
Open-celled porous NiAl intermetallics prepared by
replication of carbamide space-holders
WU Jie, CUI Hong-zhi, CAO Li-li, GU Zheng-zheng
School of Materials Science and Engineering, Shandong University of Science and Technology, Qingdao 266510, China
Received 18 October 2010; accepted 16 December 2010
Abstract: Open-celled porous NiAl intermetallics with adjustable pore characteristics and mechanical properties were successfully prepared by using spherical carbamide as space-holders via combustion synthesis. Examinations of macroscopic and microscopic morphologies as well as the quasi-static compressive test for the resultant materials were carried out. Depending on the volume fraction and particle size of the carbamide, the porosity and pore size of the porous NiAl intermetallics can be controlled freely in a range of 57.57%-84.58% and 0.4-2.0 mm, respectively. Furthermore, quasi-static compressive tests indicate that the mechanical behavior of the present porous materials is in good agreement with the Gibson-Ashby model.
Key words: porous NiAl; combustion synthesis; carbamide; porosity; compression
1 Introduction
It is well known that NiAl intermetallics have excellent characteristics of both ceramics and metals due to the mixture of metallic and covalent bonds [1-2], so porous NiAl intermetallics can be used for applications in extremely severe environments, such as in thermal barrier coatings at high temperature and filter materials in corrosive environments [3-4]. Combustion synthesis, also known as self-propagating high-temperature synthesis (SHS), is an attractive method to produce porous NiAl intermetallics [5-8]. MORSI et al [9] obtained cylindrical porous NiAl intermetallics with porosity of 60% through controlling the heat generation during combustion synthesis. KANETAKE and KOBASHI [3, 10] prepared Ni-Al foams by combustion reactions and proposed possible formation mechanisms of the pores. HUNT et al [11] fabricated porous NiAl intermetallics using nanoparticle reactants through the chemical process of SHS. The above methods, however, do not allow independent control over pore size, shape and connectivity. Furthermore, there is rare report about the mechanical properties of porous NiAl intermetallics, which is extremely important for engineering applications and needs extensive investigation.
Based on the importance of pore structures and mechanical properties, the present study aims to fabricate open-celled porous NiAl intermetallics with adjustable pore characteristics and mechanical properties using spherical carbamide as space-holders via combustion synthesis. Examinations of macroscopic and microscopic morphologies as well as the quasi-static compressive test for the resultant materials were also carried out. The results and analysis are expected to be useful for the development and applications of porous NiAl intermetallics.
2 Experimental
Pure Ni powder (particle size <75 ?m, purity 99.5%) and Al powder (particle size 75-150 ?m, purity 99%) were used as starting materials. Spherical carbamide (CO(NH2)2) particles with three particle sizes, i.e. 0.4-0.6 mm, 0.6-0.9 mm, 0.9-2.0 mm, were prepared as space holder particles. In order to guarantee the uniform distribution between Ni and Al powders, they were thoroughly mixed according to equiatomic ratio before the addition of the carbamide particles. The Ni powders, Al powders and carbamide particles with a chosen size were weighted using an electronic balance with a resolution of 0.01 g. They were blended together in a stirrer at a rotation speed of 80 r/min for 10 min. The volume fraction of carbamide particles varied from 50% to 80% with a gap of 5%. The well mixed mixture was uniaxially compressed under a pressure of 200 MPa into green compact. The green compact was heat treated at 300 °C for 1.5 h in a vacuum furnace to remove the carbamide particles. Finally, the preheated compact was ignited at one end by ignition reagent (Ti powders). Once ignited, combustion wave could self-propagate along the axis to the other end of the compact in a very short time, then the porous material was synthesized.
Phase identification of the products was analysed by X-ray diffraction (XRD). Optical microscopy and scanning electron microscopy (SEM) were used to characterize the pore morphologies of the resultant materials.
The specimen density was determined from its mass and physical dimension, and the porosity (ρ) of the porous material was calculated as
![]() (1)
(1)
where ρ and ρ0 are the density of the specimen and its corresponding theoretical density, respectively.
To evaluate the mechanical properties of the porous materials, quasi-static uniaxial compressive tests were performed at an initial strain rate of 10-3 s-1 on a universal material testing machine at room temperature. The dimensions of all the specimens for the compressive tests were d 20 mm×20 mm.
3 Results and discussion
Both the melting point and decomposition temperature of carbamide are below 200 °C [12] and the carbamide should be removed after the preheating process and have no influence on the phase composition of the products. To prove this assumption, phase identification was carried out by XRD as shown in Fig. 1. It is observed that only the desired NiAl phase was detected in the products although the volume fraction of carbamide particles is different from that of the corresponding green compacts, indicating that the reaction between Ni and Al powders is sufficient and independent on the carbamide particles.
When Ni and Al powders were mixed according to equiatomic ratio, all the possible reactions between Ni and Al during combustion synthesis can be described by the following equations [2]:
![]() =
=![]() (2)
(2)
![]() =
=![]() (3)
(3)
![]() =
=![]() (4)
(4)
![]() =
=![]() (5)
(5)
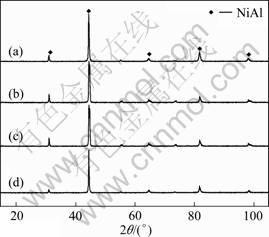
Fig. 1 XRD patterns of product with volume fraction of carbamide particles in corresponding green compacts: (a) 50%; (b) 60%; (c) 70%; (d) 80%
Among the above reactions, the Gibbs free energy of the formation of NiAl phase is the lowest [2]. So the NiAl phase is the most possible final product according to the thermodynamic principles, which is consistent with the XRD patterns shown in Fig. 1.
Figures 2 and 3 show the typical macroscopic and microscopic morphologies of porous NiAl intermetallics, respectively. Homogeneous distribution in NiAl matrix of interconnected pores can be clearly seen. The pores virtually inherit the shape and size of the carbamide particles, suggesting that the morphology and size of pores can be controlled by selecting an appropriate carbamide particle. However, the cell walls are rough and dispersed with a number of micro pores, which forms numerous channels between neighbouring cells, as shown in Figs. 3 and 4.
Besides the morphology and size of pores, the porosity of porous NiAl intermetallics can be also adjusted by varying the volume fraction of carbamide particles in green compacts. As seen in Fig. 5, the actual porosities of the specimens are 57.57%, 67.39% and 79.35% corresponding to the carbamide volume fraction of 50%, 60% and 75%, respectively. It can be seen that the actual porosity is not in accordance with the designed porosity (the carbamide volume fraction), and the difference decreases with increasing volume fraction of the carbamide particles, as shown in Fig. 6.
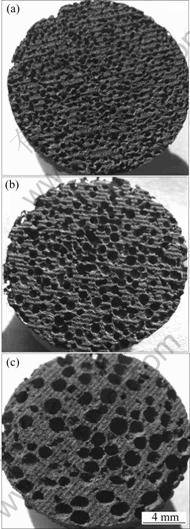
Fig. 2 Photos of cross sections in porous NiAl intermetallics with porosity of about 67.39% and different pore sizes of 0.4-0.6 mm (a), 0.6-0.9 mm (b) and 0.9-2.0 mm (c)

Fig. 3 SEM image of porous NiAl intermetallic with porosity of 67.39% and pore size of 0.9-2.0 mm
There are a number of micro pores on the cell walls, which is responsible for the difference between the actual and designed porosities. The initial pores in green compacts as well as the gases volatilization during combustion synthesis are the primary sources of the micro pores [3, 10, 13]. Compared with Ni and Al powders, carbamide particle is softer and easier to deform, and the density of green compacts can be improved by the corresponding deformation of the carbamide particles accordingly. The higher the volume fraction of the carbamide particles, the denser the green compacts, and the less the initial pores and volatilization of the gases during combustion reaction. These are the reasons for the difference between the actual and designed porosities decreases with increasing volume fraction of the carbamide particles.

Fig. 4 SEM image of micro pores on cell walls of porous NiAl intermetallics

Fig. 5 Photos of cross section in porous NiAl intermetallics with pore sizes of 0.6-0.9 mm and different porosities of 57.57% (a), 67.39% (b) and 79.35% (c)

Fig. 6 Actual and designed porosities of porous NiAl intermetallics
The nominal stress—strain curves of porous NiAl intermetallics are shown in Fig. 7. To understand the detailed stress—strain curves at lower scale, an inner figure of curves at lower strain is also presented. It is apparent that all stress—strain curves consist of typical three regions, i.e. elastic, plateau and densification regions [14]. However, the plateau region is not smooth but serrated, suggesting the brittle nature of the specimens. This serrated plateau reflects the brittle deformation mode, i.e. brittle fracture of the cell walls after the yield [12]. As mentioned above, the cell walls are not sound but dispersed with a number of micro pores. These defective regions will transform to cracks due to stress concentration upon loading. As the compression proceeds, cracks quickly spread and get connected, resulting in collapse of the cell walls and consequently in the drop of the stress. When the compression is carried on, the broken cell walls squeeze together and thus the stress increases again until the next fracture occurs. The repeated collapse and squeezing of the cell walls are responsible for the serrations in the plateau region [12]. In spite of the serrated curves, the increase of plateau stress is very slow and the densification does not begin until a strain of 0.65 is reached. This means that the present porous NiAl intermetallics are excellent in impact absorption applications.

Fig. 7 Nominal stress—strain curves of porous NiAl intermetallics (pore size 0.6-0.9 mm) with different relative densities
From Fig. 7, it can be seen that the compressive strength, i.e. the peak stress, decreases with decreasing relative density. The relationship between the relative stress σ/σ0, and the relative density ρ/ρ0 is analyzed [15-16] assuming that the edge collapses when the compressive force exceeds the fully plastic moment of the cell edges. The relationship is given as [16]:
![]() (6)
(6)
where σ is the collapse stress of a porous metal; σ0 is the yield stress of the cell wall material; ρ is the density of the porous metal; ρ0 is the density of the cell wall material and C is a constant. If Eq. (6) is applied to the porous NiAl intermetallics, the dependence of σ on (ρ/ρ0)3/2[1+(ρ/ρ0)1/2] should yield a straight line, which is demonstrated by the experimental results shown in Fig. 8, indicating that the compressive behavior of the porous NiAl intermetallics can be understood by the Gibson-Ashby model.
Nevertheless, only limited data of quasi-static compressive test are available, further work is needed to clarify the effect of pore size, pore shape, as well as strain rate on the mechanical properties of the porous NiAl intermetallics.

Fig. 8 Dependence of compressive strength on relative density for porous NiAl intermetallics (pore size 0.6-0.9 mm)
4 Conclusions
1) An open-celled porous NiAl intermetallics is successfully prepared by replication of spherical carbamide particles via combustion synthesis technique.
2) The porous NiAl intermetallics exhibit uniformly distributed and interconnected pores. The pores virtually inherit the shape and size of the carbamide particles. Depending on the volume fraction and particle size of the carbamide, the porosity and pore size of the porous NiAl intermetallics can be controlled freely in the range of 57.57%-79.35% and 0.4-2.0 mm, respectively.
3) The nominal stress—strain curve of porous NiAl intermetallics consists of typical three regions, i.e. elastic, plateau and densification regions. The serrated and quite long plateau region demonstrates the brittle nature and good energy absorption property of the materials. Furthermore, for porous NiAl intermetallics, the relationship between the compressive strength and relative density can be explained by the Gibson-Ashby model.
References
[1] STOLOFF N S, LIU C T, DEEVI S C. Emerging applications of intermetallics [J]. Intermetallics, 2000, 8(9-11): 1313-1320.
[2] GUO Jian-ting. Ordering Ni-Al intermetallics [M]. Beijing: Science Press, 2003: 3-5. (in Chinese)
[3] KANETAKE N, KOBASHI M. Innovative processing of porous and cellular materials by chemical reaction [J]. Scr Mater, 2006, 54(4): 521-525.
[4] DONG H X, JIANG Y, HE Y H, SONG M, ZOU J, XU N P, HUANG B Y, LIU C T, LIAW P K. Formation of porous Ni-Al intermetallics through pressureless reaction synthesis [J]. J Alloys Compd, 2009, 484(1-2): 907-913.
[5] HSIUNG L C, SHEU H H. A comparison of the phase evolution in Ni, Al, and Ti powder mixtures synthesized by SHS and MA processes [J]. J Alloys Compd, 2009, 479(1-2): 314-325.
[6] CURFS C, TURRILLAS X, VAUGHAN G B M, TERRY A E, KVICK ![]() , RODRíGUEZ M A. Al-Ni intermetallics obtained by SHS; A time-resolved X-ray diffraction study [J]. Intermetallics, 2007, 15(9): 1163-1171.
, RODRíGUEZ M A. Al-Ni intermetallics obtained by SHS; A time-resolved X-ray diffraction study [J]. Intermetallics, 2007, 15(9): 1163-1171.
[7] VARMA A, MUKASYAN A S. Combustion synthesis of advanced materials: Fundamentals and applications [J]. J Chem Eng, 2004, 21(2): 527-536.
[8] DONG S S, HOU P, YANG H B, ZOU G T. Synthesis of intermetallic NiAl by SHS reaction using coarse-grained nickel and ultrafine-grained aluminum produced by wire electrical explosion [J]. Intermetallics, 2002, 10(3): 217-223.
[9] MORSI K, FUJII T, MCSHANE H, MCLEAN M. Control of heat generation during reaction synthesis [J]. Scr Mater, 1999, 40(3): 359-364.
[10] KOBASHI M, KANETAKE N. Processing of intermetallic foam by combustion reaction [J]. Advanced Eng Mater, 2002, 4(10): 745-747.
[11] HUNT E M, PANTOYA M L, JOUET R J. Combustion synthesis of metallic foams from nanocomposite reactants [J]. Intermetallics, 2006, 14(6): 620-629.
[12] HAO G L, HAN F S, LI W D. Processing and mechanical properties of magnesium foams [J]. J Porous Mater, 2009, 16(3): 251-256.
[13] CHU C L, CHUNG C Y, LIN P H, WANG S D. Fabrication of porous NiTi shape memory alloy for hard tissue implants by combustion synthesis [J]. Mater Sci Eng A, 2004, 366(1): 114-119.
[14] WANG Q Z, CUI C X, LIU S J, ZHAO L C. Open-celled porous Cu prepared by replication of NaCl space-holders [J]. Mater Sci Eng A, 2010, 527(4-5): 1275-1278.
[15] YAMADA Y, SHIMOJIMA K, SAKAGUCHI Y, MABUCHI M, NAKAMURA M, ASAHINA T, MUKAI T, KANAHASHI H, HIGASHI K. Effects of heat treatment on compressive properties of AZ91 Mg and SG91A Al foams with open-cell structure [J]. Mater Sci Eng A, 2000, 280(1): 225-228.
[16] YAMADA Y, BANNO T, XIE Z K, LI Y C, WEN C E. Preparation and characterisation of open-cell microporous nickel [J]. Mater Sci Forum, 2007, 539-543: 1833-1838.
以尿素为造孔剂制备开孔NiAl金属间化合物
吴 杰,崔洪芝,曹丽丽,谷征征
山东科技大学 材料科学与工程学院,青岛266510
摘 要:以尿素为造孔剂,利用燃烧合成技术成功制备孔洞结构和力学性能可控可调的开孔NiAl金属间化合物,并对材料的宏观和微观形貌、准静态压缩性能进行分析。通过调整尿素的体积分数和颗粒大小,多孔NiAl金属间化合物的孔隙率可控制在57.57%-84.58%,孔径大小可控制在0.4-2.0 mm。准静态压缩实验表明,多孔NiAl金属间化合物的力学性能可用Gibson-Ashby模型来解释。
关键词:
(Edited by FANG Jing-hua)
Foundation item: Project (51072104) supported by the National Natural Science Foundation of China; Project (BS2010CL038) supported by the Research Award Fund for Outstanding Young and Middle-aged Scientists of Shandong Province, China
Corresponding author: WU Jie; Tel: +86-532-86057929; E-mail: wujie0537@163.com
DOI: 10.1016/S1003-6326(11)60925-4


