
J. Cent. South Univ. (2021) 28: 1170-1182
DOI: https://doi.org/10.1007/s11771-021-4688-8
Fe-Zn supersaturated solid solution prepared by mechanical alloying and laser sintering to accelerate degradation
YANG You-wen(杨友文)1, 2, CAI Guo-qing(蔡国庆)1, SHEN Li-da(沈理达)2,
GAO Cheng-de(高成德)3, PENG Shu-ping(彭淑平)4, 5, SHUAI Ci-jun(帅词俊)1, 3
1. Institute of Bioadaptive Manufacturing, Jiangxi University of Science and Technology,Nanchang 330013, China;
2. Jiangsu Key Laboratory of Precision and Micro-Manufacturing Technology, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, China;
3. State Key Laboratory of High Performance Complex Manufacturing, Central South University,Changsha 410083, China;
4. NHC Key Laboratory of Carcinogenesis, School of Basic Medical Science, Central South University, Changsha 410013, China;
5. School of Energy and Mechanical Engineering, Jiangxi University of Science and Technology,Nanchang 330013, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
The slow degration of iron limits its bone implant application. The solid solution of Zn in Fe is expected to accelerate the degradation. In this work, mechanical alloying (MA) was used to prepare Fe-Zn powder with supersaturated solid solution. MA significantly decreased the lamellar spacing between particles, thus reducing the diffusion distance of solution atoms. Moreover, it caused a number of crystalline defects, which further promoted the solution diffusion. Subsequently, the MA-processed powder was consolidated into Fe-Zn part by laser sintering, which involved a partial melting/rapid solidification mechanism and retained the original supersaturated solid solution. Results proved that the Fe-Zn alloy became more susceptible with a lowered corrosion potential, and thereby an accelerated corrosion rate of (0.112±0.013) mm/year. Furthermore, it also exhibited favorable cell behavior. This work highlighted the advantage of MA combined with laser sintering for the preparation of Fe-Zn implant with improved degradation performance.
Key words:
Cite this article as:
YANG You-wen, CAI Guo-qing, SHEN Li-da, GAO Cheng-de, PENG Shu-ping, SHUAI Ci-jun. Fe-Zn supersaturated solid solution prepared by mechanical alloying and laser sintering to accelerate degradation [J]. Journal of Central South University, 2021, 28(4): 1170-1182.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4688-81 Introduction
Biodegradable iron (Fe) metal shows great potential as bone implant owing to its excellent mechanical properties and good biocompatibility [1-3]. Nevertheless, it is degraded too slow in human body, which delays its further clinical application [4, 5]. Zinc (Zn) is one essential nutrient element, and has low standard electrode potential as compared with Fe [6-8]. Assuming that Zn can be dissolved into Fe lattice, the electrode potential of Fe matrix can be reduced to a certain extent, thereby accelerating its degradation. Meanwhile, the degraded Zn ions can also be expected to bring positive biological effects. Nevertheless, the melting point of Fe (1535 °C) is significantly higher than the boiling point of Zn ~907 °C [9]. It is difficult to obtain Fe-Zn alloy using traditional metallurgy smelting method, and rare Fe-Zn biometal has been reported [10].
Mechanical alloying (MA) can produce alloys from elements that possess high melting point difference at room temperature [11, 12]. Its basic principle is to utilize the mechanical force to impact the powder, and activates the chemical activity, so that the reaction usually required at high temperature can be carried out at low temperature [13]. Significantly, MA can expand the solid solubility of elements with very small solid solubility at equilibrium conditions [14]. On one hand, the lamellar spacing is significantly decreased during MA, which decreases the diffusion distance between solid atoms and accelerates the alloying process [15]. On the other hand, MA causes a lot of defects and dislocations in the particles, which reduces the energy barrier of atomic diffusion, and further provides a channel for the rapid atom diffusion [16]. Currently, a series of supersaturated solid solution alloy systems, including Fe-Cu [17], Cu-Nb [18], Ti-Al-Nb [14], have been successfully prepared using MA.
To achieve successful orthopedic application, the MA-processed powder should be developed into parts so as to obtain desirable shape and function. Laser sintering technique is an advanced forming technique which utilizes a high energy laser as heat source [19-22]. Under the irradiation of laser beam, the powder particles are partial melted and subsequently experience a rapid solidification. Significantly, such a partial melting mechanism makes most of the particles maintain in the solid state. Furthermore, the rapid solidification can effectively reduce the precipitation of Zn atoms caused by the high temperature environment [19]. Thus, it is reasonable to expect that the original supersaturated solid solution can be retained in the sintered Fe-Zn parts, thus achieving a controllable degradation.
Based on above consideration, herein, MA was used to prepare Fe-Zn powder, which was then developed into Fe-Zn implant using laser sintering. The phase composition, microstructural evolution and crystal structure of MA-processed powders were studied systematically. The forming mechanism of Fe-Zn supersaturated solid solution powder was elaborated in depth. It was focused on the degradation behaviour of laser sintered Fe-Zn part. Furthermore, the in vitro biocompatibility was also evaluated for its potential orthopedic application.
2 Material and methods
2.1 Preparation of Fe-Zn powders
The irregularly shaped Fe powder (99% purity, ~20 μm) and Zn powder (99% purity, ~15 μm) were obtained from Shanghai Naiou Nanotechnology Co., Ltd. (China), as shown in Figures 1(a) and (b), respectively. The Fe and Zn mixture containing 20 wt% of Zn was used for MA on a miniature planetary ball mill (PULVERISETTE 6, Germany) at room temperature. Three kinds of balls (d=15, 10 and 5 mm, respectively) were used. The container had a diameter of 250 mm. The mixed powder was milled at a rotation rate of 300 r/min with a powder-to-ball mass ratio of 1:15, and the rotation direction was changed every 30 min with a suspension of 15 min to cool down the powders. Anhydrous ethanol was used as the process control agent to reduce the agglomeration of the powder and the damage of the grinding medium. The milling time was determined at 10, 20, 30 and 40 h, respectively. Before operation, the sealed vials were vacuumed and filled with argon (99.999% purity) to avoid the oxidation. After ball milling, the obtained powders were observed using a transmission electron microscope (TEM, Tecnai G2, FEI, USA) at 300 kV. The phase composition of as-milled powder was determined utilizing an X-ray diffractometer (XRD, D8 Advance, Karlsruhe, Germany) with Cu Kα radiation.
2.2 Laser sintering of Fe-Zn parts
The as-milled powders were used for laser sintering experiment. The laser sintering system consisted of a fiber laser (IPG, 500 W, Germany), a scanning galvanometer, a building chamber filled with argon (99.999% purity). A laser scanning strategy that scanned in x-direction in one layer and then turned 90° in next layer was used. After a series of pre-experiments, cubic parts (8 mm×8 mm×8 mm) were built under optimized parameters as follows: laser power 120 W, scanning rate 200 mm/s, hatching space 50 μm and layer thickness 50 μm.
2.3 Microstructural characterization
The surface of laser-sintered parts was observed utilizing a scanning electron microscope (SEM, EVO 18, Zeiss, Germany). The surface was polished and then etched using 5% nitric acid alcohol solution. The microstructure was observed by an optical microscope (OM, Leica, DM2700M, Germany). Meanwhile, the microstructure was investigated using SEM combined with an energy dispersive spectrometer (EDS, X-Max 20, Oxford Instruments, UK).
2.4 Electrochemical and immersion tests
Electrochemical tests were conducted using an electrochemical working system (CHI604D, CH Instruments Ins., China), in which the three- electrode cell was composed of a platinum counter- electrode, a saturated calomel electrode and a working electrode. Simulated body fluid (SBF) served as the electrolyte, which contained 142.0 mmol/L Na+, 5.0 mmol/L K+, 1.5 mmol/L Mg2+, 2.5 mmol/L Ca2+, 125.0 mmol/L Cl-, 327.0 mmol/L HCO3-, 41.0 mmol/L HPO2- 4 and 40.5 mmol/L SO2- 4. The temperature was regulated at 37 °C using the water bath. The Tafel polarization tests were carried out at a scanning rate of 0.05 mV/s. The Nyquist plots and electrochemical impedance were tested ranging from 100 kHz to 10 mHz.
Immersion tests were performed to further investigate the degradation behaviour according to ASTM G31-72 [23]. The specimens soaked in SBF at 28 d were washed with distilled water. The corrosion surface was observed by SEM. Meanwhile, the mass loss was recorded after removing the corrosion product using a solution containing CrO3 and AgNO3. The corrosion rate (R) was calculated by:
R=W/(AT) (1)
where W is mass loss; A is exposure area; T is soaking time.
2.5 Cell experiments
MG-63 cells (American Type Culture Collection, Rockville, USA) were used in cell experiments. They were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% of fetal bovine serum, 100 U/mL penicillin and 100 mg/mL streptomycin at 37 °C. According to ISO 10993-5 standard, samples were sterilized and then immersed in culture medium for 3 d (1 mL/cm2) to obtain the extracts [24]. The cells were incubated in a 96-well plate containing culture medium for 1 d, and then replaced by 100 μL of extracts. After 1, 3 and 5 d, 10 μL of cell counting kit-8 (CCK-8) reagent was added with a further incubation of 3 h. Subsequently, the absorbance was recorded by a microplate reader. The fluorescence staining was also carried out to estimate the cell viability. After incubation for 1, 3 and 5 d, the cells were stained by using calcein-AM and ethidium homodimer-1 reagent, which were then visualized by a fluorescence microscope (BX60, Olympus, Japan). Besides, the differentiation behaviour of cells after culture for 7 d was estimated by alkaline phosphatase activity (ALP) staining. The stained cells were observed using the fluorescence microscope.
2.6 Statistical analysis
All the experiments were carried out at least three times to obtain the averages. SPSS soft were was used to analyze the significant difference. P<0.05 was recognized to be of statistical difference.
3 Results and discussion
3.1 Microstructural evolution of as-milled powders
The as-milled Fe-Zn powders were observed by SEM, as shown in Figure 2. Under the effect of exerted mechanical force, the particle shape was significantly altered. In detail, the as-milled powders got flattened and consequently generated a number of new surfaces at initial stage, which enabled them to be welded together, as presented in Figure 2(a). As milling progressed, continuous collision and deformation resulted in the fracture of the powders because of the fatigue failure, which significantly reduced the particle sizes and inter-layer space, as presented in Figures 2(b) and (c). Such a reduced lamellar spacing was believed to be favorable for the diffusion of solution atoms. Nevertheless, cold welding occurred with milling time further extending to 40 h. In this condition, a balance state between fracturing and welding was achieved, which in turn increased the particles size in some extent, as shown in Figure 2(d).
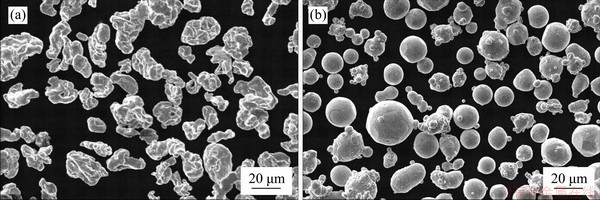
Figure 1 SEM microstructure of original Fe (a) and Zn (b) powder

Figure 2 SEM microstructures of as-milled Fe-Zn powder after milling for 10 h (a), 20 h (b), 30 h (c) and 40 h (d)
The XRD patterns of as-milled Fe-Zn powder with different milling time are depicted in Figure 3. The principal diffraction peaks of Fe and Zn were clearly observed after milling for 10 h. With the milling time extending, the peak intensity of Zn continuously decreased (Figure 3(b)), which indicated that partial Zn dissolved in the Fe matrix during milling. Meanwhile, the crystalline peaks of Fe continuously turned weak and broad after milling for 30 and 40 h. Thus, it was reasonable to deduce that MA effectively broke the original crystal structure and led to the grain refinement. A close observation further revealed that the dominant crystalline peak of Fe gradually shifted to low location (Figure 3(c)), which was believed to be related with the lattice distortion caused by the solid solution of Zn in Fe matrix.
The crystallite size (d) of the as-milled powders was quantitatively calculated using Scherer formulas, as expressed by [25]:
d=0.89λ/(Bcosθ) (2)
where d is the crystallite size; λ is the wavelength; B is the full width at half maximum intensity and θ is the Bragg angle. The calculated crystallite sizes are listed in Table 1. It was indicated that the crystallite size of Fe phase gradually decreased to ~8 nm after milling for 30 h, which revealed that nanocrystalline structure was achieved after MA operation. As milling time further prolonged to 40 h, no obvious variation in the crystallite sizes was found. Previous SEM observation proved that cold welding occurred at 40 h, which should counteract the grain refinement effect. The milling operation also generated the lattice strain (η), which could be calculated using Williamson-Hall equation [26]:
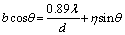 (3)
(3)
As presented in Table 1, the lattice strain of Fe phases increased gradually as the milling time increased. It was believed that a large amount of Zn dissolved in Fe matrix during MA, thus contributing to a severe lattice distortion.
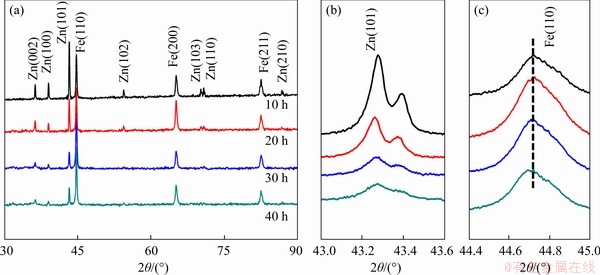
Figure 3 Overall XRD patterns of as-milled Fe-Zn powder obtained at a scanning rate of 8°/min (a); Local XRD spectrum obtained at 2°/min (b, c)
Table 1 Calculated crystallite size and lattice strain of Fe phase derived from XRD patterns
The microstructure of Fe-Zn powder milling for 30 h was further investigated using TEM, with results exhibited in Figure 4. The grain sizes of as-milled powder were less than 10 nm, as shown in Figure 4(a), which was in line with previous XRD analysis. The characteristic Debye-Scherrer rings of body-centered cubic (BCC) structure and hexagonal close-packed (HCP) structure were detected, as shown in Figure 4(b), which corresponded to the lattice structure of Fe and Zn, respectively. Notably, BCC-Fe presented in this work showed an enlarged interplanar spacing of 1.43  as compared with normal BCC-Fe (200). The HRTEM image and corresponding fast Fourier transform (FTT) images are presented in Figures 4(c) and (d). In area A, the atom lattice above the interface showed an interplanar spacing of 1.42
as compared with normal BCC-Fe (200). The HRTEM image and corresponding fast Fourier transform (FTT) images are presented in Figures 4(c) and (d). In area A, the atom lattice above the interface showed an interplanar spacing of 1.42  , which was in line with the BCC-Fe. Significantly, a large number of lattice dislocations and distortions were observed below the interface (as marked by red arrows), which was caused by the solid solution of Zn. In area B, the atom lattice arranged in HCP structure and the interplanar spacing was 2.75
, which was in line with the BCC-Fe. Significantly, a large number of lattice dislocations and distortions were observed below the interface (as marked by red arrows), which was caused by the solid solution of Zn. In area B, the atom lattice arranged in HCP structure and the interplanar spacing was 2.75 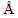 , which belonged to the (110) plane of Zn nanocrystals. Meanwhile, a large number of dislocations were observed in Zn nanocrystals. Similarly, the lattice distortions also appeared in area C. These TEM observations confirmed that Fe-Zn supersaturated solid solution formed after MA.
, which belonged to the (110) plane of Zn nanocrystals. Meanwhile, a large number of dislocations were observed in Zn nanocrystals. Similarly, the lattice distortions also appeared in area C. These TEM observations confirmed that Fe-Zn supersaturated solid solution formed after MA.
In the present work, XRD together with TEM analysis proved that Fe-Zn supersaturation solid solution was successfully prepared using MA. In general, MA involved cycled deforming, fracturing and welding of particles. In this process, the crystalline was effectively refined, which refined the solute phase and matrix phase. Consequently, the grain surface area per unit volume increased rapidly, thus obtaining extremely high interfacial energy, which was beneficial to the solute diffusion. Furthermore, MA produced a large number of local stresses and thereby generated a large number of structural defects or dislocations in the particles, as presented in Figures 4(d, e, f). Under the dislocation strain field, solute atoms would accumulate near the dislocation. Thereby, solute atoms gradually diffused into the grains accompanied with the continuous dislocation movement, and finally obtained supersaturated solid solution. In short, MA as one kind of compulsory process realized the preparation of Fe-Zn supersaturated solid solution.
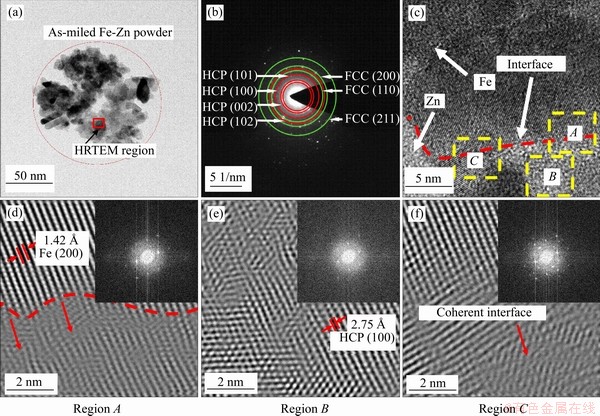
Figure 4 TEM (a), Debye-Scherrer rings (b) and high resolution TEM micrographs (c) of as-milled Fe-Zn powder; (d, e, f) FFT images showing the different regions marked in Figure 4(c)
3.2 Microstructure feature of laser-sintered parts
The Fe-Zn powder after milling for 30 h with a large amount of Zn dissolving in Fe matrix was used to develop into parts via laser sintering. The general view of laser-sintered parts and their representative surface is shown in Figure 5(a). Laser sintering which used a partial melting mechanism was adopted in this study. Under the irradiation of laser beam, the milled powders were partially melted, which was expected to maintain the original nonequilibrium phase. As observed by the SEM, the particles bonded together via sintering neck due to the limited liquid phase, showing typical sintering forming characteristics. After polishing, the microstructure of laser sintered Fe-Zn parts was captured by OM, as shown in Figure 5(b), and the laser-sinter Fe was used as control. It was found that refined crystalline structure was observed in Fe-Zn matrix as compared with Fe. A close SEM observation was carried out to further investigate the microstructure of Fe-Zn, as shown in Figures 5(e) and (f). The EDS results indicated nearly 10.2 wt.% of Zn retained in the Fe matrix, which indicated that supersaturated solid solution was maintained after laser sintering.
In the present study, a partial melting/ solidification mechanism was used to build parts. Assuming that Fe-Zn powder was completely melted, the bath temperature should be higher than the Fe melting point of 1535 °C. In this case, Zn metal would be gasified owing to its low gasification point (907 °C). Thus, we determined to partially melt the Fe-Zn powder by optimizing the laser parameters. During sintering, a small amount of liquid phase formed and wetted the particles, thus promoting the rearrangement of particles and finally realizing the densification behavior [27]. As shown in Figure 5(b), no pores or cracks appeared in the matrix, which proved its favorable formability. More significantly, a partial melting/ solidification mechanism avoided ruining the Fe-Zn supersaturated solid solution, since most of the particles remained in the solid phase during sintering. As the laser beam left, the molten pool subsequently underwent an extremely rapid solidification, which could also inhibit the precipitation of Zn. Thus, the Fe-Zn supersaturated solid solution was retained in the sintered parts, which was expected to regulate the degradation behavior.
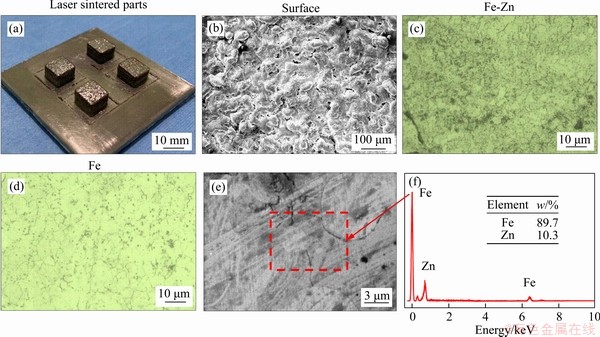
Figure 5 Laser-sintered Fe-Zn parts (a) and the typical surface texture (b); (c, d) OM images showing the crystal structure of Fe-Zn and Fe parts: (e) SEM microstructure of sintered Fe-Zn (e) and corresponding EDS pattern (f)
3.3 Degradation behavior of laser-sintered parts
It is well known that biodegradable Fe is degraded by means of electrochemical corrosion mechanism. Thus, electrochemical tests were used to evaluate the corrosion behavior of the Fe-Zn, with Fe as control. The recorded Tafel curves were depicted in Figure 6(a). It could be clearly seen that the Ecorr was negatively shifted for Fe-Zn. Meanwhile, Fe-Zn also exhibited a significantly increased Icorr as compared with Fe. Specifically, the Ecorr and Icorr were (-1.3±0.2) V and (25.3±0.9) μA/cm2 for Fe-Zn, and (-0.8±0.1) V and (7.7±0.4) μA/cm2 for Fe, respectively. From the perspective of electrochemical kinetics, the reduced Ecorr and enhanced Icorr indicated the high electrochemical activity of Fe-Zn as compared with Fe [28-30].
The Nyquist plots obtained from EIS measurements are shown in Figure 6(b). It could be seen that the diameter of the capacitive loop was decreased for Fe-Zn, which also indicated the reduced corrosion resistance. The fitted equivalent circuit is displayed in Figure 6(c), which was expressed by Rs(Qdl(Rct(QpRp))). Herein, Rs represented the electrolyte resistance; Qdl represented the double layer capacitance; Rct represented the interfacial charge transfer resistance; whereas Qp and Rp represented the capacitance and resistance associate with the surface product layer, respectively. The calculated parameters of equivalent circuit are listed in Table 2. Fe-Zn possessed relatively small Rct and Rp as compared with Fe, which further indicated its reduced corrosion resistance. The Bode diagrams are shown in Figure 6(d). In the high-frequency region, both Fe-Zn and Fe showed close impedance |Z| and phase angles, which proved no significant difference for their electrolyte resistance. However, in the low and intermediate frequency region (0.1-1000Hz), the impedance |Z| and phase angles of Fe-Zn were lower than those of Fe, which represented that Fe-Zn possessed a low stability of passive film and resulted in a weakened corrosion barrier to protect the substrate during tests.
Figure 6 Tafel curves (a), Nyquist EIS spectra (b), fitted equivalent circuit (c), bode diagrams (d) of Fe-Zn and Fe in SBF solution
Table 2 Fitted results of electrochemical impedance spectra

Immersion tests were used to further investigate the degradation behavior. After immersion in SBF for 28 d, the corrosion surface was observed by SEM, as exhibited in Figure 7. Fe part presented an integrated surface, and scratches left by polishing could be observed, indicating its slight corrosion (Figure 7(a)). As a comparison, a large amount of corrosion product covered on Fe-Zn part, which revealed that it experienced severe corrosion (Figure 7(b)). The corresponding EDS results showed that the corrosion product mainly consisted of Fe oxide and Zn oxide. Besides, a few Ca-P products were detected on the surface, which should be deposited from the SBF. Mass loss was determined during immersion tests (Figure 7(d)). Fe possessed a slow degradation rate of (0.047±0.008) mm/year, which was consistent with previously researches[31]. Nevertheless, Fe-Zn part exhibited a significantly improved degradation rate of (0.112±0.013) mm/year. Usually, the bone healing cycle ranges from 3 to 6 months, which demands a moderate degradation rate of (0.1-0.2) mm/year for bone implant [32, 33]. From this perspective, Mg alloy exhibited too fast degradation, whereas Zn alloy, and the Fe-Zn alloy presented in this work are more suitable as temporary bone substitute.
It was well accepted that the corrosion behavior of metal materials was highly related with their electrochemical stability. A high electrode potential with low corrosion thermodynamic free energy generally represented the poor corrosion resistance. However, it had been proved that solid solution of elements was able to regulate the electrochemical stability [37, 38]. For instance, the solid solution of Ga in Fe matrix decreased the corrosion potential of Fe matrix, thus enhancing the degradation [39]. In the present work, Zn with relatively negative electrochemical potential was used as solution element. As expected, the Fe-Zn solid solution possessed a reduced electrical potential and an enhanced degradation rate, as proved by our electrochemical and immersion tests. Usually, long-term immersion tests were believed to offer more accurate characterization of the degradation behavior [40]. A large number of studies had assessed the degradation rate of Fe by immersion tests, and also reported the slow degradation [3, 41]. Nevertheless, Fe-Zn part in this work showed a remarkable enhanced degradation rate. The corrosion potential of Fe matrix was lowered by the solid solution of zinc, which was the main reason for accelerated degradation. Besides, the remained Fe or Zn phase would form galvanic corrosion against Fe-Zn solid solution, which also contributed to an accelerated degradation.
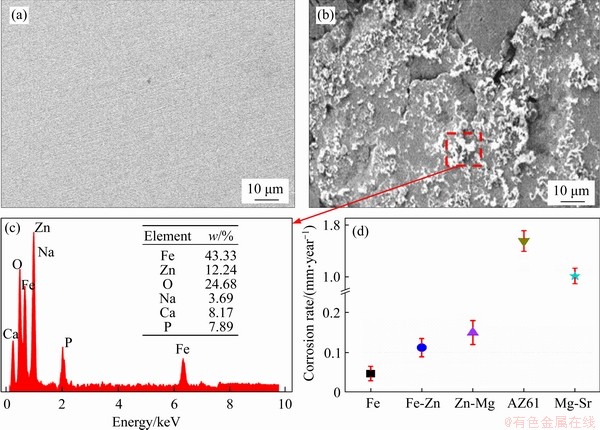
Figure 7 SEM images showing corrosion surface of Fe (a) and Fe-Zn (b) after immersion for 28 d; (c) EDS showing elemental composition of corrosion products on Fe-Zn; (d) Comparison of corrosion rate between Fe, Fe-Zn, and other biodegradable metals, including Zn-Mg [34], AZ61 [35], Mg-Sr [36]
3.4 In vitro cytotoxicity
Bone implants require good biocompatibility to ensure the safety of the host [42, 43]. The cytotoxicity of laser sintered Fe-Zn and Fe part was evaluated using indirect cell tests. The cells after culture in as-prepared extracts for 1, 3 and 5 d were stained and observed using fluorescence microscopy, as presented in Figure 8(a). In general, very few dead cells appeared throughout the whole culture period, and the cell number was enhanced with culture time increasing. Specifically, the cells exhibited round and unhealthy morphology at day 1. However, at day 3, the cells extended and showed a typical fusiform shape. It was believed that the cells adapted to the new environment with high ion concentration and recovered. As culture time further extended to day 5, the cells developed cytoplasmic extensions and formed some junctions across each other.
CCK-8 assay was used to quantitatively investigate the cytotoxicity, with results shown in Figure 8(b). The culture medium without corrosion extract was used as control. At day 1, the cells exhibited relatively low viability, only 78.3% for Fe and 81.2% for Fe-Zn, which proved that the 100% extracts reduced the cell ability in some extent owing to the concentration degradation product, namely Fe and Zn ions. In fact, the local high ion concentration in vivo would be relieved, due to the dynamic cycling environment [44]. As culture time gradually extending, both two groups exhibited increased cell viability higher than 80%, which indicated their accepted cytotoxicity. It should be noted that the Fe-Zn group showed relative high cell viability than Fe group at day 5. The ALP activity of MG-63 cells cultured for 5 d was investigated, as shown in Figure 8(c). Similarly, the cells cultured on the Fe-Zn extracts presented intensified ALP staining as compared that cultured in Fe extract, which indicated the improved cells differentiation. Together with above cell tests, it was revealed that Fe-Zn extract was more beneficial for cell proliferation and differentiation. There was no doubt that the degraded Zn ion exerted some positive effect on cell behavior. Zn is one essential nutrient element, and its recommend daily intake is almost 10-15 mg [45]. It participates in synthesis of several nucleic acid and protein. Some researchers had reported the positive influence of Zn for cell growth [46-48].
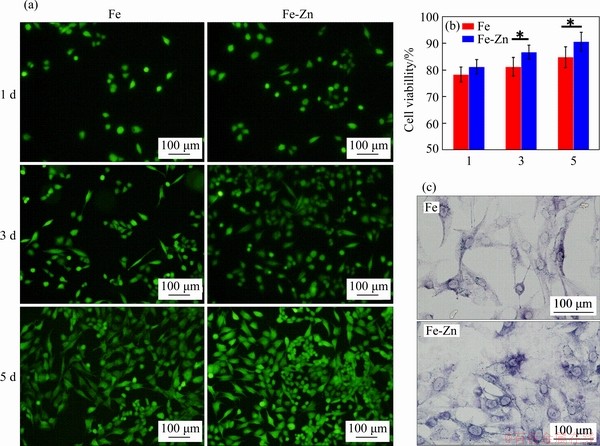
Figure 8 (a) Fluorescent images of cell after culture in extracts for 1, 3, 5 d, in which the green represented live cells; (b) CCK-8 assay; (c) ALP Fluorescent images (N=3, *P<0.05)
4 Conclusions
In this study, mechanical alloying (MA) was firstly used to prepare Fe-Zn powder. Under the impact of mechanical force, the grains were refined and a large number of defects were introduced, which promoted the diffusion of solute atoms. Thus, a large amount of Zn dissolved in Fe matrix, forming Fe-Zn supersaturated solid solution. The MA-processed powder was developed into Fe-Zn part by laser sintering, which retained the original supersaturated solid solution owing to its partial melting/solidification mechanism. The electrochemical tests confirmed that the Fe-Zn part exhibited a reduced corrosion potential of (-1.3±0.2) V as compared with Fe. Thereby, it presented an accelerated degradation rate of (0.112±0.013) mm/year. In vitro cell tests also revealed its improved cell behavior due to the released nutrient element of Zn. This study demonstrated that MA combined with laser sintering showed great potential in fabricating non-equilibrium Fe-Zn alloy as bone implant.
Contributors
The overarching research goals were developed by YANG You-wen, PENG Shu-ping, and SHUAI Ci-jun. CAI Guo-qing and SHEN Li-da provided the measured landslides displacement data, and analyzed the measured data. CAI Guo-qing and GAO Cheng-de analyzed the calculated results. The initial draft of the manuscript was written by YANG You-wen and CAI Guo-qing.
Conflict of interest
YANG You-wen, CAI Guo-qing, SHEN Li-da, GAO Cheng-de, PENG Shu-ping, and SHUAI Ci-jun declare that they have no conflict of interest.
References
[1] GOREJOVA R, HAVEROVA L, ORINAKOVA R, ORINAK A, ORINAK M. Recent advancements in Fe-based biodegradable materials for bone repair [J]. Journal of Materials Science, 2019, 54: 1913-1947. DOI: 10.1007/s10853-018-3011-z.
[2] DREVET R, ZHUKOVA Y, MALIKOVA P, DUBINSKIY S, KOROTITSKIY A, PUSTOV Y, PROKOSHKIN S. Martensitic transformations and mechanical and corrosion properties of Fe-Mn-Si alloys for biodegradable medical implants [J]. Metallurgical and Materials Transactions A, 2018, 49: 1006-1013. DOI: https://doi.org/10.1007/s11661- 017-4458-2.
[3] YANG Chen, HUAN Zhi-guang, WANG Xiao-ya, WU Cheng-tie, CHANG Jiang. 3D printed Fe scaffolds with HA nanocoating for bone regeneration [J]. ACS Biomaterials Science & Engineering, 2018, 4: 608-616.
[4] CARLUCCIO D, DEMIR A G, CAPRIO L, PREVITALI B, BERMINGHAM M J, DARGUSCH M S. The influence of laser processing parameters on the densification and surface morphology of pure Fe and Fe-35Mn scaffolds produced by selective laser melting [J]. Journal of Manufacturing Processes, 2019, 40: 113-121. DOI: https://doi.org/ 10.1016/j.jmapro.2019.03.018.
[5] SHUAI Ci-jun, LI Sheng, YANG Wen-jing, YANG You-wen, DENG You-wen, GAO Cheng-de. MnO2 catalysis of oxygen reduction to accelerate the degradation of Fe-C composites for biomedical applications [J]. Corrosion Science, 2020: 108679. DOI: https://doi.org/10.1016/j.corsci.2020.108679.
[6] ZHENG Yu-feng, GU Xue-nan, WITTE F. Biodegradable metals [J]. Materials Science and Engineering: R: Reports, 2014, 77: 1-34. DOI: https://doi.org/10.1016/j.mser.2014. 01.001.
[7] WANG Yun-yan, PENG Wen-jie, CHAI Li-yuan. Electrochemical behaviors of Zn-Fe alloy and Zn-Fe-TiO2 composite electrodeposition [J]. Journal of Central South University of Technology, 2003, 10: 183-189.
[8] HUANG He, LIU Huan, WANG Li-sha, LI Yu-hua, AGBEDOR S O, BAI Jing, XUE Feng, JIANG Jing-hua. A high-strength and biodegradable Zn–Mg alloy with refined ternary eutectic structure processed by ECAP [J]. Acta Metallurgica Sinica, 2020: 1-10. DOI: https://doi.org/ 10.1007/s40195-020-01027-x.
[9] HOSOKAWA S, INUI M, MATSUDA K, ISHIKAWA D, BARON A Q R. Damping of the collective modes in liquid Fe [J]. Physical Review B, 2008, 77: 174203. DOI: https://doi.org/10.1103/PhysRevB.77.174203.
[10] HE Jin, LI Da-wei, HE Feng-li, LIU Yang-yang, LIU Ya-li, ZHANG Chen-yan, REN Fu-zeng, YE Ya-jing, DENG Xu-dong, YIN Da-chuan. A study of degradation behaviour and biocompatibility of ZnFe alloy prepared by electrodeposition [J]. Materials Science and Engineering C. 2020: 111295. DOI: https://doi.org/10.1016/j.msec.2020. 111295.
[11] IBRAHIM N, PETERLECHNER M, EMEIS F, WEGNER M, DIVINSKI S V, WILDE G. Mechanical alloying via high-pressure torsion of the immiscible Cu50Ta50 system [J]. Materials Science and Engineering A, 2017, 685: 19-30. DOI: https://doi.org/10.1016/j.msea.2016.12.106.
[12] JOO S H, KATO H, JANG M J, MOON J, KIM E B, HONG S J, KIM H S. Structure and properties of ultrafine-grained CoCrFeMnNi high-entropy alloys produced by mechanical alloying and spark plasma sintering [J]. Journal of Alloys and Compounds, 2017, 698: 591-604. DOI: https://doi.org/ 10.1016/j.jallcom.2016.12.010
[13] DAI Xiao-qin, XIE Min, ZHOU Sheng-feng, WANG Chun-xia, GU Meng-hao, YANG Jiao-xi, LI Zheng-yang. Formation mechanism and improved properties of Cu95Fe5 homogeneous immiscible composite coating by the combination of mechanical alloying and laser cladding [J]. Journal of Alloys and Compounds, 2018, 740: 194-202. DOI: https://doi.org/10.1016/j.jallcom.2018.01.007.
[14] JIA Jian-bo, SUN Wei, PENG Wei-jin, YANG Zhi-gang, XU Yan, ZHONG Xiao-xiao, LIU Wen-chao, LUO Jun-ting. Preparation of Ti-22Al-25Nb solid solution powders using mechanical alloying and solid solution mechanism analysis [J]. Advanced Powder Technology, 2020. DOI: https://doi.org/10.1016/j.apt.2020.02.029.
[15] ANAS N S, RAMAKRISHNA M, DASH R K, RAO T N, VIJAY R. Influence of process control agents on microstructure and mechanical properties of Al alloy produced by mechanical alloying [J]. Materials Science and Engineering A, 2019, 751: 171-182. DOI: https://doi.org/ 10.1016/j.msea.2019.02.060.
[16] ROSTAMI A, BAGHERI G A, SADRNEZHAAD S K. Microstructure and thermodynamic investigation of NiTi system produced by mechanical alloying [J]. Physica B: Condensed Matter, 2019, 552: 214-220. DOI: https://doi.org/10.1016/j.physb.2018.10.015.
[17] RABIEE M, MIRZADEH H, ATAIE A. Processing of Cu-Fe and Cu-Fe-SiC nanocomposites by mechanical alloying [J]. Advanced Powder Technology, 2017, 28: 1882-1887. DOI: https://doi.org/10.1016/j.apt.2017. 04.023.
[18] LEI Ruo-shan, WANG Ming-pu, WANG Huan-ping, XU Shi-qing. New insights on the formation of supersaturated Cu-Nb solid solution prepared by mechanical alloying [J]. Materials Characterization, 2016, 118: 324-331. DOI: https://doi.org/10.1016/j.matchar.2016.06.013.
[19] FINA F, MADLA C M, GOYANES A, ZHANG Jia-xin, GAISFORD S, BASIT A W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering [J]. International Journal of Pharmaceutics, 2018, 541: 101-107. DOI: https://doi.org/ 10.1016/j.ijpharm.2018.02.015.
[20] CHEN Jie, YANG Yong-qiang, SONG Chang-hui, ZHANG Ming-kang, WU Shi-biao, WANG Di. Interfacial microstructure and mechanical properties of 316L/CuSn10 multi-material bimetallic structure fabricated by selective laser melting [J]. Materials Science and Engineering A, 2019, 752: 75-85. DOI: https://doi.org/10.1016/j.msea.2019. 02.097.
[21] WANG Di, WANG Yi-meng, YANG Yong-qiang, LU Jian-bin, XU Zhen-long, LI Sheng, LIN Kang-jie, ZHANG Dong-yun. Research on design optimization and manufacturing of coating pipes for automobile seal based on selective laser melting [J]. Journal of Materials Processing Technology, 2019, 273: 116227. DOI: https://doi.org/ 10.1016/j.jmatprotec.2019.05.008.
[22] YANG You-wen, CHENG Yun, PENG Shu-ping, XU Liang, HE Chong-xian, QI Fang-wei, ZHAO Ming-chun, SHUAI Ci-jun. Microstructure evolution and texture tailoring of reduced graphene oxide reinforced Zn scaffold [J]. Bioactive Materials, 2021, 6: 1230-1241. DOI: https://doi.org/10.1016/ j.bioactmat.2020.10.017.
[23] Testing American Society for Materials. ASTM G31-72: Standard practice for laboratory immersion corrosion testing of metals [S]. ASTM, 2004.
[24] International Organization for Standardization, Geneva. 10993–5: 2009. Biological evaluation of medical devices—Part 5: Tests for in vitro Cytotoxicity [S]. 2009.
[25] E Dian-yu. Validation of CFD–DEM model for iron ore reduction at particle level and parametric study [J]. Particuology, 2020, 51: 163-172. DOI: https://doi.org/ 10.1016/j.partic.2019.10.008.
[26] KIBASOMBA P M, DHLAMINI S, MAAZA M, LIU Chuan-Pu, RASHAD M M, RAYAN D A, MWAKIKUNGA B W. Strain and grain size of TiO2 nanoparticles from TEM, Raman spectroscopy and XRD: The revisiting of the Williamson-Hall plot method [J]. Results in Physics, 2018, 9: 628-635. DOI: https:// doi.org/10.1016/j.rinp.2018.03.008.
[27] GU Dong-dong, SHEN Yi-fu, FANG Shang-qing, XIAO Jun. Metallurgical mechanisms in direct laser sintering of Cu–CuSn–CuP mixed powder [J]. Journal of Alloys and Compounds, 2007, 438: 184-189. DOI: https://doi.org/ 10.1016/j.jallcom.2006.08.040.
[28] VETTER K J. Electrochemical kinetics: Theoretical aspects [M]. Elsevier, 2013.
[29] AFSHARI V, DEHGHANIAN C. Effects of grain size on the electrochemical corrosion behaviour of electrodeposited nanocrystalline Fe coatings in alkaline solution [J]. Corrosion Science, 2009, 51: 1844-1849. DOI: https://doi.org/10.1016/j.corsci.2009.05.015.
[30] WANG Y, JIANG SL, ZHENG Y G, KE W, SUN W H, WANG J Q. Electrochemical behaviour of Fe-based metallic glasses in acidic and neutral solutions [J]. Corrosion science, 2012, 63: 159-173. DOI: https://doi.org/10.1016/j.corsci. 2012.05.025.
[31] HERMAWAN H, PURNAMA A, DUBE D, COUET J, MANTOVANI D. Fe–Mn alloys for metallic biodegradable stents: degradation and cell viability studies [J]. Acta Biomaterialia, 2010, 6: 1852-1860. DOI: https://doi.org/ 10.1016/j.actbio.2009.11.025.
[32] GAO Cheng-de, LI Sheng, LIU Long, BIN Shi-zhen, YANG You-wen, PENG Shu-ping, SHUAI Ci-jun. Dual alloying improves the corrosion resistance of biodegradable Mg alloys prepared by selective laser melting [J]. Journal of Magnesium and Alloys, 2020. DOI: https://doi.org/ 10.1016/j.jma.2020.03.016.
[33] SHUAI Ci-jun, WANG Bing, BIN Shi-zhen, PENG Shu-ping, GAO Cheng-de. TiO2-induced in situ reaction in graphene oxide-reinforced AZ61 biocomposites to enhance the interfacial bonding [J]. ACS Applied Materials & Interfaces, 2020, 12: 23464-23473. DOI: https://doi.org/10.1021/acsami. 0c04020.
[34] XIAO Chi, WANG Li-qing, REN Yu-ping, SUN Shi-neng, ZHANG Er-lin, YAN Chong-nan, LIU Qi, SUN Xiao-gang, SHOU Fen-yong, DUAN Jing-zhu. Indirectly extruded biodegradable Zn-0.05 wt% Mg alloy with improved strength and ductility: In vitro and in vivo studies [J]. Journal of Materials Science & Technology, 2018, 34: 1618-1627. DOI: https://doi.org/10.1016/j.jmst.2018.01.006.
[35] LIU Long, MA Hao-tian, GAO Cheng-de, SHUAI Ci-jun, PENG Shu-ping. Island-to-acicular alteration of second phase enhances the degradation resistance of biomedical AZ61 alloy [J]. Journal of Alloys and Compounds, 2020: 155397. DOI: https://doi.org/10.1016/j.jallcom.2020.155397.
[36] GU X N, XIE X H, LI N, ZHENG Y F, QIN L. In vitro and in vivo studies on a Mg–Sr binary alloy system developed as a new kind of biodegradable metal [J]. Acta Biomaterialia, 2012, 8: 2360-2374. DOI: https://doi.org/10.1016/j.actbio. 2012.02.018.
[37] LIU Huan, SUN Chao, WANG Ce, LI Yu-hua, BAI Jing, XUE Feng, MA Ai-bin, JIANG Jing-hua. Improving toughness of a Mg2Ca-containing Mg-Al-Ca-Mn alloy via refinement and uniform dispersion of Mg2Ca particles [J]. Journal of Materials Science, 2020, 59: 61-71. DOI: https://doi. org/10.1016/j.jmst.2020.02.092.
[38] HUANG He, LIU Huan, WANG Li-sha, LI Yu-hua, AGBEDOR S O, BAI Jing, XUE Feng, JIANG Jing-hua. A high-strength and biodegradable Zn–Mg alloy with refined ternary eutectic structure processed by ECAP [J]. Acta Metallurgica Sinica (English Letters), 2020, 33: 1191-1200. DOI: 10.1007/s40195-020- 01027-x.
[39] WANG He-nan, ZHENG Yang, LIU Jing-hua, JIANG Cheng-bao, LI Yan. In vitro corrosion properties and cytocompatibility of Fe-Ga alloys as potential biodegradable metallic materials [J]. Materials Science and Engineering C, 2017, 71: 60-66. DOI: https://doi.org/10.1007/s40195-020- 01027-x.
[40] LIN Wei-chun, YAO Chen-min, HUANG Ting-yun, CHENG Shih-jung, TANG Cheng-ming. Long-term in vitro degradation behavior and biocompatibility of polycaprolactone/cobalt-substituted hydroxyapatite compo- site for bone tissue engineering [J]. Dental Materials, 2019, 35: 751-762. DOI: https://doi.org/10.1016/j.dental.2019.02. 023.
[41] CARLUCCIO D, XU Chun, VENEZUELA J, CAO Yu-xue, KENT D, BERMINGHAM M, DEMIR A G, PREVITALI B, YE Qing-song, DARGUSCH M. Additively manufactured iron-manganese for biodegradable porous load-bearing bone scaffold applications [J]. Acta Biomaterialia, 2020, 103: 346-360. DOI: https://doi.org/10.1016/j.actbio.2019.12.018.
[42] ZOU An-chao, LIANG Hui-xin, JIAO Chen, GE Meng-xing, YI Xin-yu, YANG You-wen, SUN Jun, WANG Chang-jiang, SHEN Li-da, LI Yao. Fabrication and properties of CaSiO3/Sr3(PO4)2 composite scaffold based on extrusion deposition [J]. Ceramics International, 2020. DOI: https:// doi.org/10.1016/j.ceramint.2020.10.048.
[43] WANG Guo-yong, QIAN Guo-wen, ZAN Jun, QI Fang-wei, ZHAO Zheng-yu, YANG Weng-jing, PENG Shu-ping, SHUAI Ci-jun. A co-dispersion nanosystem of graphene oxide @silicon-doped hydroxyapatite to improve scaffold properties [J]. Materials & Design, 2021, 199: 109399. DOI: https://doi.org/10.1016/j.matdes.2020.109399.
[44] SHUAI Ci-jun, ZAN Jun, DENG Fang, YANG You-wen, PENG Shu-ping, ZHAO Zhen-yu. Core-shell structured ZIF-8@PDA-HA with controllable zinc ion release and superior bioactivity for improving poly-l-lactic acid scaffold [J]. ACS Sustainable Chemistry & Engineering, 2021, 9: 1814-1825. DOI: https://doi.org/10.1021/acssuschemeng. 0c08009.
[45] GARCíA-RICO L, LEYVA-PEREZ J, JARA-MARINI M E. Content and daily intake of copper, zinc, lead, cadmium, and mercury from dietary supplements in Mexico [J]. Food and Chemical Toxicology, 2007, 45: 1599-1605. DOI: https://doi.org/10.1016/j.fct. 2007.02.027.
[46] HU H, ZHANG W, QIAO Y, JIANG X, LIU X, DING C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium [J]. Acta Biomaterialia, 2012, 8: 904-915. DOI: https://doi. org/10.1016/j.actbio.2011.09.031.
[47] QI Fang-wei, WANG Chen, PENG Shu-ping, SHUAI Ci-jun, YANG Wen-jing, ZHAO Zhen-yu. A co-dispersed nanosystem from strontium-anchored reduced graphene oxide to enhance bioactivity and mechanical property in polymer scaffolds [J]. Materials Chemistry Frontiers, 2021, 5: 2373-2386. DOI: https://doi.org/10.1039/D0QM00958J.
[48] ZOU Zi, LIU Wei, CAO Li-hua, LIU Ying, HE Tian-tian, PENG Shu-ping, SHUAI Ci-jun. Advances in the occurrence and biotherapy of osteoporosis [J]. Biochemical Society Transactions, 2020, 48: 1623-1636. DOI: https://doi.org/10. 1042/BST20200005.
(Edited by HE Yun-bin)
中文导读
机械合金化与激光烧结制备铁锌过饱和固溶体及其降解行为研究
摘要:过慢的降解限制铁的骨植入应用,锌固溶在铁基体中有望加速其降解。首先利用机械合金化制备铁锌过饱和固溶体粉末。在合金化过程中,机械力的作用使得粉末颗粒间反复焊合,减小了溶质原子扩散距离,同时出现大量晶格缺陷,进一步促进了溶质原子的扩散。随后,利用激光烧结技术把粉末固化成型,烧结过程中粉末部分熔化并快速凝固,从而维持了原有过饱和固溶体结构。实验结果表明,所制备铁锌合金的腐蚀电位降低,腐蚀速率提高到0.112 mm/year,且具有良好的生物相容性。这项工作展现了机械合金化与激光烧结组合工艺在制备高性能可降解铁锌植入物方面的巨大潜力。
关键词:过饱和固溶体;机械合金化;激光烧结;铁锌合金;降解行为
Foundation item: Projects(51935014, 82072084, 81871498) supported by the Natural Science Foundation of China; Projects(20192ACB20005, 2020ACB214004) supported by the Jiangxi Provincial Natural Science Foundation of China; Project(20201BBE51012) supported by the Provincial Key R & D Projects of Jiangxi Province, China; Project(2018) supported by the Guangdong Province Higher Vocational Colleges & Schools Pearl River Scholar Funded Scheme, China; Project(2017RS3008) supported by Hunan Provincial Science and Technology Plan, China; Project supported by the Open Research Fund of Jiangsu Key Laboratory of Precision and Micro-Manufacturing Technology, China; Project(2020M682114) China Postdoctoral Science Foundation
Received date: 2020-11-12; Accepted date: 2020-12-30
Corresponding author: SHUAI Ci-jun, PhD, Professor; E-mail: shuai@csu.edu.cn; ORCID: https://orcid.org/0000-0002-2029-5112
Abstract: The slow degration of iron limits its bone implant application. The solid solution of Zn in Fe is expected to accelerate the degradation. In this work, mechanical alloying (MA) was used to prepare Fe-Zn powder with supersaturated solid solution. MA significantly decreased the lamellar spacing between particles, thus reducing the diffusion distance of solution atoms. Moreover, it caused a number of crystalline defects, which further promoted the solution diffusion. Subsequently, the MA-processed powder was consolidated into Fe-Zn part by laser sintering, which involved a partial melting/rapid solidification mechanism and retained the original supersaturated solid solution. Results proved that the Fe-Zn alloy became more susceptible with a lowered corrosion potential, and thereby an accelerated corrosion rate of (0.112±0.013) mm/year. Furthermore, it also exhibited favorable cell behavior. This work highlighted the advantage of MA combined with laser sintering for the preparation of Fe-Zn implant with improved degradation performance.


