网络首发时间: 2016-06-22 08:23
熔盐电脱氧法制备合金时物质间相互影响的研究进展
李泽全 胡玲 卢姣 刘楠 韩梅
重庆大学化学化工学院
摘 要:
熔盐电脱氧法是一种高温下直接电解金属氧化物制备金属或合金的电化学方法,采用该方法制备合金时,物质间存在先后还原及相互影响。还原顺序主要取决于金属氧化物分解电压的大小,分解电压小的金属氧化物还原更容易,还原速率相对较快,更先完成还原;金属氧化物混合电解相对于各自单独电解的时间均相应缩短,说明在混合烧结、电解过程中物质间产生了相互影响,其相互影响主要有:混合烧结过程中试样的微观形貌(包括颗粒大小、颗粒形貌、试样孔隙率)相对于单独烧结发生改变,同时试样导电性提高;混合电解过程中先还原的金属提高了试样的导电性、降低了后还原金属氧化物的过电位及缩短了后还原金属氧化物的还原路径,掺杂的金属氧化物促进了钙钛矿化中间产物的还原。故今后的研究中应重点关注金属氧化物混合烧结过程中微观形貌变化、变化机制和对电解的影响,及先还原金属对后还原金属氧化物还原路径的影响,从而通过实验调控促进电解效率的提高。
关键词:
熔盐电脱氧法 ;合金 ;还原顺序 ;相互影响 ;
中图分类号: TF111.522
作者简介: 李泽全(1970-),男,四川眉山人,博士研究生,副教授,研究方向:熔盐电化学;电话:023-65678940;E-mail:lzq0313@cqu.edu.cn;
收稿日期: 2015-04-24
基金: 国家教育部中央高校基本科研业务费项目(CDJZR10 22 0003)资助;
Progress in Mutual Influences between Substances during Preparation of Alloy via Molten Salt Electro-Deoxidation
Li Zequan Hu Ling Lu Jiao Liu Nan Han Mei
College of Chemistry and Chemical Engineering,Chongqing University
Abstract:
Molten salt electro-deoxidation is an electrolytic reduction extraction method of metals or alloys from metal oxides. During the preparation of alloy via molten salt electro-deoxidation,metal oxides that were mixed together successively completed reduction and there existed mutual influences between substances. The reduction sequence depended mainly on the decomposition voltage of metal oxide,and the reduction of metal oxide with lower decomposition voltage was thermodynamically easier and kinetically faster and thus preferentially completed reduction. The electrolysis time of mixed metal oxides,relative to the electrolysis time when each metal oxide was singly electrolyzed,was shortened,which indicated the interactions between mixed metal oxides during sintering and electrolytic process. The main mutual influences between substances were that during sintering process,the morphology( including particle size,particle morphology,porosity) of mixed metal oxides,relative to the morphology when each metal oxide was singly sintered,was changed and sample conductivity was improved; during electrolysis process,preferentially reduced metal improved sample conductivity,decreased the over-potential of other mixed metal oxides and shortened the reduction path of other mixed metal oxides,and doping metal oxide promoted the reduction of perovskite intermediate product. The future research should focus on the change of morphology during sintering process,its mechanism and influence on electrolysis,and the influence of preferentially reduced metal on the reduction path of other mixed metal oxides,so that the experimental conditions could be controlled and the electrolysis efficiency could be improved.
Keyword:
molten salt electro-deoxidation; alloy; reduction sequence; mutual influence;
Received: 2015-04-24
熔盐电脱氧法(FFC法)是一种在高温熔盐中电解固态金属氧化物制备金属及合金的电化学方法,于2000年由Chen等[1] [2 ,3 ,4 ,5 ,6 ] ,该工艺不仅具有工艺简单、流程短、节能环保、可连续化生产等优点,还解决了制备合金时因活性金属扩散较快而引起的合金偏析、偏聚等问题,且对合金组分可控[4] [7 ,8 ] ,Ni-35Ti-15Hf[9] [10] [11] [12] 2 [13] 4 Cu[14] [15] [16] [17] 5 [18 ,19 ] ,Tb Ni5 [20] [21] [22 ,23 ,24 ] ,Ti V[25] [26] 3 Sn[27] [28] 5 [29] 5 [30] [3] [31 ,32 ,33 ,34 ,35 ,36 ,37 ,38 ] 曾试图通过探明熔盐电脱氧法的还原机制,优化工艺参数等来进行改善,但电解效率都未取得实质性地提高。因此,目前亟待找到一种有效提高电解效率的方法。
大量研究结果表明,在熔盐电脱氧法工艺基础上,金属氧化物混合电解时存在先后还原现象,且混合电解所需时间相对于金属氧化物各自单独电解所需时间均相应缩短[7 ,8 ,9 ,10 ,11 ,12 ,13 ,14 ,15 ,16 ,17 ,18 ,19 ,20 ,21 ,22 ,23 ,24 ,39 ,40 ] 。例如Ti Ni体系,高筠等[39] [40] 2 还原为金属钛。朱用等[7] [8] 2 为原料制备Ti Ni合金,其中Ni O优先快速还原,在电解10 min左右已基本还原出金属Ni,整个混合试样电解还原时间分别为4和6 h,均比Ti O2 和Ni O单独电解还原时间要短。此外,Wang等[9] 2 制备Ni Ti Hf三元合金,同样在电解10min左右得到金属Ni,之后Ti O2 和Hf O2 依次先后完成还原,电解9 h后还原为Ni Ti Hf合金,比Ni O[39] 2 [40] 2 [12]
目前,关于熔盐电脱氧法制备合金过程中物质间相互影响的研究鲜有报道,而该影响机制的探明将为电解效率的提高指明新的方向,对合金的合理高效制备具有重要指导性意义。本论文分析了金属氧化物混合电解的先后还原顺序,并总结归纳了已有的关于熔盐电脱氧法制备合金时物质间相互影响的现象和观点,希望能从中找到提高电解效率的新思路。
1 金属氧化物的先后还原顺序
很多研究者注意到金属氧化物混合电解时存在先后还原的现象,并普遍认为是由金属氧化物分解电压的大小所决定的
[7 ,10 ,17 ,21 ,22 ,23 ,24 ,25 ,26 ,46 ,47 ,48 ,49 ,50 ]
。Dring等
[22 ]
制备Ti W合金时证实,理论分解电压较小的WO3 先于Ti O2 还原,并认为这是由于WO3 混合的量较少而导致其发生快速完成还原。朱用等
[7 ]
在制备Ti Ni合金的研究中提出,钛镍氧化物的分解电压在900℃存在约1.6 V的差异,在相同的电解电压下,Ni O还原速度势必快于Ti O2 。Hyslop等
[10 ]
制备Co Cr合金时表明,Co3 O4 的还原电位更正,故还原最先发生。李晴宇等
[17 ]
也认为,从理论上说,在相同电解条件下,分解电压较小的还原更容易。因此,金属氧化物分解电压的大小对混合金属氧化物的先后还原顺序起决定性作用。
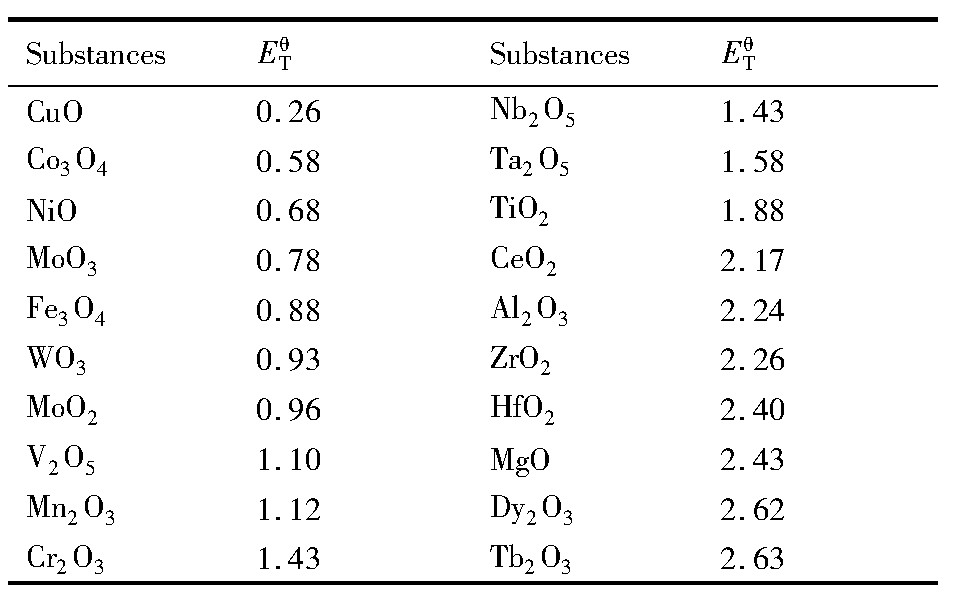
在相同外加电压下,金属氧化物的电解还原同时进行,分解电压小的金属氧化物电解还原更容易,还原速率相对更快,较先完成还原,而分解电压大的金属氧化物还原较慢。目前主要通过理论分解电压来进行粗略判断,依据查询的热力学数据
[51 ]
和理论分解电压的计算方法
[52 ]
可得1200 K下常见金属氧化物的理论分解电压,见表1。由所计算的理论分解电压推测的金属氧化物的先后还原顺序与已有文献中的实验结果基本吻合,故采用分解电压大小判定的观点具有较强的说服力。还有研究者通过更精确、直观的Predominance Diagrams图
[21 ,22 ,23 ,49 ,50 ]
或直接采用计算出的金属氧化物的标准生成吉布斯自由能变值
[8 ,9 ,47 ]
来判定金属氧化物的先后还原顺序,它们的原理与用分解电压大小判定的原理是一致的。但也存在反例,如杜继红等
[25 ]
在采用FFC法制备Ti V合金时推测,理论上900℃时V2 O5 的还原电位比Ti O2 小0.722 V,在相同的电解电压下V应优先于Ti析出,然而实验表明电解10 min的产物主要是Ti,Ti O,Ca Ti O3 和V4 O9 ,即Ti先于V析出,对此作者认为是由于氧化物中钒含量很低,可能受动力学因素影响,析出速度慢。
综上所述,金属氧化物的先后还原顺序大致可以通过理论分解电压大小来粗略判定,但具体还需考虑动力学等因素带来的影响。
表1 1200 K下常见金属氧化物的理论分解电压Table 1Theoretical decomposition voltage(ET θ )of com-mon metal oxides at 1200 K(V) 下载原图
表1 1200 K下常见金属氧化物的理论分解电压Table 1Theoretical decomposition voltage(ET θ )of com-mon metal oxides at 1200 K(V)
2 物质间的相互影响
2.1 混合烧结过程中物质间的相互影响
相对于单独烧结而言,金属氧化物混合烧结过程中试样的微观形貌(包括颗粒大小、颗粒形貌、试样孔隙率)发生了改变;且混合烧结易形成固溶体,使得传导电子和氧空位的浓度增大,试样导电性提高。
2.1.1 混合烧结过程中试样微观形貌的变化
金属氧化物混合烧结颗粒尺寸相对于单独烧结时颗粒尺寸变小
[15 ,27 ]
,主要有以下两方面原因:一方面,金属氧化物晶体的生长需要通过晶粒间的吞并作用,若晶粒之间被阻隔,则金属氧化物的生长将受到抑制。如郭春芳和董云会
[53 ]
在Zr O2 -Ni2 O3 烧结片中加入石墨粉,可分别有效阻隔Zr O2 和Ni2 O3 颗粒,从而阻碍大颗粒的形成。同样,在混合烧结过程中,由于另一种金属氧化物的掺入,阻隔了同种金属氧化物颗粒之间的接触,进而影响了晶体颗粒的生长,使颗粒尺寸变小,掺杂过量时甚至出现熔蚀现象
[27 ]
;另一方面,朱道云等
[54 ]
和周方桥等
[55 ]
考察Ti O2 陶瓷中Nb2 O5 掺杂的作用时认为,Ti O2 晶粒粒径变小是由于Nb2 O5 掺杂过量发生复合缺陷反应:2Nb2 O5 4Nb Ti +VTi ″″ +10 O0 × ,造成钛空位的大量浓集,抑制Ti O2 晶粒的生长。
混合试样颗粒尺寸变小,颗粒自身的形貌也因此有所改变,晶粒不再是多面体形状,掺杂过量时边缘看上去似乎被熔蚀了,呈现出圆弧形状
[54 ,55 ]
。
对于整个试样而言,孔隙率相对于单独烧结也发生了变化。王雪
[27 ]
将Nb2 O5 -Sn O2 混合烧结,由于两种晶粒颗粒尺寸差别较大,颗粒尺寸较小的Sn O2 填充在晶体颗粒较大的Nb2 O5 的孔隙之间,从而使Nb2 O5 -Sn O2 混合物阴极片的孔隙率小于纯Nb2 O5 烧结片的孔隙率。对于不同配比的阴极片,随着Sn O2 小颗粒在阴极片中配比增大,有更多的孔隙中填充进Sn O2 的晶体颗粒,试样孔隙率不断减小。邓丽琴
[15 ]
在制备Ti Nb合金时也提及,随着Ti O2 小颗粒添加量的增大,逐渐包裹着Nb2 O5 大颗粒,同时填充在大颗粒间的孔隙中,使得各颗粒间的孔隙尺寸逐渐减小。
2.1.2 混合烧结过程中试样导电性提高
金属氧化物混合烧结容易形成置换式或间隙式固溶体,使得试样的导电性提高。Johnson
[56 ]
研究证明,金红石中任意Ti4+ 被另一更高价的金属离子取代或在晶格间隙中掺入半径较小的金属离子(如Be2+ ),会引入传导电子来平衡过剩的正电荷,可以提高其导电性。朱道云等
[54 ]
和周方桥等
[55 ]
报道,由于Nb5+ 与Ti4+ 的离子半径相当,在高温烧结过程中Nb5+ 易固溶于Ti O2 中取代Ti4+ ,该过程在材料中引入了传导电子,同时产生氧空位,随着Nb2 O5 掺杂量的增大,传导电子和氧空位浓度成比例地增大,有利于电子的传导和O2- 在阴极的扩散。Bhagat等
[21 ]
提到Mo O2 与Ti O2 烧结后形成了固体溶剂,相应地提高了试样的导电性,且Nb2 O5 与WO3 能将Ti O2 的导电性提高大约3~4个数量级。
2.2 混合电解过程中物质间的相互影响
相对于单独电解而言,混合电解中先还原的金属提高了试样的导电性,降低了后还原金属氧化物的过电位,并缩短了后还原金属氧化物的还原路径;掺杂的金属氧化物提高了钙钛矿化中间产物氧空位的量和O2- 的导电性,有利于钙钛矿相的电解还原。
2.2.1 先还原的金属对电解过程产生的影响
金属氧化物混合电解存在先后还原,先还原的金属对电解过程产生的影响有:(1)提高整个试样的导电性;(2)降低后还原金属氧化物的过电位;(3)缩短后还原金属氧化物的还原路径。
混合电解过程中,分解电压小的金属氧化物优先快速还原,一方面先还原的金属提高了阴极片的导电性,另一方面增加了电极的孔隙率,从而加快阴极片内部的电脱氧速率。刘品
[28 ]
在Zr O2 中掺杂Ni O使得混合试样中的Zr O2 在18 h内就基本完成脱氧,且随着Ni O含量的增加,电脱氧速率明显加快,而纯Zr O2 要70多个小时才能完全脱氧,其认为这是反应初始阶段首先脱氧生成的金属镍所致。郭春芳
[57 ]
研究证明,混合电解制备Zr Ni合金的电脱氧速率明显大于单纯制备金属锆的电脱氧速率,其认为这可能与反应原料Ni O的电化学活性有关。Bhagat等
[21 ]
研究表明,先还原的Mo与Ti O结合形成(Ti,Mo)O,提高了(Ti,Mo)O相的导电性。Abdelkader等
[29 ]
在制备Nb Co5 合金时也指出,钴离子快速还原为金属Co,促进了Nd3+ 的后续还原。
优先还原的金属除了提高试样导电性外,还能降低后还原金属氧化物的过电位。朱用等
[7 ]
在制备Ti Ni合金过程中认为,Ni O可在很低的槽压(900℃下分解电压为0.7 V)下优先还原,而镍的优先析出可降低Ti O2 还原的过电位,使电解形成Ti Ni合金的槽压可低于电解提炼单质钛的槽压。Bhagat等
[21 ]
在制备Ti Mo合金时,认为由于Ti和Mo颗粒产生了相互作用,使得Ti的还原电势变得更正,促进了电解还原过程。
先还原的金属生成后,后还原的金属氧化物或其还原中间产物以其为晶核还原形成合金,相对于后还原金属氧化物单独逐级脱氧还原而言中间产物的量明显减少,使得后还原金属氧化物的还原路径缩短,且该还原路径所需外界提供的能量也更小
[7 ,8 ,13 ,18 ,20 ,21 ,30 ]
。如Ti O2 单独电解时,按Ti O2 -Ti4 O7 -Ti3 O5 -Ti2 O3 -Ti O-Ti2 O-Ti逐级脱氧的过程还原,同时伴有钙钛矿化中间产物生成,还原需约30 h
[40 ]
。而掺入Ni O混合电解后,Ti O2 微粒在新鲜生成的镍微粒上还原,中间产物主要为Ca T-i O3-x 和Ni2 Ti4 O,在热力学上比Ti O2 单独还原更容易,所需分解电压更小,电解还原时间缩短为4h
[7 ]
。Bhagat等
[21 ]
电解9 h获得Ti-15%Mo(质量分数)的合金,相对于文献中记载的纯Ti O2 还原时间要短,认为先还原的Mo减少了Ti O2 低价氧化物的生成量,进而减少晶变的发生。且Mo在氧含量接近Ti O时能稳定化β-Ti,促进Ti O还原为Ti。2.2.2掺杂的金属氧化物促进钙钛矿化中间产物的还原金属氧化物还原过程中的钙钛矿化中间产物(ABO3 )体积大、结构致密,其结晶化过程不仅会降低试样的孔隙率,还会降低晶体的有序性,减缓O2- 在晶体内的迁移,不利于金属氧化物的电解还原
[12 ]
。若在钙钛矿(ABO3 )的A位点或B位点掺杂不同价态的离子,能很好地提高钙钛矿中氧空位的量和O2- 的导电性
[58 ]
。用单一的Hf O2 难于电解得到Hf,电解16 h仍有大量的Hf O2 和Ca Hf O3 。当在Hf O2 加入5%(质量分数)较好的B位点掺杂剂Nb2 O5
[59 ,60 ]
后,电解电流大于纯Hf O2 电解的电流,电解24 h的产物主要为Hf Nb。如果Ca Hf O3 的Ca位点和Hf位点都被掺杂了,则还原可能进行得更快。此外,对比合金中的Hf与纯Hf发现,掺杂剂Nb2 O5 不仅促进了钙钛矿相的还原,所形成的形貌还抑制金属Hf在操作过程中的重新氧化,并提高了Hf的动力学稳定性,避免了其在空气中的自燃现象
[12 ]
。
3 结语
综上所述,熔盐电脱氧法制备合金时,金属氧化物混合电解的先后还原顺序主要由各金属氧化物的分解电压大小决定,但具体还需考虑动力学等因素带来的影响。混合烧结、电解过程中物质间的相互影响主要表现在:改变物质微观形貌、提高试样导电性、缩短金属氧化物还原路径及促进钙钛矿中间产物的还原等方面,但关于它们的影响机制及如何实验调控促进电解效率的提高等问题还有待进一步研究,因此,今后重点展开以下两方面工作:
1.进一步研究金属氧化物混合烧结过程中微观形貌变化及变化机制和对电解效率的影响,从而通过实验调控以提高电解效率。
2.深入研究先还原金属对后还原金属氧化物还原路径的影响机制,以尽量缩短后还原金属氧化物的还原路径,从而加快后还原金属氧化物的还原速率和提高制备合金的电解效率。
参考文献
[1] Chen G Z,Fray D J,Farthing T W.Direct electro-chemical reduction of titanium dioxide to titanium in molten calcium chloride[J].Nature,2000,407(21):361.
[2] Huang J S,Zhou J C,Liu W S,Huang B Y.Preparation and treatment of high-density alloy powder[J].Rare Metals and Cemented Carbides,2003,31(4):40.(黄劲松,周继承,刘文胜,黄伯云.高密度合金粉末的制备与处理[J].稀有金属与硬质合金,2003,31(4):40.)
[3] Jin S,Ge X L,Zhang M,Guo M,Wang X D.Research development of preparation of metal and alloy powder by molten salts electrodeoxidation[J].Materials Review,2007,21(11):64.(金烁,盖鑫磊,张梅,郭敏,王习东.熔盐电脱氧法制备金属及合金的研究进展[J].材料导报,2007,21(11):64.)
[4] Liao C F,Xie Q W,Wang X,Xiao Z H,Yang W Q,Deng P.The latest development of preparation of refractory metals and alloys by molten salt electro-deoxidation[J].Rare Metals and Cemented Carbides,2011,39(4):20.(廖春发,谢泉文,王旭,肖志华,杨文强,邓攀.熔盐电脱氧法制备难熔金属及合金的研究进展[J].稀有金属与硬质合金,2011,39(4):20.)
[5] Wang Z,Li J,Hua Y X,Zhang Z,Zhang Y,Ke P C.Research progress in production technology of titanium[J].Chinese Journal of Rare Metals,2014,38(5):915.(王震,李坚,华一新,张志,张远,柯平超.钛制取工艺研究进展[J].稀有金属,2014,38(5):915.)
[6] Yan L J.Research status of titanium production[J].Science and Technology of West China,2008,7(32):35.(闫丽静.钛制备方法研究现状[J].中国西部科技,2008,7(32):35.)
[7] Zhu Y,Ma M,Wang D H,Jiang K,Hu X H,Jin X B,Chen Z.Electrolytic reduction of mixed solid oxides in molten salts for energy efficient production of the TiNi alloy[J].Chinese Science Bulletin,2006,51(16):2535.(朱用,马猛,汪的华,蒋凯,胡晓宏,金先波,陈政.熔盐电还原固态混合氧化物低能耗制备Ti Ni合金[J].科学通报,2006,51(16):1966.)
[8] Jackson B K,Inman D,Jackson M,Dye D,Dashwood R J.Ni Ti production via the FFC cambridge process:refinement of process parameters[J].Journal of the Electrochemical Society,2010,157(3):36.
[9] Wang B X,Bhagat R,Lan X Z,Dashwood R J.Production of Ni-35 Ti-15 Hf alloy via the FFC cambridge process[J].J.Electrochem.Soc.,2011,158(10):595.
[10] Hyslop D J S,Abdelkader A M,Cox A,Fray D J.Electrochemical synthesis of a biomedically important CoCr alloy[J].Acta Materialia,2010,58(8):3124.
[11] Yin H Y,Yu T,Tang D Y,Ruan X F,Zhu H,Wang D H.Electrochemical preparation of Ni Al intermetallic compound from solid oxides in molten Ca Cl2and its corrosion behaviors in Na Cl aqueous solution[J].Materials Chemistry and Physics,2012,133(1):465.
[12] Abdelkader A M,Fray D J.Electro-deoxidation of hafnium dioxide and niobia-doped hafnium dioxide in molten calcium chloride[J].Electrochimica Acta,2012,64:10.
[13] Qiu G H,Wang D H,Ma M,Jin X B,Chen G Z.Electrolytic synthesis of Tb Fe2from Tb4O7and Fe2O3powders in molten Ca Cl2[J].Journal of Electroanalytical Chemistry,2006,589(1):139.
[14] Kang X,Xu Q,Yang X M,Song Q S.Electrochemical synthesis of Ce Ni4Cu alloy from the mixed oxides and in situ heat treatment in a eutectic Li Cl-KCl melt[J].Materials Letters,2010,64(20):2258.
[15] Deng L Q.Preparation of Niobium and Nb-Ti Alloy by Electro-Deoxidation in a Eutectic Melt[D].Shenyang:Northeastern University,2006.28.(邓丽琴.熔盐电脱氧法制备金属Nb及Nb-Ti合金[D].沈阳:东北大学,2006.28.)
[16] Xie Q W.Preparation of WNi Alloy by Electro-Deoxidation in Molten Salt[D].Ganzhou:Jiangxi Polytechnic University,2011.25.(谢泉文.熔盐电脱氧法制备WNi合金的研究[D].赣州:江西理工大学,2011.25.)
[17] Li Q Y,Du J H,Xi Z P,Li Z X.Preparation of Ti Zr alloy by electro-deoxidization in molten salt[J].Rare Metal Materials and Engineering,2008,37(S4):594.(李晴宇,杜继红,奚正平,李争显.混合氧化物直接电化学还原制备Ti Zr合金[J].稀有金属材料与工程,2008,37(S4):594.)
[18] Dai L,Wang S,Li Y H,Wang L,Shao G J.Direct electrochemical preparation of Ce Co5alloy from mixed oxides[J].Trans.Nonferrous Met.Soc.China,2012,22(8):2007.
[19] Qu M L.Study on Preparation of Co-Based Function Alloy by Electro-Deoxidization in Molten Salt[D].Tangshan:Hebei Polytechnic University,2009.22.(曲梅玲.熔盐电脱氧法制备钴基功能合金的研究[D].唐山:河北理工大学,2009.22.)
[20] Qiu G H,Wang D H,Jin X B,Chen G Z.A direct electrochemical route from oxide precursors to theterbium-nickel intermetallic compound Tb Ni5[J].Electrochimica Acta,2006,51(26):5785.
[21] Bhagat R,Jackson M,Inman D,Dashwood R.The production of Ti-Mo alloys from mixed oxide precursors via the FFC cambridge process[J].Journal of the Electrochemical Society,2008,155(6):63.
[22] Dring K,Bhagat R,Jackson M,Dashwood R,Inman D.Direct electrochemical production of Ti-10W alloys from mixed oxide preform precursors[J].Journal of Alloys and Compounds,2006,419(1-2):103.
[23] Bhagat R,Jackson M,Inman D,Dashwood R.Production of Ti-W alloys from mixed oxide precursors via the FFC cambridge process[J].J.Electrochem.Soc.,2009,156(1):1.
[24] Liu X B,Luo Z T,Xing P F,Chang P B.Preparation of Ti W alloy by electrolysis method in molten-salt[J].Development and Application of Materials,2012,(4):21.(刘喜波,罗志涛,邢朋飞,常鹏北.熔盐电解法制备Ti W合金[J].材料开发与应用,2012,(4):21.)
[25] Du J H,Li Q Y,Yang S H,Li Z X,Xi Z P.Preparation of Ti V alloy by electrolysis-deoxidization in molten salt[J].Rare Metal Materials and Engineering,2009,38(12):2230.(杜继红,李晴宇,杨升红,李争显,奚正平.熔盐电解脱氧制备Ti V合金[J].稀有金属材料与工程,2009,38(12):2230.)
[26] Du J H,Xi Z P,Li Q Y,Li Z X,Tang Y.Preparation of Ti Fe alloy by electro-deoxidization in molten salt[J].Rare Metal Materials and Engineering,2008,37(12):2240.(杜继红,奚正平,李晴宇,李争显,唐勇.熔盐电解还原制备Ti Fe合金[J].稀有金属材料与工程,2008,37(12):2240.)
[27] Wang X.The Investigation of Preparation of SinglePhase Nb3Sn by Electro-Deoxidation of Nb2O5-Sn O2in the Molten Salt[D].Shenyang:Northeastern University,2008.27.(王雪.熔盐电脱氧制备单相Nb3Sn研究[D].沈阳:东北大学,2008.27.)
[28] Liu P.Study on Production of Zr-Ni Alloy by ElectroDeoxidization in Molten Salt[D].Zibo:Shangdong Polytechnic University,2010.26.(刘品.熔融盐电脱氧法制备锆镍合金的研究[D].淄博:山东理工大学,2010.26.)
[29] Abdelkader A M,Hyslop D J S,Cox A,Fray D J.Electrochemical synthesis and characterization of a NdCo5permanent magnet[J].Journal of Materials Chemistry,2010,20(29):6039.
[30] Zhao B J,Wang L,Dai L,Cui G H,Zhou H Z,Kumar R V.Direct electrolytic preparation of cerium/nickel hydrogen storage alloy powder in molten salt[J].Journal of Alloys and Compounds,2009,468(1-2):379.
[31] Chen G Z,Fray D J.Voltammetric studies of the oxygen-titanium binary system in molten calcium chloride[J].Journal of the Electrochemical Society,2002,149(11):455.
[32] Ono K,Suzuki R O.A new concept for producing Ti sponge:calciothermic reduction of Ti O2[J].Journal of the Minerals Metals&Materials Society,2002,54(2):59.
[33] Liu M F,Guo Z C,Lu W C.Process of direct electrochemical reduction of Ti O2[J].Chinese Journal of Nonferrous Metals,2004,14(10):1752.(刘美凤,郭占成,卢维昌.Ti O2直接电解还原过程的研究[J].中国有色金属学报,2004,14(10):1752.)
[34] Ma G Q,Zou M,Liu G Q,Wang Q L.Study on the voltage of direct electrochemical reduction of Ti O2to spongy titanium in vacuum condition[J].China Nonferrous Metallurgy,2010,74(3):56.(马光强,邹敏,刘国钦,王琪琳.真空条件下电解Ti O2制备海绵钛电解电压的研究[J].中国有色冶金,2010,74(3):56.)
[35] Zou M,Liu G Q,Ma G Q,Wang Q L.Study of impact factors of electric current efficiency for electrolysis reduction of Ti O2to titanium in molten[J].China Nonferrous Metallurgy,2009,53(2):71.(邹敏,刘国钦,马光强,王琪琳.真空熔盐电解法制备海绵钛电流效率影响因素的研究[J].中国有色冶金,2009,53(2):71.)
[36] Liao X J,Zhai Y C,Xie H W,Shen H T,Zou X Y.Preparation of Ti by direct electrochemical deoxidation in low temperature molten salt[J].Chinese Journal of Materials Research,2012,26(6):590.(廖先杰,翟玉春,谢宏伟,沈洪涛,邹祥宇.低温熔盐电解法制备金属Ti及其动力学[J].材料研究学报,2012,26(6):590.)
[37] Hu M L,Bai C G,Dong L Y,Li Z Q,Nie X M.Research development of Ti preparation via Ti O2electrolyzation[J].Titanium Industry Progress,2005,22(5):44.(扈玫珑,白晨光,董凌燕,李泽全,聂新苗.电解Ti O2提取钛的研究进展[J].钛工业进展,2005,22(5):44.)
[38] Suzuki R O.Calciothermic reduction of Ti O2and in situ electrolysis of Ca O in the molten Ca Cl2[J].Journal of Physics and Chemistry of Solids,2005,66(1):461.
[39] Gao Y,Zhou Z,Wang L,Dai L,Zhu S Q.Research on preparation of nickel powder by electrolytic deoxidation from Ni O[J].Powder Metallurgy Industry,2007,17(2):15.(高筠,周正,王岭,戴磊,朱书全.熔盐电解氧化亚镍制备镍粉新工艺研究[J].粉末冶金工业,2007,17(2):15.)
[40] Liu X B,Luo Z T,Gao G H,Chang P B.Study on preparation of metal titanium by electrolyzing in molten salt[J].China Nonferrous Metallurgy,2013,8(4):61.(刘喜波,罗志涛,高贵华,常鹏北.熔盐电解法制备金属钛的研究[J].中国有色冶金,2013,8(4):61.)
[41] Zhang Q J,Qu M L,Wang L,Dai L,Tian Y,Cui C X.Preparation of Co alloy by direct electro-deoxidization in molten salt[J].Journal of Functional Materials,2009,40(5):830.(张庆军,屈梅玲,王岭,戴磊,田颖,崔春翔.熔盐电解法制备金属钴的研究[J].功能材料,2009,40(5):830.)
[42] Zhao B J,Wang L,Dai L,Cui G H,Li Y H,Zhu L G.Research of preparation of Cr by electro-deoxidation in molten salt[J].Powder Metallurgy Industry,2008,18(2):23.(赵炳建,王岭,戴磊,崔广华,李岳华,朱立光.熔盐电脱氧法制备金属Cr粉的研究[J].粉末冶金工业,2008,18(2):23.)
[43] Yan X Y,Fray D J.Production of niobium powder by direct electrochemical reduction of solid Nb2O5in a eutectic Ca Cl2-Na Cl melt[J].Metallurgical and Materials Transactions B,2002,33(5):685.
[44] Yin H Y,Tang D Y,Zhu H,Zhang Y,Wang D H.Production of iron and oxygen in molten K2CO3-Na2CO3by electrochemically splitting Fe2O3using a cost affordable inert anode[J].Electrochemistry Communications,2011,13(12):1521.
[45] Zhao B J.Research of Preparation of Cr and Ce Ni5by Electro-Deoxidization in Molten Salt[D].Tangshan:Hebei Polytechnic University,2008.28.(赵炳建.熔盐电脱氧法制备金属Cr及Ce Ni5合金的研究[D].唐山:河北理工大学,2008.28.)
[46] Zhao Z Y,Gao J,Zhao Y.Research development on preparation of metal and alloy by molten salts electrodeoxidation[J].Journal of Hebei United University(Natural Science Edition),2013,35(2):13.(赵志颖,高筠,赵颖.熔盐电脱氧法制备金属及合金的研究进展[J].河北联合大学学报,2013,35(2):13.)
[47] Descallar-Arriesgado R F,Kobayashi N,Kikuchi T,Suzuki R O.Calciothermic reduction of Ni O by molten salt electrolysis of Ca O in Ca Cl2melt[J].Electrochimica Acta,2011,56(24):8422.
[48] Li G M,Wang D H,Jin X B,Chen G Z.Electrolysis of solid Mo S2in molten Ca Cl2for Mo extraction without CO2emission[J].Electrochemistry Communications,2007,9(8):1951.
[49] Dring K,Dashwood R,Inman D.Predominance diagrams for electrochemical reduction of titanium oxides in molten Ca Cl2[J].Journal of the Electrochemical Society,2005,152(10):184.
[50] Jackson B,Jackson M,Dye D,Inman D,Dashwood R.Production of Ni Ti via the FFC cambridge process[J].Journal of the Electrochemical Society,2008,155(12):171.
[51] Ihsan Barin,Fried Sauert,Ernst Schultze-Rhonhof,Wang Shu Sheng.Thermochemical Data of Pure Substances,PartⅠAg-Kr/PartⅡLa-Zr[M].Federal Repubilc of Germany:Wiley-VCH,1993.48.
[52] Fu X C,Shen W X,Yao T Y,Hou W H.Physical Chemistry(5th Edition)[M].Beijing:Higher Education Press,2005.69.(傅献彩,沈文霞,姚天扬,侯文华.物理化学(第五版)[M].北京:高等教育出版社,2005.69.)
[53] Guo C F,Dong Y H.Effects of graphite powder dopingon Zr Ni alloy preparation by direct electro-deoxidation method[J].Rare Metals and Cemented Carbides,2012,40(5):33.(郭春芳,董云会.添加石墨粉对直接电脱氧法制备Zr Ni合金的影响[J].稀有金属与硬质合金,2012,40(5):33.)
[54] Zhu D Y,Zhou F Q,Ding Z W,Liang H D.The effects of burying sintering process and Nb2O5-doped amounts on the electric properties of Ti O2varistor ceramic[J].J.Huazhong Univ.of Sci.&Tech.(Nature Science Edition),2004,32(2):54.(朱道云,周方桥,丁志文,梁鸿东.Nb2O5掺杂及Ti O2压敏陶瓷埋烧工艺的研究[J].华中科技大学学报,2004,32(2):54.)
[55] Zhou F Q,Li L,Zhuang Y.Behaviours of Nb5+and Sr2+in the varistors based on Sr O-Nb2O5-Ti O2ceramics[J].Journal of Inorganic Materials,2002,17(16):1174.(周方桥,李莉,庄严.Sr O-Nb2O5-Ti O2系压敏陶瓷中Nb5+和Sr2+的研究[J].无机材料学报,2002,17(6):1174.)
[56] Johnson G H.Influence of impurities on electrical conductivity of rutile[J].Journal of the American Ceramic Society,1953,36(3):97.
[57] Guo C F.Study on Production of Zirconium by Direct Electrodeoxidation of Solid Zirconium Dioxide[D].Zibo:Shangdong Polytechnic University,2007.30.(郭春芳.直接电脱氧法由Zr O2制备金属Zr的研究[D].淄博:山东理工大学,2007.30.)
[58] Chen G Z,Gordo E,Fray D J.Direct electrolytic preparation of chromium powder[J].Metall.Mater.Trans.B,2004,35(2):223.
[59] Zhang K,Ran R,Ge L,Shao Z,Jin W,Xu N.Systematic investigation on new Sr Co1-yNbyO3-δceramic membranes with high oxygen semi-permeability[J].J.Membr.Sci.,2008,323(2):436.
[60] Nagai T,Ito W,Sakon T.Relationship between cation substitution and stability of perovskite structure in SrCo O3-δ-based mixed conductors[J].Solid State Ionics,2007,177(39-40):3433.