Trans. Nonferrous Met. Soc. China 24(2014) 848-853
Reaction kinetics of roasting high-titanium slag with concentrated sulfuric acid
Li-li SUI1,2, Yu-chun ZHAI1
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China;
2. Department of Chemistry, Shenyang Medical College, Shenyang 110034, China
Received 27 April 2013; accepted 6 June 2013
Abstract:
A novel method of roasting high-titanium slag with concentrated sulfuric acid was proposed to prepare titanium dioxide, and the roasting kinetics of titania was studied on the basis of roasting process. The effects of roasting temperature, particle size, and acid-to-ore mass ratio on the rate of roasting reaction were investigated. The results showed that the roasting reaction is fitted to a shrinking core model. The results of the kinetic experiment and SEM and EDAX analyses proved that the reaction rate of roasting high-titanium slag with concentrated sulfuric acid is controlled by the internal diffusion on the solid product layer. According to the Arrhenius expression, the apparent activation energy of the roasting reaction is 18.94 kJ/mol.
Key words:
roasting kinetics; high-titanium slag; concentrated sulfuric acid; titania;
1 Introduction
Titanium dioxide is an important inorganic chemical material, and is extensively used in white pigment, paper, plastics, rubbers, porcelain, and fibers, etc [1-4]. The main processes for the production of titanium dioxide are sulfate process and chloride process. In the sulfate process, titanium sulfate solution is subsequently hydrolyzed in highly acidic solutions, and then the precipitate of hydrous titanium oxides is obtained. The comprehensive management of waste acid and by-product of copperas are the most fatal weakness in the sulfate process [5-8]. On the other hand, the chloride process requires a feedstock of high TiO2 grade and must satisfy the content limit of MgO+CaO less than 1.5%. Moreover, it is a high energy consuming process [9-11]. All the above-mentioned shortcomings prevent the development of the chloride process in industry. Therefore, it is significant to put forward novel technique and method for the production of titanium dioxide.
The reserve volume of vanadium-titanium magnetite resources is sufficient in Panxi region of Sichuan Province, China. In the course of the smelting of pig iron, titanium is discharged into the blast furnace slag, which pollutes the environment seriously [12-15]. High- titanium slag contains a large number of valuable metals, such as silicon, aluminium, calcium. Therefore, it is very meaningful to realize the comprehensive utilization of high-titanium slag. The procedure of extracting titanium dioxide from high-titanium slag can reduce a step of elimination of copperas and therefore the energy consumption is decreased consequently. It is apparent that the productivity is promoted and the pollution is relieved [16-18]. Hence, the high-titanium slag is adopted as the basic high-quality raw materials in titanium industry, which has a certain economic value.
The presented method of roasting high-titanium slag with concentrated sulfuric acid in this work has the advantages of saving time, low consumption of acid and high acidolysis rate. The more important term is that the waste sulfuric acid can be recycled. The effects of reaction conditions, such as roasting temperature, particle size and acid-to-ore mass ratio, on the reaction rate of roasting high titanium slag with concentrated sulfuric acid were considered sufficiently and analyzed. Furthermore, the appropriate kinetic equation and the rate-controlling step of roasting reaction were derived. Finally, the reaction mechanism was discussed in detail.
2 Experimental
2.1 Materials
Titanium slag after crushing and ball milling was used in the experiment. The chemical compositions of the high-titanium slag are listed in Table 1. The main component of titanium slag is TiO2, and its content is 48.65%. All the chemical reagents were of analytical grade, and deionized water was used throughout the experimental process.
Table 1 Chemical compositions of high-titanium slag (mass fraction, %)

The mineralogical phases of the high-titanium slag were investigated by X-ray powder diffraction. The XRD pattern shown in Fig. 1 indicates that the main crystalline phases of the high-titanium slag are anosovite solid solution of magnesium and iron (Mg0.5Fe0.5)Ti2O5 and complex silicate phase Al2Ca(SiO4)2. FeO and MgO in titanium slag are beneficial to the increase of acidolysis rate of titanium slag.
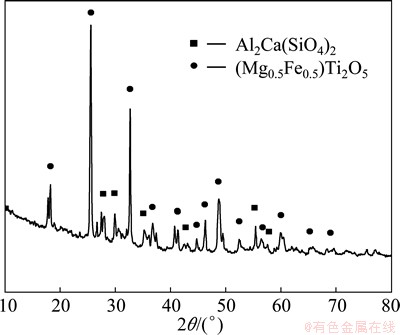
Fig. 1 XRD pattern of high titanium slag
2.2 Procedure
Experiments were performed in a resistance wire heating furnace. The concentrated sulfuric acid and high- titanium slag were homogeneously mixed in the porcelain crucibles. Then the crucibles were put into the resistance wire heating furnace and heated. When the temperature reached the predetermined temperature, we began to activate the timing device. The porcelain crucible was taken out at specified time and cold water was added quickly in order to stop the reaction immediately, with free access to air in the whole process. The temperature of the resistance wire heating furnace was controlled by a programmable temperature controller, with a precision of ±1 °C. The roasting product was leached by water (leaching conditions: temperature 70 °C, solid-to-liquid ratio 1:4, and stirring time 1 h). The extraction rate of TiO2 was determined by the ammonium ferric sulfate dodecahydrate titration. The calculating formula of the extraction rate of TiO2 is expressed as
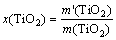 (1)
(1)
where x(TiO2) is the extraction rate of TiO2; m′(TiO2) is the mass of TiO2 in the filtrate; m(TiO2) is the total mass of TiO2 in high-titanium slag.
3 Results and discussion
3.1 Effect of temperature
The influence of roasting temperature on the reaction rate of high-titanium slag with concentrated sulfuric acid was investigated in the temperature range of 250-310 °C, with acid-to-ore mass ratio of 2:1 and particle size of 45-48 μm. Figure 2 shows that temperature has a significant effect on the reaction rate of roasting high-titanium slag. The extraction rate of TiO2 increases rapidly with the increase of temperature.
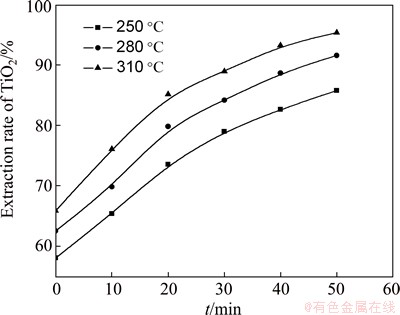
Fig. 2 Relationship between extracting rate of TiO2 and time at different temperatures
In order to determine the kinetic parameters and rate-controlling step for roasting high-titanium slag with concentrated sulfuric acid, the experimental data presented in Fig. 2 were analyzed on the basis of the shrinking-core model. The experimental data were substituted into the Crank-Ginsting-Braunshtein’s kinetic equation [19-22]:
1+2(1-x)-3(1-x)2/3=Kt (2)
where x is the extraction rate of TiO2; K is the apparent rate constant; t is the reaction time. The corresponding relationship between the value of 1+2(1-x)-3(1-x)2/3 and the roasting time t is described in Fig. 3, which shows that the linear relationship between 1+2(1-x)-3(1-x)2/3 and roasting time t is significant. The results indicate that the reaction rate is fitted to the Crank-Ginsting- Braunshtein’s kinetic equation, which proves that the reaction rate is controlled by the internal diffusion on the solid product layer in the roasting process.
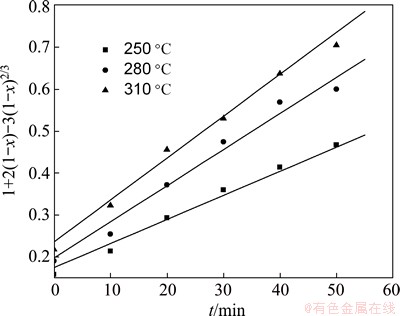
Fig. 3 Relationship between 1+2(1-x)-3(1-x)2/3 and t at different temperatures
According to the Arrhenius expression:
ln K=ln A-E/(RT) (3)
where K is the rate constant, A is the frequency factor, E is the apparent activation energy, R is the mole gas constant and T is the thermodynamic temperature. The corresponding relationship between ln K and 1/T is revealed in Fig. 4, where the approximation of the apparent activation energy can be obtained from the slope of the plotted straight line (E=18.94 kJ/mol). The result verifies that the reaction rate is controlled by the internal diffusion.
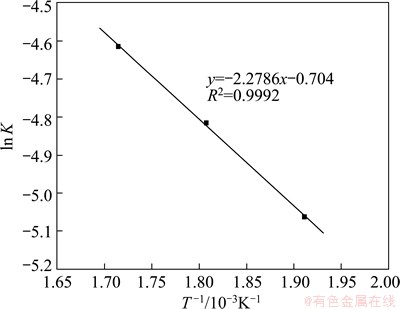
Fig. 4 Relationship between ln K and T -1
3.2 Effect of particle size
The influence of particle size on the reaction rate of roasting high titanium slag with concentrated sulfuric acid was studied (the roasting temperature was 310 °C and the acid-to-ore mass ratio was 2:1), using the three particle size fractions: 160-180, 75-80, 45-48 μm, respectively. The results presented in Fig. 5 show that the particle size of high-titanium slag has a significant effect on the extraction of TiO2. The extraction rate of TiO2 increases with the decrease of particle size because the decrease of particle size increases both the specific surface of the titanium slag and its reactivity. The experimental data are substituted into the Crank- Ginsting-Braunshtein’s kinetic equation: 1+2(1-x)- 3(1-x)2/3 =Kt.
According to the experimental data in Fig. 5, the plots of 1+2(1-x)-3(1-x)2/3 vs time t are presented in Fig. 6. The linear relationship between 1+2(1-x)- 3(1-x)2/3 and roasting time t is significant. The results further indicate that the roasting process is controlled by the internal diffusion on the solid product layer.
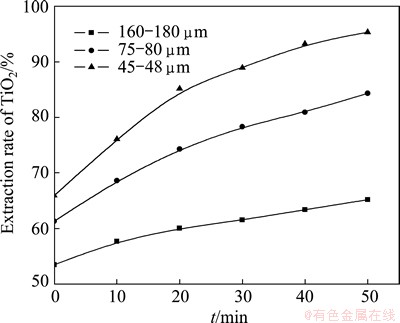
Fig. 5 Relationship between extracting rate of TiO2 and t at different particle sizes
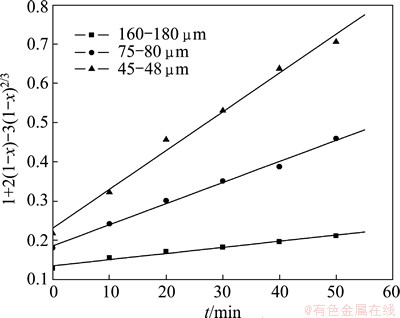
Fig. 6 Relationship between 1+2(1-x)-3(1-x)2/3 and t at different particle sizes
3.3 Effect of acid-to-ore mass ratio
The influence of acid-to-ore mass ratio on the reaction rate of roasting high-titanium slag with concentrated sulfuric acid was studied (The roasting temperature was 310 °C and the range of particle size was 45-48 μm), using three acid-to-ore mass ratios: 1.9:1, 2.0:1 and 2.1:1, respectively. The results in Fig. 7 show that the extraction rate of TiO2 increases with the increase of acid-to-ore mass ratio. The experimental data are substituted into the Crank-Ginsting-Braunshtein’s kinetic equation. The corresponding relationship between the value of 1+2(1-x)-3(1-x)2/3 and the roasting time t is described in Fig. 8, which shows that there is a good linear correlation between the above two variables. The results further indicate that the roasting process is controlled by the internal diffusion on the solid product layer.
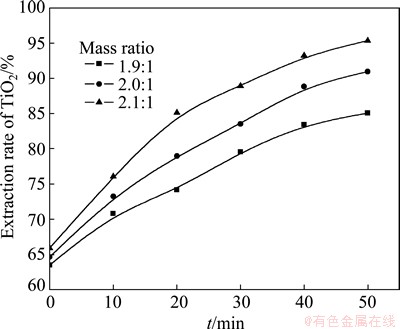
Fig. 7 Relationship between extracting rate of TiO2 and t at different acid-to-ore mass ratios
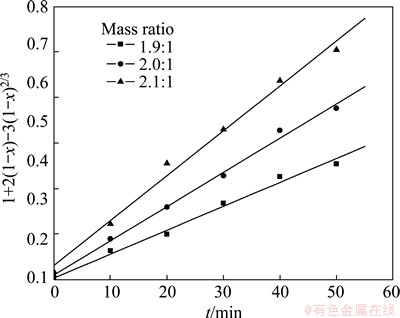
Fig. 8 Relationship between 1+2(1-x)-3(1-x)2/3 and t at different acid-to-ore mass ratios
Based on the above experiments, it can be concluded that the kinetic experimental data are fitted to the Crank-Ginsting-Braunshtein’s kinetic equation under all the experimental conditions. The reaction rate of roasting high titanium slag with concentrated sulfuric acid is controlled by the internal diffusion on the solid product layer. In the range of experimental temperature, the kinetic equation of roasting process can be described as follows:
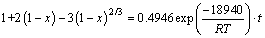 (4)
(4)
4 Analysis of roasting reaction mechanism
The main reaction of the roasting high-titanium slag with concentrated sulfuric acid could be described as follows:
2(Mg0.5Fe0.5)Ti2O5+6H2SO4=4TiOSO4+MgSO4+FeSO4+6H2O (5)
Al2Ca(SiO4)2+4H2SO4=Al2(SO4)3+CaSO4+2SiO2+4H2O (6)
The scanning electron microscopy (SEM) was employed to investigate the change of morphology and elemental composition of titanium slag and the residue. From the results given in Fig. 9, the titanium slag is granular and high-density. The surface of the residue is rough after the reaction and the morphology of titanium slag is destroyed.
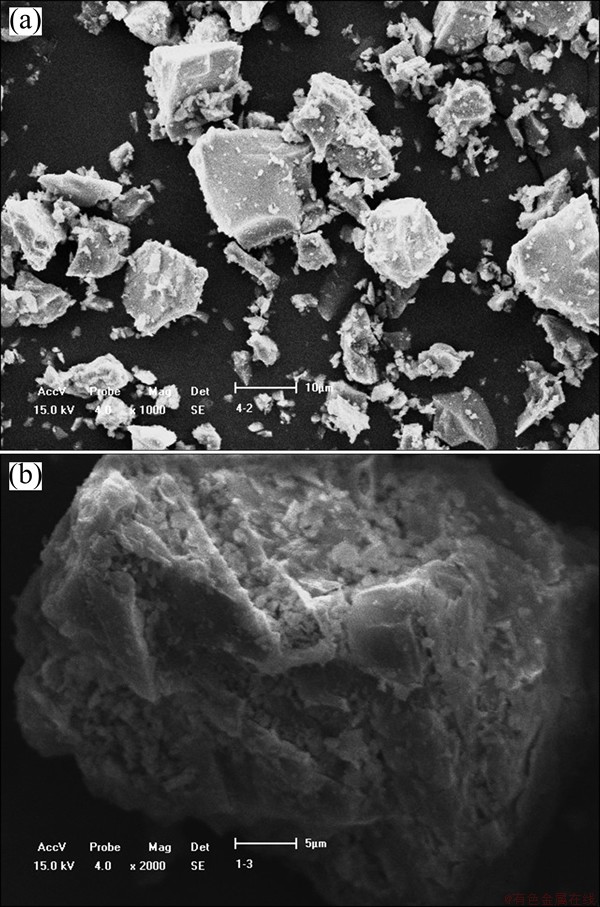
Fig. 9 SEM images of high-titanium slag (a) and residue (b)
In order to find out the constituent of the solid products, the analysis of mass fraction of elements was carried out by X-ray photoelectron spectroscopy (XPS). From the results shown in Fig. 10, the mass fraction of elements in the surface of high-titanium slag before the reaction is Ti 25.156%, Al 6.544%, Fe 3.710%, Si 8.203%, Mg 3.000%, Ca 4.074%. The mass fraction of elements in the surface of the residue after the reaction is: Ti 5.200%, Al 5.281%, Fe 1.477%, Si 26.460%, Mg 2.140%, Ca 5.346%. According to the analysis results, the main elements of the surface of the residue are Si and Ca, while Ti content reduces greatly, which proves the existence of a solid product layer.
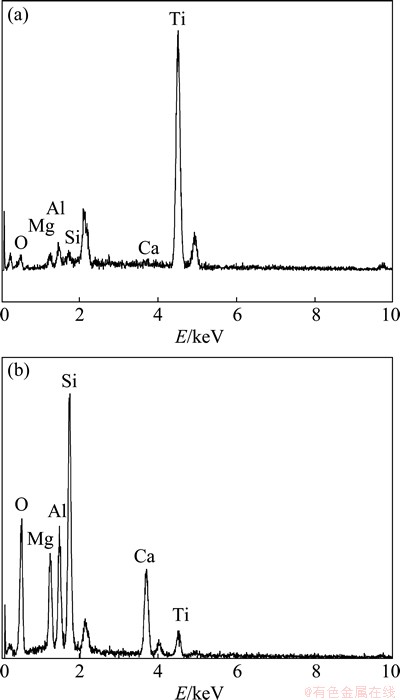
Fig. 10 EDAX patterns of high-titanium slag (a) and residue (b)
The reaction of roasting high-titanium slag with concentrated sulfuric acid is a liquid-solid reaction, which can be analyzed with the shrinking-core model under the assumption that the titanium slag is homogeneously spherical solid phase. The reaction is carried out in the solid particle surface. The reaction can generate not only the titanium ions, but also silica and calcium sulfate solid. The molecules of sulfuric acid must pass through the film formed by solid product to diffuse into the surface of titanium slag particles which do not participate in the reaction and react with titanium slag. So it can be concluded that the rate of roasting reaction is controlled by the internal diffusion on the solid product layer.
5 Conclusions
1) The reaction kinetics of roasting high-titanium slag with concentrated sulfuric acid was studied. The results of the kinetic experiment, SEM and EDAX analysis prove that the roasting kinetics is fitted to a shrinking core model with the reaction rate controlled by the internal diffusion on the solid product layer.
2) The apparent activation energy of the roasting reaction is 18.94 kJ/mol, which accords with the range of diffusion controlled processes.
3) The reaction rate satisfies the Crank-Ginsting- Braunshtein’s kinetic equation. The roasting kinetic equation can be described as follows:
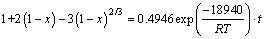
References
[1] ZHANG Yong-jie, QI Tao, ZHANG Yi. A novel preparation of titanium dioxide from titanium slag [J]. Hydrometallurgy, 2009, 96(1): 52-56.
[2] HAN Yan-fang, LI Jie, WANG Li-na, QU Jing-kui. Preparation of titanium dioxide from titania-rich slag by molten NaOH method [J]. International Journal of Minerals, Metallurgy and Materials, 2012, 19(3): 205-211. (in Chinese)
[3] WANG Bin, CHENG Xiao-zhe, HAN Ke-xi, QIN Xing-hua, MA Yong. Research of acidolysis performance of acid soluble titanium slag [J]. Iron Steel Vanadium Itanium, 2009, 30(2): 6-11. (in Chinese)
[4] ZHANG Wen-sheng, ZHU Zhao-wu, CHENG Chu-yong. A literature review of titanium metallurgical processes [J]. Hydrometallurgy, 2011, 108(3): 177-188.
[5] AKHGAR B N, PAZOUKI M, RANIBAR M, HOSSEINNIA A, KEYANPOUR-RAD M. Preparetion of nanosized synthetic rutile from ilmenite concentrate [J]. Minerals Engineering, 2010, 23(7): 587-589.
[6] LIU Xiao-hua, SUI Zhi-tong. Leaching of Ti bearing blast furnace slag by pressuring [J]. The Chinese Journal of Nonferrous Metals, 2002, 12(6): 1281-1284. (in Chinese)
[7] KOLEN’KO Y V, BURUKHIN A A, CHURAGULOV B R, OLEYNIKOV N N. Synthesis of nanocrystalline TiO2 powders from aqueous TiOSO4 solution under hydrothermal conditions [J]. Materials Letters, 2003, 57(5): 1124-1129.
[8] AHMED Y V, MOHANDAS P N, YUSUFF K K M. Preparation of high surface area TiO2 (anatase) by thermal hydrolysis of titanyl sulphate solution [J]. International Journal of Inorganic Materials, 2001, 3(7): 593-596.
[9] GRZMIL B U, GRELA D, KIC B. Hydrolysis of titanium sulphate compounds [J]. Chemical Papers, 2008, 62(1): 18-25.
[10] DONG Rui, JIANG Ji-sen. Preparation of rutile titania nanoparticles at low temperature [J]. Journal of East China Normal University: Natural Science, 2007, 2007(1): 135-140. (in Chinese)
[11] ZHOU Zhong-cheng, RUAN Jian-ming, ZOU Jian-peng, LI Song-lin, FU Nai-ke. Preparation of nanosized rutile TiO2 powders by direct hydrolysis of TiCl4 [J]. Chinese Journal of Rare Metals, 2006, 30(5): 653-656. (in Chinese)
[12] LU Hui, XIE Gang, YU Xiao-hua, XIE Hong-yan. Removel of silicon and aluminum from EAF titanium-containing slag and pre-oxidization roasting kinetics of the leached slag [J]. The Chinese Journal Process Engineering, 2010, 10(3): 513-520. (in Chinese)
[13] SATHYAMOORTHY S, HOUNSLOW M J, MOGGRIDGE G D. Influence of stirrer speed on the precipitation of anatase particles from titanyl sulphate solution [J]. Journal of Crystal Growth, 2001, 223: 225-234.
[14] LIU Xiao-hua, SUI Zhi-tong. Kinetics of leaching Ti-bearing slag by dilute sulphuric acid [J]. Acta Metallurgica Sinica, 2003, 39(3): 293-296. (in Chinese)
[15] YANG Cheng, ZOU Jian-xin. Kinetics for acidolysis of titania slag during production of titanium dioxide [J]. Multipurpose Utilization of Mineral Resources, 2007, 148(6): 25-27. (in Chinese)
[16] LIAO Chun-fa, JIANG Guo-ping, JIAO Yun-fen, LIANG Yong. Study on the technological conditions and kinetics of leaching titanium from mud red with sulfuric acid [J]. Mining Research and Development, 2008, 28(2): 45-47. (in Chinese)
[17] XUN Shun, HUANG Zhuo-shu. The kinetic of Panzhihua ilmenite leaching with sulifuric acid [J]. Mining and Metallurgical Engineering, 1993, 13(1): 44-48. (in Chinese)
[18] HUA Yi-xin. Metallurgical process kinetics introduction [M]. Beijing: Metallurgical Industry Press, 2004: 9. (in Chinese)
[19] ZHANG Cheng-gang, ZHENG Shao-hua, DU Chuang-shan, ZHANG Yong-kui, LIANG Bin. Leaching kinetics of ilmenite in sulfuric acid [J]. Chemical Reaction Engineering and Technology, 2000, 16(4): 319-324. (in Chinese)
[20] ZHANG Su-chun, NICOL M J. Kinetics of the dissolution of ilmenite in sulfuric acid solutions under reducing conditions [J]. Hydrometallurgy, 2010, 103(2): 196-204.
[21] LIU Xiao-hua, GAI Guo-sheng, YANG Yu-fen, SUI Zhi-tong, LI Li, FU Jian-xia. Kinetics of the leaching of TiO2 from Ti-bearing blast furnace slag [J]. Journal of China University of Mining and Technology, 2008, 18(2): 275-278. (in Chinese)
[22] YAN Xiong, ZHOU Zhi-rong, WU Fu-hai. Kinetic spectrophotometric determination of trace titanium (IV) based on oxidation discoloration of acid chrome blue K with hydrogen peroxide [J]. Journal of China University of Mining and Technology, 2007, 17(3): 418-423. (in Chinese).
浓硫酸焙烧高钛渣的反应动力学
隋丽丽1,2,翟玉春1
1. 东北大学 材料与冶金学院,沈阳 110819;
2. 沈阳医学院 化学系,沈阳 110034
摘 要:提出一种新方法,利用浓硫酸焙烧高钛渣提取二氧化钛,并在焙烧工艺的基础上研究焙烧反应动力学。考察焙烧温度、粒度以及酸矿比对反应速率的影响。结果表明,焙烧反应符合未反应核收缩模型。动力学实验数据、SEM和EDAX结果分析表明,用浓硫酸焙烧高钛矿渣的反应受通过固体产物层的内扩散控制。Arrhenius方程得到焙烧反应的表观活化能为18.94 kJ/mol。
关键词:焙烧动力学;高钛渣;浓硫酸;二氧化钛
(Edited by Hua YANG)
Foundation item: Project (2007CB613603) supported by the National Basic Research Program of China
Corresponding author: Yu-chun ZHAI; Tel: +86-13324089199; E-mail: zhaiyc@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(14)63134-4
Abstract: A novel method of roasting high-titanium slag with concentrated sulfuric acid was proposed to prepare titanium dioxide, and the roasting kinetics of titania was studied on the basis of roasting process. The effects of roasting temperature, particle size, and acid-to-ore mass ratio on the rate of roasting reaction were investigated. The results showed that the roasting reaction is fitted to a shrinking core model. The results of the kinetic experiment and SEM and EDAX analyses proved that the reaction rate of roasting high-titanium slag with concentrated sulfuric acid is controlled by the internal diffusion on the solid product layer. According to the Arrhenius expression, the apparent activation energy of the roasting reaction is 18.94 kJ/mol.


