此,如何降低晚期垃圾渗滤液中氨氮的含量,并且降低外碳源的投加量是晚期垃圾渗滤液处理的关键[17]。本试验使用生活污水稀释所取渗滤液,达到既降低所取渗滤液中氨氮和有机物的含量,又处理一部分生活污水的双重目的。理论上,完成硝酸盐反硝化需要ρ(COD)/ρ(TKN)为4.0,亚硝酸盐反硝化只要求ρ(COD)/ρ(TKN)>2.5即可[18]。van Benthum等[19]采用前置反硝化工艺对渗滤液处理的研究表明,最优的ρ(COD)/ρ(TN)为3.0左右。所取渗滤液COD质量浓度为3 g/L,经生活污水稀释后减到1.6 g/L左右,氨氮质量浓度经稀释后降到1 g/L左右,因此,在此阶段系统进水中投加相当于1 560 mg/L COD的外碳源无水乙酸钠,目的是使ρ(C)/ρ(N)=3.0,同时也补充反硝化所需碳源。
2.2 氮的变化情况
两级UASB-A/O系统中氮的变化如图2所示。从图2可见,经过生活污水稀释后,系统进水氨氮质量浓度降为1.03 g/L。通过系统出水的回流稀释,UASB1进水、出水、UASB2出水的氨氮质量浓度在360 mg/L左右。因为回流污泥的稀释作用,A/O反应器进水氨氮质量浓度为120 mg/L左右。系统出水的氨氮质量浓度为3 mg/L,系统氨氮的去除率为99.7%。在硝化结束时,A/O反应器出水硝态氮质量浓度基本在57 mg/L左右,亚硝态氮质量浓度在162 mg/L左右,亚硝态氮累积率为74%。通常认为,亚硝态氮累积率大于50%即发生了短程硝化反应。因此,本试验在A/O反应器中发生了较为明显的短程硝化反应。
此外,经生活污水稀释后,系统进水总氮质量浓度降为1.3 g/L左右,A/O反应器末格(第10格)总氮质量浓度降为265 mg/L左右。因为有机氮质量浓度很低,总氮质量浓度与氨氮、亚硝酸盐氮及硝酸盐氮的质量浓度总和接近,故总氮的去除情况与此三氮和的去除情况接近,因此,可以用此三氮和来间接反映总氮的情况。为了进一步降低总氮质量浓度,在二沉池出水后再串联1个SBR。因为在A/O反应器的末格氨氮质量浓度已降到很低,所以,SBR的主要作用就是去除在A/O反应器中硝化产生的亚硝态氮和硝态氮。采用的SBR体积为8 L,每一个周期处理水量为3 L,其余为泥水混合物。二沉池出水中的COD已基本不可生化,因此,能利用的有机碳源已很少,故在SBR中首先投加相当于1.0 g/L COD的外碳源无水乙酸钠进行反硝化,9 h后反硝化结束。为了去除残余COD,反硝化结束后再曝气1 h,然后沉淀1 h,最后排水。
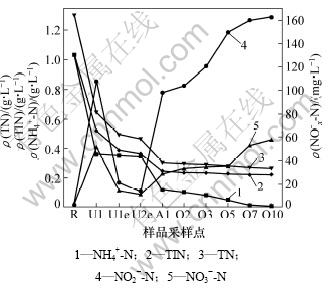
图2 两级UASB - A/O系统中氮的变化
Fig.2 Variation of nitrogen in two-stage UASB-A/O system
SBR中氮的变化如图3所示。由图3可看出:SBR进水中的亚硝态氮质量浓度为54 mg/L,经过9 h反硝化后降到3.2 mg/L左右。而硝态氮质量浓度则由25 mg/L左右降到2 mg/L左右。
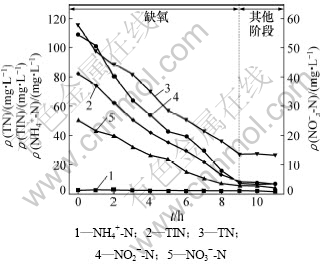
图3 在SBR中氮的变化
Fig.3 Variation of nitrogen in SBR
经检测,SBR出水中总氮质量浓度仅为26 mg/L,总氮去除率达到98%。因此,此工艺不仅使处理水总氮浓度完全达标,而且总氮去除率很高。
2.3 IC质量浓度与pH的变化规律
图4所示为两级UASB-A/O系统中IC质量浓度和pH的变化。试验中系统进水的IC质量浓度为2.46 g/L,通过与A/O反应器出水混合后,UASB1进水IC质量浓度降到870 mg/L,pH从系统进水中的8.01提高到8.12。在UASB1中,由于发生了反硝化和产甲烷反应,碱度基本没变,pH升高到8.32。由于在UASB1中反硝化不完全,在UASB2中继续进行反硝化反应,继续消耗碱度,IC质量浓度降到625 mg/L,pH升高到8.35。
由于回流污泥的进一步稀释作用,在A/O反应器的缺氧区(第1格),IC质量浓度进一步降低到258.6 mg/L。在A/O反应器的好氧区(第2~10格),由于在此段发生硝化反应,而硝化反应不断消耗碱度,IC质量浓度不断下降。在A/O反应器末格IC质量浓度降低到不足100 mg/L。在A/O反应器的第7格,pH出现“ammonia valley(氨谷)”,表明硝化反应结束。在该点pH降低到7.39,硝化反应结束后继续曝气pH又回升到7.48。由此可知,在ρ(COD)/ρ(TN)=3左右时,系统内碱度仍然可以满足硝化的需求,在A/O反应器内不需外加碱度就可完成氨氮的完全硝化并且是短程硝化。
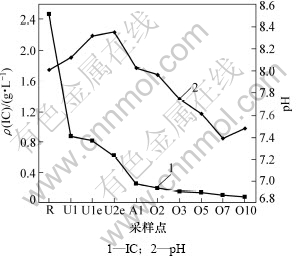
图4 IC质量浓度和pH在两级UASB-A/O系统内的转化规律
Fig.4 Evolution of IC and pH in two-stage UASB-A/O system
2.4 短程硝化过程的关键影响因素
在试验中,FA和FNA质量浓度采用下式计算:
![]()
![]()
式中:ρ(FA)为FA质量浓度,mg/L;![]() 为氨氮质量浓度,mg/L;ρ(FNA)为游离亚硝酸质量浓度,mg/L;
为氨氮质量浓度,mg/L;ρ(FNA)为游离亚硝酸质量浓度,mg/L;![]() 为亚硝态氮质量浓度,mg/L;θ为温度,℃[20]。
为亚硝态氮质量浓度,mg/L;θ为温度,℃[20]。
FA与FNA是实现和维持稳定短程硝化的重要影响因素。通常人们认为过高的游离氨质量浓度会抑制亚硝酸氧化菌(Nitrite-oxidizing bacteria,NOB)和氨氧化菌(Ammonia-oxidizing bacteria,AOB)。目前,对此抑制性浓度说法不一,有人认为0.1~1.0 mg/L的FA就会对NOB产生抑制,而AOB承受FA的抑制能力则更强,其抑制范围一般为10~160 mg/L[21-22]。
FA与FNA在A/O反应器内的转化规律如图5所示。由图5可以看出,在A/O反应器中,FA质量浓度逐渐由9.7 mg/L降低至第7格的0.2 mg/L左右。此时,FA浓度仍然大于Anthonisen等[23]提出的对NOB产生抑制的临界质量浓度(0.1 mg/L);随着硝化反应的进行,氨氮质量浓度逐渐减少,在第7格出现氨谷,硝化反应结束,氨氮质量浓度几乎没有,这样在最后1格FA质量浓度为76 μg/L,此质量浓度虽然也可能对NOB产生抑制,但其抑制作用已非常弱。此时,第7格的FNA质量浓度为12.7 μg/L,第10格的FNA质量浓度为11 μg/L,已大于等于Vadivelu等[24]报道的对NOB产生抑制作用的临界质量浓度(11 μg/L)。也就是说,在A/O反应器的第7~10格对NOB产生主要抑制作用的是FNA。这样,在整个A/O反应器中都对NOB产生抑制,随着长时间的运行,NOB将被逐渐淘洗出去,系统中的亚硝态氮累积率越来越高,而亚硝态氮累积率大于50%则通常认为系统内发生了短程硝化反应,因此,在A/O反应器中,通过利用反应器前段的高质量浓度FA与后段的FNA联合对NOB产生抑制作用而实现并维持了稳定高效的短程硝化。
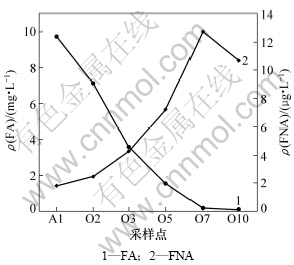
图5 FA与FNA在A/O反应器内的转化规律
Fig.5 Evolution of FA and FNA in A/O reactor
3 结论
(1) 采用生活污水与原渗滤液按体积比1:1混合的同时投加无水乙酸钠,将ρ(C)/ρ(N)从原来的1.7提高到3.0,并利用两级UASB-A/O-SBR工艺深度处理此混合液。在A/O反应器中,通过短程硝化去除氨氮,其出水的氨氮质量浓度仅为3 mg/L,氨氮去除率达到99.7%。在SBR中通过反硝化处理二沉池的出水。最终出水的亚硝态氮与硝态氮质量浓度为3 mg/L左右。经生活污水稀释后系统进水总氮质量浓度从原来的2.3 g/L降到1.3 g/L,而SBR出水总氮质量浓度为26 mg/L。因此,此工艺未经任何物化处理,就实现了高氨氮的生化去除,不仅完全达到了国家2008年最新公布的生活垃圾填埋场污染控制标准(GB 16889—2008)中对总氮质量浓度不超过40 mg/L的要求,同时还得到98%的总氮去除率。
(2) 系统进水的ρ(C)/ρ(N)为3左右时,在A/O反应器末格IC质量浓度仍然有75 mg/L。也就是说,系统内碱度仍然可以满足硝化的需求,不需在A/O反应器内额外投加碱度就可完成氨氮的完全硝化并且是短程硝化。
(3) FA与FNA对NOB和AOB的选择性抑制作用是在A/O反应器内实现和维持稳定短程硝化的关键因素。利用FA与FNA对NOB的协同抑制作用,在A/O反应器内亚硝态氮累积率大于70%。
参考文献:
[1] Berge N D, Reinhart D R, Dietz J D, et al. The impact of temperature and gas-phase oxygen on kinetics of in situ ammonia removal in bioreactor landfill leachate[J]. Water Research, 2007, 41(9): 1907-1914.
[2] 杨朝晖, 李晨, 曾光明, 等. 前置MAP-SBBR工艺处理早期及晚期垃圾渗滤液试验[J]. 环境工程, 2006, 24(1): 14-17.
YANG Zhao-hui, LI Chen, ZENG Guang-ming, et al.. Treatment of early and late landfill leachates by composite process of MAP-SBBR[J]. Environmental Engineering, 2006, 24(1): 14-17.
[3] 孙英杰, 徐迪民, 张隽超. 生活垃圾填埋场渗滤液中氨氮的脱除[J]. 给水排水, 2002, 28(7): 35-37.
SUN Ying-jie, XU Di-ming, ZHANG Jun-chao. Ammonia nitrogen removal from municipal refuse landfill leachate[J]. Water and Wastewater Engineering, 2002, 28(7): 35-37.
[4] 王宝贞, 王琳. 城市固体废物渗滤液处理与处置[M]. 北京: 化学工业出版社, 2005: 15-35.
WANG Bao-zhen, WANG Lin. Treatment and disposal of landfill leachate from municipal solids waste[M]. Beijing: Chemical Industry Press, 2005: 15-35.
[5] 范家明, 周少奇. 广州大田山垃圾填埋场渗滤液污染现状的调查[J]. 环境卫生工程, 2001, 9(4): 160-163.
FAN Jia-ming, ZHOU Shao-qi. Investigation on the pollution of landfill leachate in Guangzhou datianshan[J]. Environmental Sanitation Engineering, 2001, 9(4): 160-163.
[6] 周劲风, 李耀初, 张淑娟. 广州李坑垃圾填埋场水环境污染调查[J]. 上海环境科学, 1999, 18(2): 94-97.
ZHOU Jin-feng, LI Yao-chu, ZHANG Shu-juan. Investigation on water environmental pollution of landfill site Likang, Guangzhou[J]. Shanghai Environmental Sciences, 1999, 18(2): 94-97.
[7] Bohdziewics J, Bodzek M, Gorska L. Application of pressure-driven membrane techniques to biological treatment of landfill leachate[J]. Process Biochemistry, 2001, 36(7): 641-646.
[8] Liang Z, Liu J X. Landfill leachate treatment with a novel process: Anaerobic ammonium oxidation (Anammox) combined with soil infiltration system[J]. Journal of Hazardous Materials, 2008, 151(1): 202-212.
[9] Sun G Z, Austin D. Completely autotrophic nitrogen-removal over nitrite in lab-scale constructed wetlands: Evidence from a mass balance study[J]. Chemposphere, 2007, 68(6): 1120-1128.
[10] Ruiz G, Jeison D, Chamy R. Nitrification with high nitrite accumulation for the treatment of wastewater with high ammonia concentration[J]. Water Research, 2003, 37(6): 1371-1377.
[11] Chung J W,Bae W. Nitrite reduction by a mixed culture under conditions relevant to shortcut biological nitrogen removal[J]. Biodegradation, 2002, 13(3): 163-170.
[12] Ismail T, Imen S,Tarek D, et al. Coupling of anoxic and aerobic biological treatment of landfill leachate[J]. Desalination, 2009, 246: 506-513.
[13] 孙洪伟, 王淑莹, 时晓宁, 等. 单一缺氧/厌氧UASB同步反硝化产甲烷与A/O组合工艺处理实际晚期渗滤液[J]. 化工学报, 2009, 60(11): 2891-2896.
SONG Hong-wei, WANG Shu-ying, Shi Xiao-ning, et al. Single anoxic/anaerobic UASB simultaneous denitrification and methanogenesis combined with A/O for treatment of real landfill leachate[J]. Journal of Chemical Industry and Engineering (China), 2009, 60(11): 2891-2896.
[14] GB 16889—2008, 生活垃圾填埋场污染控制标准[S].
GB 16889—2008, Standard for pollution control on the landfill site of municipal solid waste[S].
[15] Yang Q, Peng Y Z, Liu X H, , et al. Nitrogen removal via nitrite from municipal wastewater at low temperatures using real-time control to optimize nitrifying communities[J]. Environmental Science and Technology, 2007, 41(23): 8159-8164.
[16] APHA. Standard methods for the examination of water and wastewater[M]. 19th ed. Washington, D C: American Public Health Association/American Water Works Association/Water Environment Federation, 1995: 10-30.
[17] 史一欣, 倪晋仁. 晚期垃圾渗滤液短程硝化影响因素研究[J]. 环境工程学报, 2007, 1(7): 110-114.
SHI Yi-xin, NI Jin-ren. Study on factors affecting shortcut nitrification of mature landfill leachate[J]. Chinese Journal of Environmental Engineering, 2007, 1(7): 110-114.
[18] 张树军, 彭永臻, 王淑莹, 等. 城市生活垃圾晚期渗滤液中氨氮的常温短程去除[J]. 化工学报, 2007, 58(4): 1042-1047.
ZHANG Shu-jun, PENG Yong-zhen, WANG Shu-ying, et al. Short-cut removal of nitrogen from mature municipal landfill leachate at ambient temperature[J]. Journal of Chemical Industry and Engineering (China), 2007, 58(4): 1042-1047.
[19] van Benthum W A J, Derissen B P, van Loosdrecht M C M, et al. Nitrogen removal using nitrifying biofilm growth and denitrifying suspended growth in a biofilm airlift suspension reactor coupled with a chemotat[J]. Water Research, 1998, 32(7): 2009-2018.
[20] 张树军, 曾薇, 彭永臻, 等. 高氮城市生活垃圾渗滤液短程生物脱氮[J]. 环境科学学报, 2006, 26(5): 751-756.
ZHANG Shu-jun, ZENG Wei, PENG Yong-zhen, et al. Nitrogen removal from high nitrogen municipal landfill leachate via nitritation and denitritation[J]. Acta Scientiae Circumstantiae, 2006, 26(5): 751-756.
[21] Kim D J, Lee D L, Keller J. Effect of temperature and free ammonia on nitrification and nitrite accumulation in landfill leachate and analysis of its nitrifying bacterial community by fish[J]. Bioresource Technology, 2005, 97(3): 459-468.
[22] 吴莉娜, 彭永臻, 王淑莹, 等. 游离氨对城市生活垃圾渗滤液短程硝化的影响[J]. 环境科学, 2008, 29(12): 135-140.
WU Li-na, PENG Yong-zhen, WANG Shu-ying, et al. Effect of free ammonia on the short-cut nitrification of the municipal landfill leachate[J]. Environmental Science, 2008, 29(12): 135-140.
[23] Anthonisen A C, Loehr R C, Prakasam T B S, et al. Inhibition of nitrification by ammonia and nitrous-acid[J]. Water Pollut Control Fed, 1976, 48(5): 835-852.
[24] Vadivelu V, Keller J, Yuan Z G. Effect of free ammonia and free nitrous acid concentration on the anabolic and catabolic processes of an enriched nitrosomonas culture[J]. Biotechnol Bioeng, 2006, 95(5): 830-839.
(编辑 赵俊)
收稿日期:2010-08-12;修回日期:2010-11-18
基金项目:北京市自然科学基金重点项目(8091001);国家自然科学基金资助项目(50978003)
通信作者:彭永臻(1949-),男,黑龙江哈尔滨人,博士,教授,从事污水生物处理理论与应用研究;电话:010-67392627;E-mail: cai@emails.bjut.edu.cn; pyz@bjut.edu.cn


