DOI:10.19476/j.ysxb.1004.0609.2019.09.09
稀土镁合金中关键相及其界面与性能的相关性
李 谦1, 2, 3,周国治1, 2
(1. 上海大学 材料科学与工程学院,上海 200444;
2. 上海大学 省部共建高品质特殊钢冶金与制备国家重点实验室,上海 200444;
3. 上海大学 材料基因组工程研究院,上海 200444)
摘 要:
镁合金是实现航空航天、交通运输、民用建筑等轻量化、缓解日益严重的能源问题的重要材料之一。本文以稀土镁合金为对象,聚焦多元体系中关键相对合金性能的作用机制,从热力学上分析稀土镁合金中的金属间化合物种类及其物相稳定性,进一步总结相变动力学模型及其在稀土镁合金相转变机理分析中的应用。讨论相结构和界面对稀土镁合金力学性能、耐蚀性能和储氢性能的影响规律,展望通过调控关键相和第二相转变设计新型稀土镁合金的前景和发展方向。
关键词:
文章编号:1004-0609(2019)-09-1934-19 中图分类号:TG146 文献标志码:A
镁是地球上储量最丰富的元素之一,在地壳中含量丰度为2.3%,在盐湖及海洋中的含量也十分可观。中国是世界上镁资源最丰富的国家,拥有的镁资源矿石类型全,分布广,储量产量均居世界第一。同时,镁合金因其具有比强度高、比刚度高、导热导电性好、电磁屏蔽、阻尼减振、切屑加工性以及加工成本低、易于回收等优点[1],广泛应用于航空航天、导弹、交通运输、民用建筑等行业,被美誉为“21世纪绿色结构材料”,也被很多行业专家视为未来金属界的明星材料之一。
然而,无论作为结构材料还是功能材料,镁合金的大规模应用还面临一些主要障碍[2-3]。师昌绪院士指出,镁合金的发展存在三大瓶颈,即缺乏有效析出相、易腐蚀和难变形。首先,目前研究的镁合金绝对强度低,其主要是由于合金元素在镁中的固溶度有限,仅靠固溶强化难以显著提升镁合金的力学性能。2017年,WU等[4]制备双相纳米晶镁薄膜,将镁合金强度提高至3.3 GPa。虽然这一结构在块体材料中还难以实现,但为镁合金的强韧化提供了新思路。因此,探索弥散强化、析出强化、固溶强化及组织均匀化等多种综合强韧机制[5-6]是目前提高镁合金强韧性的主要方向。其次,镁合金中的杂质元素或化合物通常电势电位都高于镁,而且镁本身性质活泼,氧化膜疏松,易形成强烈的电偶腐蚀,也是阻碍镁合金商用的主要问题。改善镁合金的耐蚀性主要有两种方式:一种是通过合金化和纯净化处理来提高镁合金基体本身的电极电位,或者形成表面自愈合防护膜,增强自身对环境腐蚀的抵抗能力;另一种是通过表面防护处理,形成表面保护膜而防止基体的腐蚀。再次,镁合金的塑形差,根源在于其密排六方结构(HCP)。在温度低于25 ℃时,塑形变形主要在基面(0001)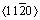 滑移系和棱面
滑移系和棱面
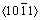 孪晶系中,只有三个几何滑移系和两个独立滑移系[7]。此外,作为新能源材料,虽然镁的理论储氢容量高达7.6%(质量分数),但MgH2在0.1 MPa氢压下的放氢温度高于280 ℃,且吸放氢速率慢,粉末易结块,导致循环寿命短。
孪晶系中,只有三个几何滑移系和两个独立滑移系[7]。此外,作为新能源材料,虽然镁的理论储氢容量高达7.6%(质量分数),但MgH2在0.1 MPa氢压下的放氢温度高于280 ℃,且吸放氢速率慢,粉末易结块,导致循环寿命短。
在镁合金中加入稀土元素,可改善镁合金的力学、耐热和阻燃、抗疲劳、耐摩擦磨损、耐腐蚀及储氢等性能。丁文江院士指出,镁与稀土结合,有望形成中国“王牌”[8]。镁合金中添加RE和其它合金元素如Al、Zn等可显著改善其性能:1) 形成一系列有效的有序金属间化合物,在镁基体中起到析出强化和弥散强化的作用[9-11];2) 它们易与氢形成一系列新的氢化物相,作为活性催化剂提高吸放氢速率[12-14],同时延长循环寿命;3) 细化晶粒,改善组织和织构[15-17];4) 使镁表面的氧化膜和腐蚀产物膜变得致密,可提高镁的耐蚀性[18]。
不难看出,前述瓶颈问题的核心点都是相及其物理化学性质。其实,界面也对镁合金的性能起着极其重要的作用。物相的周期性结构在界面处中断,造成界面处化学键的不连续性,并在界面处形成畸变能,使界面具有不同于体相的特性并显著影响合金性能。鉴于此,本文围绕稀土镁合金多元体系中关键相及其界面,从热力学和动力学两方面综合分析体系中金属间化合物种类及其物相稳定性,以及相变动力学模型及其在稀土镁合金相转变机理分析中的应用,并介绍了相结构和界面对稀土镁合金力学性能、耐蚀性能和储氢性能的影响规律。
1 稀土镁合金中的关键相及其相转变
1.1 稀土镁合金中的关键金属间化合物
常见的稀土元素Y[19]、La[20-22]、Ce[23-27]、Pr[28-30]、Nd[31-33]等与Mg形成一系列结构相似的金属间化合物,如REMg12、RE2Mg17、RE5Mg41、REMg3、REMg2、REMg等。除了尚未报道的Mg-Pm相图和金属间化合物,其他轻稀土和镧系稀土的化合物和结构类型列在图1中。REMg相几乎存在于所有Mg-RE合金体系,且都为简单的CsCl结构类型。REMg2有两种结构类型,分别为MgZn2和MgCu2。REMg3具有BiF3结构,是Mg-RE体系中常见的析出强化相,作为金属间化合物稳定存在于Mg-La/Ce/Pr/Nd/Sm/Gd/Tb/Dy体系中。越靠近富Mg侧,由于结构复杂性提高,能形成富Mg的Mg-RE金属间化合物的体系越少,RE5Mg41只在Mg-Ce/Pr/Nd/Sm体系稳定存在,RE2Mg17只在Mg-La/Ce/Eu体系稳定存在,REMg12只在Mg-La/Ce/Pr体系稳定存在,而NdMg12为亚稳相。ZHAI等[34]研究了冷速和磁场对Mg-Nd体系中NdMg12和Nd5Mg41形成的影响规律,并从经典形核理论和瞬态形核理论方面阐释亚稳相NdMg12在高冷速下形成的原因。
三元及以上体系中常存在着结构复杂的相,实验研究的工作量巨大,往往实验精度也不确定。随着计算机的出现,人们开始尝试利用热力学的基本原理计算不同体系的相关系,并最终发展成为相图理论的重要分支,即相图计算技术(Calphad,Calculation of phase diagram)。KATTNER对CALPHAD技术作了比较全面的描述,它是指利用所有可得的实验和理论数据来评估各相吉布斯自由能模型中所用到的参数的方法[38]。在一定温度范围内获得低元系物相的自由能参数,可以内插得到高元系物相关系,以及预测亚稳范围[39-40]。因此,对于三元及以上体系中物相和相关系的研究常采用Calphad方法。
Mg-RE-TM(TM=Zn, Cu, Ni, Mn)体系在过去的几十年中一直是镁合金研究的热点。由于这些体系中可以形成长周期堆垛有序结构(LPSO)相、准晶相等,显著提高镁合金的强度、高温性能和储氢性能,可用于制备高强镁合金、块体非晶和储氢材料等。例如,Mg97Y2Zn1合金屈服强度超过600 MPa,同时伸长率约为5%[41];Mg-Y/Ce/Nd-Ni合金的吸放氢循环寿命大于500次,且储氢容量高于4.8%(质量分数)[42-44]。为了厘清Mg-RE-TM体系中的金属间化合物和相关系,研究者对这些体系进行了大量的实验和计算工作。
Mg-Y/Gd-Zn体系中存在多种金属间化合物[45],其中最受研究者关注的是一种长周期堆垛有序结构相(LPSO)。SHAO等[46]优化Mg-Y-Zn的热力学参数时只考虑了一种18R结构的LPSO相Mg12YZn、二十面体的准晶I-Mg3YZn6、与α-Mg直接平衡的W-Mg3Y2Zn3以及富Zn的Z-Mg28Y7Zn65和H-Mg15Y15Zn70。

图1 Mg-RE体系中的金属间化合物及其结构类型[35-37]
Fig. 1 Intermetallic compounds and structure types in Mg-RE systems[35-37]
GROBNER等[47]计算Mg-Y-Zn体系时考虑了18R和14H型LPSO,而Mg-Gd-Zn体系只存在一种14H的LPSO[48]。他们还研究了18R和14H的热力学稳定性以及与其他金属间化合物的相关系,阐明了18R和14H在铸态和热处理合金中的形成与转变过程。然而,到目前为止已经有4种结构的LPSO —— 10H[49-51]、14H[49-50, 52]、18R[49-50, 52]、24R[49, 52]在Mg-Y-Zn体系中被报道。可以看出,Mg-Y/Gd-Zn体系的相平衡和热力学数据还有待进一步完善。Mg-La/Ce/Nd/Sm-Zn体系中目前还没有LPSO相的报道,但这些体系的金属间化合物比较复杂。当添加Zn含量在4%(摩尔分数)以下时,通常形成REMg12相分布在α-Mg的枝晶间;而当Zn含量增高时,Mg-La/Ce-Zn合金中将形成结构未知的τ1-(Mg,Zn)11~12RE相[53-59]。
Mg-Y-Ni体系类似于Mg-Y-Zn体系,存在多种LPSO相。在300~500 ℃范围内,14H和18R相可以与α-Mg形成相平衡[60]。XU等[61]实验测定了Mg-Y-Ni体系400 ℃等温截面,观察到了13种金属间化合物,包括14H、18R和10H的LPSO相。LI等[62-66]系统研究了Mg-RE(RE=La, Ce, Nd, Y, Sm)-Ni体系的相平衡,并构建了这些体系的热力学相图,计算的Mg-RE-Ni体系400 ℃等温截面相图如图2所示。在Mg-Y-Ni体系中发现并解析了一种新型12R结构的LPSO相,Ni和Y原子富集层之间无Mg原子堆垛,是Ni、Y含量最高的LPSO结构类型[67]。图2所示为Mg-RE-TM体系已有报道的LPSO成分和对应的STEM原子图像,可以看到L12结构富集层间的Mg原子层分别从24R结构对应的4层逐渐减少到12R结构对应的0层。
添加RE元素有利于提高Mg-Al合金的耐蚀性、抗蠕变性能和燃点[73]。然而对于Mg-RE-Al体系相关系的研究并不多。Mg-Al-Y体系中只检测到一种金属间化合物Al4MgY[74]。Mg-Sm-Al[75]和Mg-La-Al[76]体系中还没有新的三元化合物报道。对于Mg-RE-Al体系,无论是否有三元化合物,其相似之处在于能与α-Mg直接平衡的相都为REAl3、REAl2、REMg12(对于不存在REMg12的体系,为RE5Mg41)和Mg17Al12[74-77]。这 些相的形成与制备工艺密切相关,比如,在快速凝固时,Mg17Al12的形成会被抑制,出现RE3Al11和REAl2相[78]。
1.2 金属间化合物的热力学稳定性
相图给出的是稳定相之间的平衡关系,而在合金体系中还存在着各种亚稳相。这些金属间化合物的稳定性与其晶体结构以及第二相/基体界面能有直接关系。金属间化合物的稳定性可以通过生成焓、混合焓、生成吉布斯自由能、晶胞体积、电子结构等判断[79-81]。由于实验测定精度的不确定性和复杂性,且随着计算方法和计算硬件条件的高速发展,有大量工作采用第一性原理(DFT)计算评估不同结构物相的稳定性。形成能Ef判据通常被用来比较金属间化合物相对于纯物质是否稳定,如MA等[82]计算得到的Mg-Zn-Y体系的14H和18R型LPSO的形成能分别为-0.0712 eV/atom和-0.0793 eV/atom,证明了14H和18R型LPSO的结构稳定性。

图2 Mg-TM-RE体系中已报道的LPSO成分[50-51, 67-72](a)和24R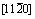 方向[52](b),14H
方向[52](b),14H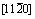 方向[52](c), 18R
方向[52](c), 18R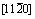 方向[52] (d),10H
方向[52] (d),10H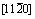 方向[51](e),12R
方向[51](e),12R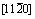 方向[67](f)及12R
方向[67](f)及12R 方向[67](g)的STEM像
方向[67](g)的STEM像
Fig. 2 Reported LPSO in Mg-TM-RE system[50-51, 67-72](a) and STEM images of 24R from 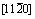 direction[52](b), 14H from
direction[52](b), 14H from 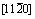 direction[52](c), 18R from
direction[52](c), 18R from 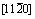 direction[52](d), 10H from
direction[52](d), 10H from 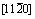 direction[51](e), 12R from
direction[51](e), 12R from 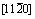 direction[67](f), 12R from
direction[67](f), 12R from  direction[67](g)
direction[67](g)
但在金属间化合物成分处,组成该成分的纯物质组合并不一定具有最低的能量。因此,仅通过DFT计算的形成能Ef判定金属间化合物能否稳定有时并不准确,还需要对比其它近邻的单相或者金属间化合物的稳定能Estab才能判定该化合物是否热力学稳定。Estab为负值说明金属间化合物是稳定的,Estab为正值但小于25 meV/atom代表金属间化合物是可能稳定存在的,Estab大于25 meV/atom表明金属间化合物是不稳定的。在预测LPSO稳定存在体系时,SAAL等[83]和LIU等[84]分别使用Estab研究了140个Mg-TM-RE体系和34个Mg-Zn/Al-RE体系中18R和14H型LPSO的稳定能,KIM等[49]以Mg、MgYZn和Mg3Y为近邻参考相计算Mg-Y-Zn体系10H、14H、18R和24R热力学稳定性,预测的Mg-TM-RE体系各种LPSO相稳定存在体系与已有文献报道吻合,而其它预测体系还尚待实验验证。他们的LPSO稳定性预测结果总结在图3中(蓝色表示LPSO稳定存在体系,黄色表示LPSO可能存在体系,红色表示LPSO无法稳定存在的体系,*为有实验观察到LPSO的体系)。
除了合金的成分和化合物的结构影响金属间化合物的稳定性外,化合物与基体界面也常常影响其形成的顺序。即使在相同铸造条件下Mg-3.44%La(质量分数)和Mg-2.87%Ce(质量分数)合金中凝固形成的是Mg12La和Mg12Ce,而Mg-2.6%Nd(质量分数)合金中形成的却是Mg3Nd[85]。基于热力学相图和晶体学匹配计算,EASTON等[86]通过相形核驱动力和形核障碍之间的竞争关系,阐明RE5Mg41、REMg12和REMg3的形成的规律为:虽然RE5Mg41在Mg-Nd体系中是稳定性,但是与基体α-Mg具有最优匹配关系的NdMg3最容易形核,其次是REMg12和RE5Mg41。目前,实验研究Mg-RE合金中析出相析出次序的文献较多,如Mg-0.99%Sm(质量分数)合金中的析出次序为过饱和固溶体(S.S.S.S)→GP区(D019-Mg3RE)→β′(Mg7RE)→ β1(fcc-Mg3RE)[87];而Mg-Nd (0.125<xNd<0.25)合金中存在β′′有序结构,析出次序复杂S.S.S.S→GP→ β′′′→β1→β(Mg12Nd)→βe(Mg41Nd5)[88]。NIE[89]详细总结了镁合金中各体系的析出序列和强化机理。无论是LPSO,还是亚稳相β″(D019)、β′(Mg7RE,体心正交结构)和β1(Mg3RE,面心立方结构),都是Mg-RE基合金中的强化相,但各相对力学性能的贡献不同,这与析出相的结构、析出相/基体界面结构以及析出相在基体析出位置有关。通过分析Mg-RE体系中β″和β′亚稳结构相的形成能、弹性模量和共格应变,可以发现:Mg-Ce/Pr/Nd合金的Mg/β″界面是稳定的,允许平板状的GP区存在于时效早期,但对于其它稀土合金,Mg/β″之间的界面是不稳定的,α-Mg和β″之间的有序化原子排列使能量降低,促进更稳定的β′相形成[90-92]。由此可以看出,界面能对析出相的异质形核和析出形貌都有重要影响[93-94],但不同稀土元素构成的金属间化合物、稀土元素在界面处的掺杂等对界面能的影响以及界面如何影响相稳定性问题还有待进一步深入研究。
1.3 稀土镁合金中相变动力学模型

图3 Mg-XL-XS三元系中LPSO稳定性计算[83-84] (XL为原子半径大于Mg的原子,XS为原子半径小于Mg的原子)
Fig. 3 Calculated stability of LPSO in Mg-XL-XS ternary systems[83-84] (XL refers to atom whose size larger than that of Mg atom, XS refers to atom whose size smaller than that of Mg atom)
镁合金在加工和服役过程中一直伴随着各种相转变,如溶质元素析出、氧化和氢化等。不同热处理温度、时间等条件直接影响合金中产物相的种类和含量,进而合金表现出不同的性能。因此,弄清楚这些相变的动力学机理,调控相转变进程,对提高镁合金的性能至关重要。长期以来应用于固态相变的经典模型有Jander模型[95]、Ginstling-Broundshtein模型[96]、Johnson-Mehl-Avrami-Kolomogorov模型(JMAK)[97-101]等。LIU等[102]对固态相变动力学进行归纳,认为一般固态相变分为3个有重叠的形核-生长-碰撞过程,还从相转变速率数据中推导了处理动力学过程的数值方程,并给出确定生长因子和有效活化能的简单方法。此外,他们考虑扩展等动力学和热力学效应,以及界面迁移、溶质扩散、错配调节三者交互效应,提出了相变动力学解析模型理论框架[102-103];从溶质与晶界交互作用原理出发,提出了考虑晶界偏聚热力学效应的亚稳平衡晶粒度模型及晶粒长大动力学模型[104-105],还研究了各相异性生长[106-107]和热力学参数[108]如温度、相转变分数在传统动力学模型中对相转变速率的影响。WANG等[109]用JMAK 模型分析Mg65Cu25Y10非晶合金的等温结晶行为,而JMAK模型只能用于单个DSC峰拟合。因此,他们提出了多峰动力学模型,其与Mg65Cu25Y10非晶合金的等温结晶动力学吻合度好,说明结晶过程机理为连续形核和三维界面扩散控制生长。钢中的析出相形成和奥氏体马氏体转变时典型的固态相变,LIU等通过形核、两个分阶段的生长和硬碰撞模型分析了Fe-0.04%C(摩尔分数)合金中奥氏体-铁素体的等温相转变[110],通过分模块的相转变模型研究了Fe-0.7%Al(摩尔分数)合金马氏体形核速率和界面迁移速率[111],以及考虑形核点饱和、界面控制的生长和碰撞校正的动力学模型研究Fe-2.96%Ni(摩尔分数)合金奥氏体-铁素体转变[112]。
虽然已报道了众多固相反应模型,但由于镁合金析出过程的复杂性,镁合金中析出相含量的定量模拟仍然进展较缓慢,比如1) 析出相有多种,即使在同一温度下等温析出,也存在特定的析出序列;2) 析出相与基体具有特殊的界面关系,影响析出相的生长形貌随析出相种类不同而改变;3) 析出相的形核驱动力难以准确计算。为了解决镁合金析出相转变的问题,ZHANG等[113]和CAO等[114]采用KWN和LSW模型,将形核的化学驱动力、基体的成分和析出相类别作为形核生长模型关键的输入参数,耦合热力学数据库、热力学计算和扩散动力学计算,预测了析出相数密度、尺寸、体积分数。PANG等[115]和LI等[116]针对前人模型中的一些共性问题,如提出的动力学参数(速率常数k和指数因子m)没有明确的物理意义和模型需要多次拟合以获得控速步骤、相变速率、活化能等信息,通过考虑自催化形核修正了经典JMAK模型,提出了形核自催化JMAK模型(NI-JMAK)。对于等温过程,模型表达式为
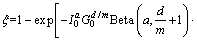
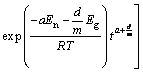 (1)
(1)
当En/(RT)>>0,对于非等温过程

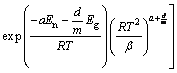 (2)
(2)
当En/RT≈0,对于非等温过程

 (3)
(3)
式中:En和Eg是形核和生长活化能;a是形核指数;d/m是生长指数;I0是形核速率常数;G0是生长速率常数;β是加热速率。该模型既可以分析等温转变,也可以模拟非等温过程动力学参数。
针对镁合金在加工和服役过程中与各种气体的反应,PANG等[116]和CHOU等[117-119]提出了一系列基于物理图景的固相反应动力学模型。考虑不同的速率限制性环节如表面渗透、原子在生成物层中的扩散、化学反应,并引入温度、表观活化能和氢压等影响,给出了不同的反应方程[117]。其表达式对于扩散控速的反应为:
 (4)
(4)
对于其它控速环节的反应为:
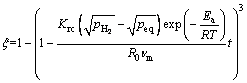 (5)
(5)
式中: 是氢原子的扩散系数;
是氢原子的扩散系数;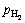 是氢在气相中的分压;peq是氢在生成物中的平衡分压;Kd和Krc是扩散和其他控速步骤的平衡系数;
是氢在气相中的分压;peq是氢在生成物中的平衡分压;Kd和Krc是扩散和其他控速步骤的平衡系数; 是取决于物质反应的相关系数。可以看到,反应分数可以清晰地表达为反应时间t、温度T、氢分压
是取决于物质反应的相关系数。可以看到,反应分数可以清晰地表达为反应时间t、温度T、氢分压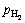 、颗粒尺寸R0和活化能Ea的方程[120]。提出“特征时间”的概念,其物理意义为反应完全所需要的时间。因此,可以用特征时间来判断反应速率的快慢,对于扩散控速的反应其表达式为[117]
、颗粒尺寸R0和活化能Ea的方程[120]。提出“特征时间”的概念,其物理意义为反应完全所需要的时间。因此,可以用特征时间来判断反应速率的快慢,对于扩散控速的反应其表达式为[117]
 (6)
(6)
结合等温拟合的方法,式(4)~(6)可以用于精确计算反应的动力学参数。当考虑生成物与反应物的密度比PBR因素时,扩散控速的反应方程推导为[119]

 (7)
(7)
该模型由于考虑了生成物与反应物的密度比z,计算精度更高[121]。
1.4 相变动力学模型在稀土镁合金中的应用
镁合金中析出相的析出过程是研究者广泛关注的问题,大量的工作通过实验测定不同温度下析出次序[122],对析出相析出过程的解析做出了巨大的贡献,但是目前对析出动力学的研究却很少。早期,PIKE和NOBLE[123]通过Avrami方程简单拟合Mg-0.5%Nd (摩尔分数)合金在升温和等温过程中的相转变动力学,给出该合金的TTT曲线(Time-Temperature- Transformation,见图4),其中给出了G.P.区(也被称为D019,Mg3RE)、β″、β′和β的稳定温度区间。类似的,YAMASAKI等[124]通过测定Mg97Zn1Gd2合金在473~800 K之间的析出次序,构建了该合金β′、β1、β和14H-LPSO析出及层错形成的TTT曲线(见图4)。TTT曲线清晰地阐明了析出相种类随温度和时间的变化规律,但无法量化析出相含量随时间的变化。
ZHANG和CAO等[113]将析出模拟耦合热力学数据库和热力学计算结合,并在Pandat软件中实现析出相的析出长大模拟,计算的AZ91合金中γ-Mg17Al12的析出数密度、析出相尺寸、体积分数与实验结果吻合较好,如图5。XIA等[125]也通过此方法预测了Mg-Sm-Zn-Zr合金中β′H-Mg5(Sm,Zn)析出相随时间的体积分数、颗粒尺寸、数密度的变化。
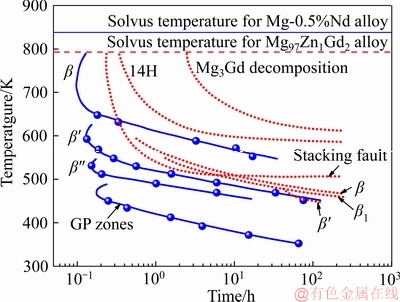
图4 Mg-0.5%Nd(摩尔分数)合金中析出相转变50%[123]和Mg97Zn1Gd2合金中析出相[124]的TTT曲线
Fig. 4 TTT curves of precipitation transformation in Mg-0.5%Nd (mole fraction) alloy (occurring 50%)[123] and Mg97Zn1Gd2 alloy[124]

图5 AZ91合金中γ-Mg17Al12的析出数密度(a)、析出相尺寸(b)、体积分数(c)和时间-温度-析出量(d)与实验结果的对比[113]
Fig. 5 Number density of precipitates(a), particle size with time(b), volume fraction of precipitates with time(c), and time-temperature-precipitate fraction curves compared(d) with experiments for γ-Mg17Al12 in AZ91 alloy[113]
镁合金作为储氢材料,其吸放氢性能如储氢容量、吸放氢速率、循环寿命等是研究者关注的焦点。有大量的工作应用已有的固相反应动力学模型来分析吸氢速率与温度、氢分压等条件的关系[126-127]。而通过实验分析结合动力学模型分析镁合金吸放氢动力学机理,可以为储氢合金的设计和性能的调控提供指导。LI等[63-66, 128-129]在设计Mg-RE(La,Ce,Nd,Y)-Ni储氢合金时,一方面从实验测定这些合金在不同温度、压力、颗粒尺寸、催化剂、制备方式等条件下的吸放氢性能数据,另一方面利用构建的动力学方程分析这些因素影响吸放氢动力学的机理,为改善合金吸放氢动力学性能提供了指导方法。
图6(a)所示为利用Nd-Mg-Ni-H热力学数据库预测的Nd4Mg80Ni8的压力-成分-温度曲线[63],给出了不同氢压下对应的相转变。通过同步辐射测定不同氢压下Nd4Mg80Ni8合金中的物相种类,精修获得各物相含量与氢压的关系如图6(b)[130],验证了热力学预测结果。为了研究Nd4Mg80Ni8合金的分解动力学,通过同步辐射测定该合金在300 ℃和2 MPa下物相随氢化时间变化的图谱。结合TEM和3DAP的微观组织分析发现,NdH2 首先从合金中析出,导致Nd4Mg80Ni8原位分解为NdH2-Mg-Mg2Ni纳米复合物,其中NdH2平均晶粒尺寸约30 nm[44]。图6(d)中通过JMAK模型分析NdH2-Mg-Mg2Ni的形成动力学,计算结果与实验吻合好,说明这三相形成为扩散控制的长大控速。该合金展现了优异的吸放氢动力学(见图6(e)和(f)),在300 ℃以上时3 min内放氢完全。

图6 Nd-Mg-Ni-H热力学数据库预测的Nd4Mg80Ni8随氢压的物相转变(a),350 ℃时相含量随氢压的变化关系(b),300 ℃时2 MPa氢压下Nd4Mg80Ni8合金首次氢化的同步辐射图谱(c),同步辐射中获得的相含量随时间的关系(d),Nd4Mg80Ni8合金的吸氢动力学曲线(e),Nd4Mg80Ni8合金的放氢动力学曲线[44, 63, 130](f)
Fig. 6 Predicted phase transformation with hydrogen pressure by Nd-Mg-Ni-H thermodynamic database(a), relations of phase fractions with hydrogen pressure(b), in-situ synchrotron powder X-ray diffraction pattern of Nd4Mg80Ni8 in the first hydrogenation at 300 ℃ under 2 MPa(c), relations of phase fraction with time(d), hydriding kinetics of Nd4Mg80Ni8(e), dehydriding kinetics of Nd4Mg80Ni8[44, 63, 130](f)
2 相和相界面对稀土镁合金性能的影响
2.1 稀土镁合金的力学性能
不同析出相对力学性能的贡献程度不同[92, 131-132],β″相和β′相的形成一般可使稀土镁合金强度达到峰 值[90],含14H型LPSO的铸态合金展现较强的抗磨损能力[133],这都与它们的结构密切相关。图7(a)给出了不同析出相的剪切模量。β″相的成分为Mg3RE,具有HCP超结构,其晶体结构用Strukturbericht符号可表示为D019。β″相在合金中一般以盘状形貌析出,具有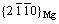 惯习面,与α-Mg之间具有共格界面,取向关系为[0001]β″//[0001]α和
惯习面,与α-Mg之间具有共格界面,取向关系为[0001]β″//[0001]α和 //
//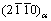 [89, 134]。β′相的成分为Mg7RE,具有正交结构。根据晶格常数b的不同,β′相可分为β′-long和β′-short,两者的晶格常数
[89, 134]。β′相的成分为Mg7RE,具有正交结构。根据晶格常数b的不同,β′相可分为β′-long和β′-short,两者的晶格常数 均约为
均约为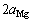 ,
, 均约为
均约为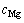 ,而
,而 约为
约为 ,
,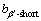 约为
约为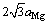 [90]。β′相的晶格常数和组成元素有关,析出形貌也会随体系的不同而发生变化,在Mg-Nd和Mg-Gd体系中为透镜状,在Mg-Y体系中呈球状。β′相的惯习面通常为
[90]。β′相的晶格常数和组成元素有关,析出形貌也会随体系的不同而发生变化,在Mg-Nd和Mg-Gd体系中为透镜状,在Mg-Y体系中呈球状。β′相的惯习面通常为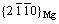 ,其与α-Mg之间的取向关系为[001]β″//[0001]α和
,其与α-Mg之间的取向关系为[001]β″//[0001]α和 //
// 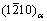 [89, 134]。在Mg-Nd等体系中β′相与α-Mg之间为共格界面,而在Mg-Y-Nd等体系中β′相与α-Mg之间仅具有半共格界面[92]。
[89, 134]。在Mg-Nd等体系中β′相与α-Mg之间为共格界面,而在Mg-Y-Nd等体系中β′相与α-Mg之间仅具有半共格界面[92]。
根据位错和析出相之间交互作用的不同,析出强化机制可以分为两类:一是位错绕过第二相的绕过强化机制,二是位错切过第二相的切过强化机制[135-136]。一般情况下,当析出相与基体共格且尺寸较小时,切过强化机制启动;而当析出相与基体具有非共格关系或尺寸较大时,绕过强化机制启动。对于时效强化镁合金,在时效处理初期,合金中存在弥散分布的细小析出相,而且这些析出相与基体的错配度较小,此时切过强化机制起作用;在时效后期,析出相颗粒尺寸增大,或合金中的析出相转变为与基体之间存在较大错配度的类型[137],会导致绕过强化机制的启动。
NIE[89, 138]发展了适用于时效强化镁合金的绕过强化机制公式,该公式包含了析出相与基体的取向关系以及析出相的形状信息,可用于评估具有不同取向和不同形状的析出相对镁合金基面滑移及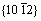 孪晶的临界分切应力(CRSS)的影响。绕过机制强化公式的基本形式如式(9)所示:
孪晶的临界分切应力(CRSS)的影响。绕过机制强化公式的基本形式如式(9)所示:
 (9)
(9)
式中: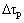 为CRSS强化增量;G为镁基体的剪切模量;b为滑移位错柏氏矢量的量级;
为CRSS强化增量;G为镁基体的剪切模量;b为滑移位错柏氏矢量的量级; 为泊松比;λ为有效面内颗粒间距;dp为颗粒平均面内直径;r0为位错核半径。通过将公式中的λ和dp替换为不同的形式,可以用于研究不同取向以及不同形状析出相的绕过强化效果。计算结果显示,柱面盘状析出相对基面滑移及
为泊松比;λ为有效面内颗粒间距;dp为颗粒平均面内直径;r0为位错核半径。通过将公式中的λ和dp替换为不同的形式,可以用于研究不同取向以及不同形状析出相的绕过强化效果。计算结果显示,柱面盘状析出相对基面滑移及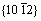 孪晶的强化效果均高于基面盘状析出相,而且其长径比的增加可以提升CRSS。对于
孪晶的强化效果均高于基面盘状析出相,而且其长径比的增加可以提升CRSS。对于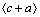 锥面滑移系,WANG等[139]的计算表明,柱面盘状析出相的强化效果同样大于基面盘状析出相。
锥面滑移系,WANG等[139]的计算表明,柱面盘状析出相的强化效果同样大于基面盘状析出相。

图7 稀土镁合金中各种析出相的剪切模量[92, 131-132](a),切过机制和绕过机制对强度的贡献[136](b),具有较高强化效果的析出相在基体结构中的析出位置[89](c)
Fig. 7 Shear modulus of different precipitates[92, 131-132](a), contribution of dislocation shearing mechanism and bypassing mechanism on yield strength of Mg alloy[136](b), effective precipitation site in α-Mg with a near-continuous network of prismatic and basal precipitate plates[89](c)
切过机制强化公式如式(10)所示,
 (10)
(10)
式中:Lp为颗粒中心的平均面内间距;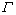 为镁基体的位错线张力;F为析出相抵抗位错切过抗力。图7(b)给出了两种机制对力学性能的贡献与颗粒尺寸的关系。除析出相的取向、尺寸及形状外,切过强化机制还与很多其它因素有关,如基体和析出相之间的界面能、共格能、模量错配、析出相的层错能以及有序强化等。NIE[89]通过计算发现,对于切过机制中的界面强化部分,柱面盘状析出相的强化效果要远高于基面盘状析出相以及球状析出相,所以最终提出析出相的有效形式如图7(c)所示。
为镁基体的位错线张力;F为析出相抵抗位错切过抗力。图7(b)给出了两种机制对力学性能的贡献与颗粒尺寸的关系。除析出相的取向、尺寸及形状外,切过强化机制还与很多其它因素有关,如基体和析出相之间的界面能、共格能、模量错配、析出相的层错能以及有序强化等。NIE[89]通过计算发现,对于切过机制中的界面强化部分,柱面盘状析出相的强化效果要远高于基面盘状析出相以及球状析出相,所以最终提出析出相的有效形式如图7(c)所示。
2.2 稀土镁合金的耐腐蚀性能
镁合金在应用于湿度较大或者生物体内环境时,存在严重的电化学腐蚀问题,其主要原因是Mg的低电极电位和疏松的腐蚀产物膜。而合金化是改善Mg耐腐蚀性能最常用的方法,其中稀土元素由于高活性并能形成致密的氧化膜,用其改善镁合金耐蚀性能是目前研究的热点。
当稀土原子固溶在α-Mg中时,合金在NaCl溶液中的自腐蚀电位向正向偏移50~150 mV不等[140-142]。SUDHOLZ等[142]认为这是由于固溶有稀土元素的α-Mg晶粒在热力学上更稳定;ESMAILY等[140]指出,自腐蚀电位的正移也可能是由于稀土的掺杂加快阴极动力学过程,降低了阴极极化程度;但也有其他研究者[143-145]认为机理为稀土元素参与并促进形成更稳定的腐蚀产物膜。添加稀土元素有利于熔炼时与有害的杂质元素如Fe、Cu、Ni等结合,生成难熔化合物成渣,降低杂质元素含量[146]。总体而言,添加少量稀土形成的单相镁合金的耐腐蚀性能往往较为优秀。但当稀土含量超过固溶度极限后,析出Mg-RE相。Mg-RE相的电势电位高于Mg基体导致基体腐蚀加剧,而固溶处理可以提高其耐蚀性。CHANG等[147]对Mg-Nd体系和LIANG等[148]对Mg-Gd-Y体系的研究都证实了这一点,通过固溶处理减少阴极相实现了在5%NaCl (质量分数)溶液中腐蚀速率分别降低50%和80%。在模拟体液中,经过固溶前处理的挤压Mg-Zn-Gd合金的腐蚀电流密度也降低了30%~40%[145]。但Mg-5Gd和Mg-4Nd合金经过固溶处理后,相比双相铸态合 金,其在0.9%NaCl(质量分数)溶液中的腐蚀速率分别发生了急剧上升和轻微下降[149]。KUBASEK和VOJTECH[149]认为这分别与Mg5Gd的生成和Mg12Nd的减少有关。

图8 镁合金及其金属间化合物在0.1 mol/L NaCl溶液和生理溶液中的腐蚀电位和腐蚀电流密度[141, 145, 156-163]
Fig. 8 Corrosion potential and corrosion current density of Mg alloys or intermetallic compounds in 0.1 mol/L NaCl solution and Hank’s solution[141, 145, 156-163]
通常铸态或变形镁合金中,通过固溶和时效形成析出相提高镁合金的力学性能,即形成双相α-Mg与Mg-RE金属间化合物双相合金[150-151]。由于Mg-RE相普遍比α-Mg的自腐蚀电位更高,也即热力学更稳定,所以对合金腐蚀行为的影响体现在正反两方面:1) 与α-Mg构成电偶对并作为阴极相加速其阳极溶解。这种现象在Mg-Nd(Mg12Nd相)[152]、Mg-La(Mg12La相)、Mg-Ce(Mg12Ce相)[153]、Mg-Gd(Mg5Gd相)[149]和Mg-Y(Mg24Y5相)[154]等多种稀土镁合金中都有相关报道。但实际上,Mg-RE相的电势电位比AZ系列、AM系列等商用镁合金中的Mg17Al12、MgZn2和Al8Mn5的要高一些[155](见图8),因此,稀土镁合金中的电偶腐蚀通常比这些合金要轻微一些。2) 由于Mg-RE在腐蚀介质中更高的稳定性,往往会起到腐蚀屏障的作用,阻止电解质与内部基体接触从而抑制腐蚀向内部扩展[164]。添加的合金元素越多,则第二相体积占比更大,这一效应也就更明显。但Mg-RE相的增多会加强电偶腐蚀的程度,导致合金整体耐蚀性下降[154, 165]。从这两方面考虑,第二相的含量、微观分布、阴极效率等特征都对镁合金的腐蚀行为起到重要影响。除此之外,一些研究者也报道了Mg-RE相在腐蚀介质中会生成较为稳定的氧化膜的现象[141]。但由于通常情况下添加的稀土元素在10%(质量分数)下,因此,形成的Mg-RE相的体积分数往往不超过30%~40%,所以当其在铸态合金晶界处形成网状共晶相时,即使其表面生成稳定的氧化膜,也很难覆盖并保护整个合金表面。对于铸态稀土镁合金来说,Mg-RE相诱发的电偶腐蚀往往主导合金表面的腐蚀过程。而在经过变形处理如挤压的稀土镁合金中,大块的网状Mg-RE变形为弥散分布的较小点状相,同时,α-Mg晶粒也随之细化,因此α-Mg与Mg-RE相之间的电偶腐蚀由于阴阳极面积同时降低会有所减轻[166]。此外,分散开来的点状相也会在合金表面促进生成相对更均匀的腐蚀产物膜[167-169]。
2.3 镁合金的储氢性能
镁合金作为储氢材料面临的瓶颈问题是吸放氢速率慢、放氢温度高和寿命短,通常采用添加一些活性组元和细化颗粒等方法来改善其动力学性能和循环寿命。Mg-RE体系用于储氢合金主要由于其高的储氢容量和REHx作为活性形核基底促进MgH2的形核和氢的吸收[37, 170]。最近,LI等[43-44]提出了一种通过直接氢化含RE元素的镁合金粉末原位形成REHx-Mg- Mg2Ni纳米复合物的新方法,可显著提高稀土镁合金的吸放氢速率和循环寿命。ZHU等[42, 171-172]发现CeH2.73-MgH2- Ni和YH2-Mg-Mg2NiH0.3展现了良好的储氢动力学性能。铸态的Mg80Ce18Ni2合金是一种多相合金,含有57% CeMg3、29% Ce2Mg17、7% CeMg 和5% CeMgNi4 (质量分数)[42]。

图9 Mg-Ni-Nd-H体系构架图和金属间化合物(a),热力学预测的富Mg角储氢容量分布图[63](b), 3DAP测定原位分解的Nd4Mg80Ni8相中各成分的分布图[44](c),NdH3-x与Mg界面结构和存在不同H空位时的能量[44](d),H原子在MgH2中扩散的能量变化(e)
Fig. 9 Mg-Ni-Nd-H system and its intermetallic compounds(a), hydrogen storage capacity distribution in Mg-rich corner predicted by thermodynamic database[63](b), composition distribution of elements in Nd4Mg80Ni8 alloy after in-situ decomposition determined by 3DAP[44](c), interface structure of NdH3-x and Mg and relative energies with different H atom vacancies[44](d), energy change for H atom diffusion between MgH2 and Mg interface(e)
为了使原位生成的REHx分布更加均匀,LUO和LI等[140, 173-174]根据构建的Mg-Ni-Nd-H热力学数据库计算该体系的相平衡,预测富Mg角的储氢容量,设计了两种具有高容量和均匀组织的储氢合金(见图9(a)和(b)),其中Nd4Mg80Ni8展现出最优的储氢性能。计算结算结果显示,富Mg角的合金氢化后都将分解为NdH2、Mg和Mg2Ni,并据此提出了直接氢化单相Mg-RE-Ni中间合金制备REHx-Mg-Mg2Ni复合物的方法。这在Mg-Ni-Y体系中也得到验证[174],原位形成的YH2和NdH2晶粒尺寸都小于35 nm,并且弥散分布于Mg和Mg2Ni基体中。进一步使用通过3DAP和SR-PXRD原位分析金属间化合物的原位分解机理为[44, 174]:由于RE元素与H的亲和力最强,首先生成大量的纳米级REHx颗粒,使得金属间化合物分解为REHx、Mg和Mg2Ni(见图9(c))。设计的均质YH2/NdH2-Mg-Mg2Ni纳米复合物展现了优异的吸放氢动力学,循环寿命长达620[43]和819[44]次。其主要原因有两方面:其一,形成的纳米复合物内具有高密度的晶界,成为H原子快速扩散的通道,提高了合金的吸放氢速率;其二,NdH3-x与Mg界面处存在H的能量势阱,相比较H在MgH2中的扩散,更容易捕获氢,并将H从4b位置沿着 方向传递到NdH3-x/Mg界面处的八面体间隙位置空位处[174],促进Mg的吸氢(见图9(d)和(e))。
方向传递到NdH3-x/Mg界面处的八面体间隙位置空位处[174],促进Mg的吸氢(见图9(d)和(e))。
3 结语与展望
稀土镁合金的力学性能、耐蚀性能和储氢性能都与合金中的金属间化合物种类、含量有关。析出相本身的强度、颗粒尺寸以及与基体的界面关系影响其对合金的强度贡献值;金属间化合物与基体的电位差决定了合金的腐蚀性能;金属间化合物与氢的亲和力决定了氢化物的类型和合金的储氢容量。稀土镁合金中关键的金属间化合物包括LPSO、β″-Mg3RE和β′-Mg7RE等,这些化合物的稳定性不仅与化合物的自身结构相关,还与成分、温度有关。通过第一性原理计算化合物的生成能Estab,结合热力学相图计算分析了LPSO稳定性和各相的存在区间,可设计具有特定金属间化合物如LPSO、Nd4Mg80Ni8等物相的合金,并预测合金的热物性。合金在固相转变中含量和尺寸受动力学控制。稀土镁合金的气固相动力学研究揭示了该系合金的反应控速环节和影响因素,结合热物性计算和吸放氢动力学,已开发出了具有高容量、长寿命的储氢合金。而由于镁合金析出过程的复杂性,目前析出相析出过程的分析还较少。因此,构建稀土镁合金体系的热物性数据库,结合动力学模型分析金属间化合物的形核生长动力学过程,对开发高性能稀土镁合金具有重要意义。
经过长期研究,研究者们总结出提升稀土镁合金性能的关键是调控其中的金属间化合物和组织分布。他们最初关注“冶金组织的遗传效应”,通过材料热力学获得合金的相关系、相组成和含量、相转变驱动力等热物理化学数据;然后,逐渐向动力学领域扩展, 建立起以多元扩散相变模拟、形核析出模拟和相场模拟结合的动力学计算方法。近年来,随着先进表征仪器的不断涌现,研究者们发现界面对轻合金性能起着极其重要的作用。因此,研究稀土镁合金中相及其界面与性能的相关性未来仍然是开发高性能镁合金的重要课题。基于大生产实况,将热/动力学集成计算与先进试验技术相结合,从目标服役性能出发,“逆向”设计稀土镁合金,有望提升稀土镁合金理性设计的科学范式,并推进新工艺的快速实用化进程。
REFERENCES
[1] 冯 艳, 陈 超, 彭超群, 王日初. 镁基复合材料的研究进展[J]. 中国有色金属学报, 2017, 27(12): 2385-2407.
FENG Yan, CHEN Chao, PENG Chao-qun, WANG Ri-chu. Research progress on magnesium matrix composites[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(12): 2385–2407.
[2] 左铁镛. 21世纪的轻质结构材料 —— 镁及镁合金发展[J]. 新材料产业, 2007(12): 22-26.
ZUO Tie-yong. The light-weight structural materials in the 21st century[J]. Advanced Materials Industry, 2007(12): 22–26.
[3] 丁文江, 吴玉娟, 彭立明, 曾小勤, 林栋樑, 陈 彬. 高性能镁合金研究及应用的新进展[J]. 中国材料进展, 2010, 29(8): 37-45.
DING Wen-jiang, WU Yu-juan, PENG Li-ming, ZENG Xiao-qin, LIN Dong-liang, CHEN Bin. Research and application development of advanced magnesium alloys[J]. Materials China, 2010, 29(8): 37-45.
[4] WU G, CHAN K C, ZHU L, SUN L, LU J. Dual-phase nanostructuring as a route to high-strength magnesium alloys[J]. Nature, 2017, 545(7652): 80-83.
[5] TRANG T T T, ZHANG J H, KIM J H, ZARGARAN A, HWANG J H, SUH B C, KIM N J. Designing a magnesium alloy with high strength and high formability[J]. Nature Communications, 2018, 9(1): 2522.
[6] ZENG Z, NIE J F, XU S W, DAVIES C H, BIRBILIS N. Super-formable pure magnesium at room temperature[J]. Nature communications, 2017, 8(1): 972.
[7] 丁文江. 镁合金科学与技术[M]. 北京: 科学出版社, 2007.
DING Wen-jiang. Magnesium science and technology[M]. Beijing: Science Press, 2007.
[8] WU Z, AHMAD R, YIN B, SANDLOBES S, CURTINW A. Mechanistic origin and prediction of enhanced ductility in magnesium alloys[J]. Science, 2018, 359(6374): 447-452.
[9] 周丽萍, 曾小勤, 李德江, 杨春明. Mg-12Gd合金的时效析出行为[J]. 中国有色金属学报, 2015, 25(6): 1409-1416.
ZHOU Li-ping, ZENG Xiao-qin, LI De-jiang, YANG Chun-ming. Ageing precipitation behavior of Mg-12Gd alloy[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(6):1409-1416.
[10] 周海涛, 曾小勤, 刘文法, 丁文江, 朱燕萍. 稀土铈对AZ61变形镁合金组织和力学性能的影响[J]. 中国有色金属学报, 2004, 14(1): 99-104.
ZHOU Hai-tao, ZENG Xiao-qin, LIU Wen-fa, DING Wen-jiang, ZHU Yan-ping. Effect of Ce on microstructures and mechanical properties of AZ61 wrought magnesium[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(1): 99-104.
[11] WANG Z Q, ZHANG B, LI D J, FRITZSCH R, ZENG X Q, ROVEN H J, DING W J. Effect of heat treatment on microstructures and mechanical properties of high vacuum die casting Mg-8Gd-3Y-0.4Zr magnesium alloy[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(12): 3762-3768.
[12] HENG W, HU L X, YUAN Y, WANG E D. Desorption behaviour and microstructure change of nanostructured hydrided AZ31 Mg alloy powders[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(4): 597-601.
[13] YUAN Y, HENG W, HU L X, SUN H F, FANG W B. Hydriding and microstructure nanocrystallization of ZK60 Mg alloy by reaction milling in hydrogen[J]. Transactions of Nonferrous Metals Society of China, 2009, 19: s363-s367.
[14] PAN Y C, ZOU J X, ZENG X Q, DING W J. Hydrogen storage properties of Mg-TiO2 composite powder prepared by arc plasma method[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(12): 3834-3839.
[15] 黄 伦, 黄光胜, 邓钱元, 汤爱涛, 蒋 斌, 潘复生. 微量Ce和Ca对AZ31组织演变及成形性能的影响[J].中国有色金属学报, 2019, 29(3): 429-438.
HUANG Lun, HUANG Guang-sheng, DENG Qian-yuan, TANG Ai-tao, JIANG Bin, PAN Fu-sheng. Effect of trace Ce and Ca on microstructure evolution and formability of AZ31 alloys[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(3): 429–438.
[16] YIN H M, JIANG B, HUANG X Y, YING Z, YANG Q S, ZHANG M X, PAN F S. Effect of Ce addition on microstructure of Mg-9Li alloy[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(7): 1936-1941.
[17] LI R H, PAN F S, JIANG B, YIN H M, LIU T T. Effects of yttrium and strontium additions on as-cast microstructure of Mg-14Li-1Al alloys[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(4): 778-783.
[18] 黄晓锋, 周 宏, 何镇明. 富铈稀土对镁合金起燃温度的影响[J]. 中国有色金属学报, 2001, 11(4): 638-641.
HUANG Xiao-feng, ZHOU Hong, HE Zhen-ming. Influence of Ce rare-earth on ignition temperature of magnesium alloy[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(8): 638-641.
[19] FABRICHNAYA O B, LUKAS H L, EFFENBERG G, ALDINGER F. Thermodynamic optimization in the Mg-Y system[J]. Intermetallics, 2003, 11(11/12): 1183-1188.
[20] ZHANG F, LIU S, DU Y. Experimental investigation and thermodynamic modeling of the La-Mg system[J]. Journal of Alloys and Compounds, 2016, 663: 279-288.
[21] BERCHE A, BENIGNI P, ROGEZ J, RECORD M C. Re-investigation of the La-Mg phase diagram[J]. Journal of Thermal Analysis and Calorimetry, 2012, 107(2): 797-807.
[22] BERCHE A, BENIGNI P, ROGEZ J, RECORD M C. Thermodynamic assessment of the La-Mg system[J]. Calphad, 2011, 35(4): 580-587.
[23] ZHANG X, KEVORKOV D, PEKGULERYUZ M. Stoichiometry study on the binary compounds in the Mg-Ce system—Part I[J]. Journal of Alloys and Compounds, 2009, 475(1/2): 361-367.
[24] ZHANG X, KEVORKOV D, PEKGULERYUZ M O. Study on the binary intermetallic compounds in the Mg-Ce system[J]. Intermetallics, 2009, 17(7): 496-503.
[25] WOOD D H, CRAMER E M. Phase relations in the magnesium-rich portion of the cerium-magnesium system[J]. Journal of the Less Common Metals, 1965, 9(5): 321-337.
[26] NAYEB-HASHEMI A A, CLARK J B. The Ce-Mg (Cerium-Magnesium) system[J]. Journal of Phase Equilibria, 1988, 9(2): 162-172.
[27] ZHANG X, KEVORKOV D, PEKGULERYUZ M O. Study on the intermetallic phases in the Mg-Ce system: Part II. Diffusion couple investigation[J]. Journal of Alloys and Compounds, 2010, 501(2): 366-370.
[28] GUO C, DU Z. Thermodynamic assessment of the Mg-Pr system[J]. Journal of Alloys and Compounds, 2005, 399(1/2): 183-188.
[29] NAYEB-HASHEMI A A, CLARK J B. The Mg-Pr system (Magnesium-Praseodymium)[J]. Journal of Phase Equilibria, 1989, 10(1): 23-27.
[30] WANG P, SCHMID-FETZER R. Thermodynamic assessment of the Mg-Pr system[J]. Computational Materials Science, 2015, 104: 138-142.
[31] NAYEB-HASHEMI A A, CLARK J B. The Mg-Nd system (Magnesium-Neodymium)[J]. Journal of Phase Equilibria, 1988, 9(5): 618-623.
[32] DELFINO S, SACCONE A, FERRO R. Phase relationships in the neodymium-magnesium alloy system[J]. Metallurgical Transactions A, 1990, 21(8): 2109-2114.
[33] GORSSE S, HUTCHINSON C R, CHEVALIER B, NIE J F. A thermodynamic assessment of the Mg-Nd binary system using random solution and associate models for the liquid phase[J]. Journal of Alloys and Compounds, 2005, 392(1/2): 253-262.
[34] ZHAI C, LUO Q, CAI Q, GUAN R, LI Q. Thermodynamically analyzing the formation of Mg12Nd and Mg41Nd5 in Mg-Nd system under a static magnetic field[J]. Journal of Alloys and Compounds, 2019, 773: 202-209.
[35] 刘楚明, 朱秀荣, 周海涛. 镁合金相图集[M]. 长沙: 中南大学出版社, 2006.
LIU Chu-ming, ZHU Xiu-rong, ZHOU Hai-tao. The phase diagram of magnesium alloys[M]. Changsha: Central South University Press, 2006.
[36] SACCONE A, DELFINO S, BORZONE G, FERRO, R. The samarium-magnesium system: A phase diagram[J]. Journal of the Less Common Metals, 1989, 154(1): 47-60.
[37] DENYS R V, POLETAEV A A, SOLBERG J K, TARASOV B P, YARTYS V A. LaMg11 with a giant unit cell synthesized by hydrogen metallurgy: Crystal structure and hydrogenation behavior[J]. Acta Materialia, 2010, 58(7): 2510-2519.
[38] KATTNER U R. The thermodynamic modeling of multicomponent phase equilibria[J]. JOM, 1997, 49(12): 14-19.
[39] LUKAS H, FRIES S G, SUNDMAN B. Computational thermodynamics: the Calphad method[M]. Cambridge university press, 2007.
[40] LUO Q, ZHAI C, SUN D, CHEN W, LI Q. Interpolation and Extrapolation with the CALPHAD Method[J]. Journal of Materials Science & Technology, 2019, 35(9): 2115-2120.
[41] KAWAMURA Y, HAYASHI K, INOUE A, MASUMOTO T. Rapidly solidified powder metallurgy Mg97Zn1Y2 alloys with excellent tensile yield strength above 600 MPa[J]. Materials Transactions, 2001, 42(7): 1172-1176.
[42] OUYANG L Z, YANG X S, ZHU M, LIU J W, DONG H W, SUN D L, YAO X D. Enhanced hydrogen storage kinetics and stability by synergistic effects of in situ formed CeH2.73 and Ni in CeH2.73-MgH2-Ni nanocomposites[J]. The Journal of Physical Chemistry C, 2014, 118(15): 7808-7820.
[43] LI Q, LI Y, LIU B, LU X, ZHANG T, GU Q F. The cycling stability of the in situ formed Mg-based nanocomposite catalyzed by YH2[J]. Journal of Materials Chemistry A, 2017, 5(33): 17532-17543.
[44] LUO Q, GU Q F, LIU B, ZHANG T F, LIU W, LI Q. Achieving superior cycling stability by in situ forming NdH2-Mg-Mg2Ni nanocomposites[J]. Journal of Materials Chemistry A, 2018, 6(46): 23308-23317.
[45] ZHOU T, XU H, CHEN H L. Phase Equilibria of the Mg-Y-Zn System at 500 ℃ in the Region of <50at. Mg and <50at.%Y[J]. Journal of Phase Equilibria and Diffusion, 2018, 39(6): 778-788.
[46] SHAO G, VARSANI V, FAN Z. Thermodynamic modelling of the Y-Zn and Mg-Zn-Y systems[J]. Calphad, 2006, 30(3): 286-295.
[47] GROBNER J, KOZLOV A, FANG X Y, GENG J, NIE J F, SCHMID-FETZER R. Phase equilibria and transformations in ternary Mg-rich Mg-Y-Zn alloys[J]. Acta Materialia, 2012, 60(17): 5948-5962.
[48] GROBNER J, KOZLOV A, FANG X Y, ZHU S, NIE J F, GIBSON M A, SCHMID-FETZER R. Phase equilibria and transformations in ternary Mg-Gd-Zn alloys[J]. Acta Materialia, 2015, 90: 400-416.
[49] KIM J K, KO W S, SANDLOBES S, HEIDELMANN M, GRABOWSKI B, RAABE D. The role of metastable LPSO building block clusters in phase transformations of an Mg-Y-Zn alloy[J]. Acta Materialia, 2016, 112: 171-183.
[50] KISHIDA, K, NAGAI K, MATSUMOTO A, YASUHARA A, INUI H. Crystal structures of highly-ordered long-period stacking-ordered phases with 18R, 14H and 10H-type stacking sequences in the Mg-Zn-Y system[J]. Acta Materialia, 2015, 99: 228-239.
[51] YAMASAKI M, MATSUSHITA M, HAGIHARA K, IZUNO H, ABE E, KAWAMURA Y. Highly ordered 10H-type long-period stacking order phase in a Mg-Zn-Y ternary alloy[J]. Scripta Materialia, 2014, 78: 13-16.
[52] NIE J F, ZHU Y M, MORTON A J. On the structure, transformation and deformation of long-period stacking ordered phases in Mg-Y-Zn alloys[J]. Metallurgical and Materials Transactions A, 2014, 45(8): 3338-3348.
[53] HUANG M L, LI H X, DING H, ZHAO J W, HAO S M. Study of intermetallics and phase equilibria of Mg-Zn-La system in Mg-rich corner at 345°C[J]. Journal of Alloys and Compounds, 2014, 612: 479-485.
[54] HUANG M L, LI H X, DING H, REN Y P, HAO S M. Isothermal section of Mg-Zn-La system in Mg rich corner at 350 ℃[J]. Acta Metallurgica Sinica (English Letters), 2008, 21(5): 329-335.
[55] HUANG M L, LI H X, REN Y P, DING H, HAO S M, CHEN H. Isothermal section of Mg-Zn-La system in Mg-rich corner at 400 ℃[J]. Transactions of Nonferrous Metals Society of China, 2007, 17(S1): s8-s11.
[56] LI H X, REN Y P, HUANG M, QIN C H, SHI M H. Phase equilibria in the Mg-rich corner of the Mg-Zn-La system at 350 ℃[J]. Rare Metals, 2006, 25(5): 572-575.
[57] HUANG M L, LI H X, HUA D, LI B, HAO S M. Intermetallics and phase relations of Mg-Zn-Ce alloys at 400 ℃[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(3): 539-545.
[58] CHIU C N, GROBNER J, KOZLOV A, SCHMID-FETZER R. Experimental study and thermodynamic assessment of ternary Mg-Zn-Ce phase relations focused on Mg-rich alloys[J]. Intermetallics, 2010, 18(4): 399-405.
[59] HUANG M L, LI H X, HUA D, REN Y P, QIN G W, HAO S M. Partial phase relationships of Mg-Zn-Ce system at 350 ℃[J]. Transactions of Nonferrous Metals Society of China, 2009, 19(3): 681-685.
[60] JIANG M, ZHANG S, BI Y, LI H, REN Y, QIN G. Phase equilibria of the long-period stacking ordered phase in the Mg-Ni-Y system[J]. Intermetallics, 2015, 57: 127-132.
[61] XU K, LIU S, HUANG D, DU Y. Experimental investigation of the isothermal section of the Mg-Ni-Y system with LPSO phases at 400 ℃[J]. Journal of Materials Science, 2018, 53(12): 9243-9257.
[62] 罗 群, 周国治, 陈双林, 李 谦, 张捷宇. 基于热力学和动力学计算的Mg-Ni-RE(La,Nd,Ce,Y)-H多元系设计及应用[J]. 中国材料进展, 2016, 35(1): 49-56.
LUO Qun, CHOU Kuo-chih, CHEN Shuang-lin, LI Qian, ZHANG Jie-yu. Material design of Mg-Ni-RE (La, Nd, Ce, Y)-H system based on thermodynamics and kinetics calculation[J]. Materials China, 2016, 35(1): 49-56.
[63] LI Q, LUO Q, GU Q F. Insights into the composition exploration of novel hydrogen storage alloys: evaluation of the Mg-Ni-Nd-H phase diagram[J]. Journal of Materials Chemistry A, 2017, 5(8): 3848-3864.
[64] LI Q, ZHANG X, AN X H, CHEN S L, ZHANG J Y. Experimental investigation and thermodynamic modeling of the phase equilibria at the Mg-Ni side in the La-Mg-Ni ternary system[J]. Journal of Alloys and Compounds, 2011, 509(5): 2478-2486.
[65] WU K B, LUO Q, CHEN S L, GU Q F, CHOU K C, WANG X L, LI Q. Phase equilibria of Ce-Mg-Ni ternary system at 673 K and hydrogen storage properties of selected alloy[J]. International Journal of Hydrogen Energy, 2016, 41(3): 1725-1735.
[66] WANG Z, LUO Q, CHEN S, CHOU K C, LI Q. Experimental investigation and thermodynamic calculation of the Mg-Ni-Y system (Y<50 at.%) at 400 and 500 ℃[J]. Journal of Alloys and Compounds, 2015, 649: 1306-1314.
[67] LIU C, ZHU Y, LUO Q, LIU B, GU Q, LI Q. A 12R long-period stacking-ordered structure in a Mg-Ni-Y alloy[J]. Journal of Materials Science & Technology, 2018, 34(12): 2235-2239.
[68] ITOI T, SEIMIYA T, KAWAMURA Y, HIROHASHI M. Long period stacking structures observed in Mg97Zn1Y2 alloy[J]. Scripta Materialia, 2004, 51(2): 107-111.
[69] ZHU Y M, MORTON A J, NIE J F. The 18R and 14H long-period stacking ordered structures in Mg-Y-Zn alloys[J]. Acta Materialia, 2010, 58(8): 2936-2947.
[70] HAGIHARA K, YOKOTANI N, UMAKOSHI Y. Plastic deformation behavior of Mg12YZn with 18R long-period stacking ordered structure[J]. Intermetallics, 2010, 18(2): 267-276.
[71] EGUSA D, ABE E. The structure of long period stacking/order Mg-Zn-RE phases with extended non-stoichiometry ranges[J]. Acta Materialia, 2012, 60(1): 166-178.
[72] YAMASHITA K, ITOI T, YAMASAKI M, KAWAMURA Y, ABE E. A novel long-period stacking/order structure in Mg-Ni-Y alloys[J]. Journal of Alloys and Compounds, 2019, 788: 277-282.
[73] 吴菊英, 单丽梅, 汤爱涛, 潘复生, 杨明波, 吴 璐. 含锶Mg-Al系镁合金中第二相研究述评[J]. 中国有色金属学报, 2017, 27(9):1757-1767.
WU Ju-ying, SHAN Li-mei, TANG Ai-tao, PAN Fu-sheng, YANG Ming-bo, WU Lu. Review of secondary phases in strontium-contained Mg-Al series magnesium alloys[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(9): 1757-1767.
[74] AL SHAKHSHIR S, MEDRAJ M. Computational thermodynamic model for the Mg-Al-Y system[J]. Journal of Phase Equilibria and Diffusion, 2006, 27(3): 231-244.
[75] JIA B R, LIU L B, YI D Q, JIN Z P, NIE J F. Thermodynamic assessment of the Al-Mg-Sm system[J]. Journal of Alloys and Compounds, 2008, 459(1/2): 267-273.
[76] WONG, C, NOGITA K, STYLES M J, ZHU S, QIU D, MCDONALD S D, EASTON M A. Solidification path and microstructure evolution of Mg-3Al-14La alloy: Implications for the Mg-rich corner of the Mg-Al-La phase diagram[J]. Journal of Alloys and Compounds, 2019, 784: 527-534.
[77] GROBNER J, KEVORKOV D, SCHMID-FETZER R. Thermodynamic modeling of Al-Ce-Mg phase equilibria coupled with key experiments[J]. Intermetallics, 2002, 10(5): 415-422.
[78] SUN W, SHI X, CINKILIC E, LUO A A. Investigation of the non-equilibrium solidification microstructure of a Mg-4Al-2RE (AE42) alloy[J]. Journal of Materials Science, 2016, 51(13): 6287-6294.
[79] KAWAMURA Y, YAMASAKI M. Formation and mechanical properties of Mg97Zn1RE2 alloys with long-period stacking ordered structure[J]. Materials Transactions, 2007, 48(11): 2986-2992.
[80] KISHIDA K, YOKOBAYASHI H, INUI H. A formation criterion for order-disorder (OD) phases of the long-period stacking order (LPSO)-type in Mg-Al-RE (Rare Earth) Ternary Systems[J]. Scientific Reports, 2017, 7(1): 12294.
[81] TANG P Y, WU M M, TANG B Y, WANG J W, PENG L M, DING W J. Microstructure of 18R-type long period ordered structure phase in Mg97Y2Zn1 alloy[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(4): 801-806.
[82] MA Z N, WANG X, YAN T T, LI Q, XU Q C, TIAN J L, WANG L. First-principles study on thermodynamic stability and electronic characteristics of long-period stacking ordered phases in Mg-Zn-Y alloys[J]. Journal of Alloys and Compounds, 2017, 708: 29-33.
[83] SAAL J E, WOLVERTON C. Thermodynamic stability of Mg-based ternary long-period stacking ordered structures[J]. Acta Materialia, 2014, 68: 325-338.
[84] LIU Z R, LI D Y. Stability and formation of long period stacking order structure in Mg-based ternary alloys[J]. Computational Materials Science, 2015, 103: 90-96.
[85] ZHU S M, GIBSON M A, EASTON M A, NIE J F. The relationship between microstructure and creep resistance in die-cast magnesium-rare earth alloys[J]. Scripta Materialia, 2010, 63(7): 698-703.
[86] EASTON M A, GIBSON M A, QIU D, ZHU S M, GROBNER J, SCHMID-FETZER, R, ZHANG M X. The role of crystallography and thermodynamics on phase selection in binary magnesium-rare earth (Ce or Nd) alloys[J]. Acta materialia, 2012, 60(11): 4420-4430.
[87] NISHIJIMA M, HIRAGA K, YAMASAKI M, KAWAMURA Y. Characterization of precipitates in Mg-Sm alloy aged at 200 ℃, studied by high-resolution transmission electron microscopy and high-angle annular detector dark-field scanning transmission electron microscopy[J]. Materials Transactions, 2009, 50(7): 1747-1752.
[88] NATARAJAN A R, VAN DER VEN A. A unified description of ordering in HCP Mg-RE alloys[J]. Acta Materialia, 2017, 124: 620-632.
[89] NIE J F. Precipitation and hardening in magnesium alloys[J]. Metallurgical and Materials Transactions A, 2012, 43(11): 3891-3939.
[90] ISSA A, SAAL J E, WOLVERTON C. Formation of high-strength β′ precipitates in Mg-RE alloys: The role of the Mg/β″ interfacial instability[J]. Acta Materialia, 2015, 83: 75-83.
[91] JI Y Z, ISSA A, HEO T W, SAAL J E, WOLVERTON C, CHEN L Q. Predicting β′ precipitate morphology and evolution in Mg-RE alloys using a combination of first-principles calculations and phase-field modeling[J]. Acta Materialia, 2014, 76: 259-271.
[92] ISSA A, SAAL J E, WOLVERTON C. Physical factors controlling the observed high-strength precipitate morphology in Mg-rare earth alloys[J]. Acta Materialia, 2014, 65: 240-250.
[93] LIU H, GAO Y, ZHU Y M, WANG Y, NIE J F. A simulation study of β1 precipitation on dislocations in an Mg-rare earth alloy[J]. Acta Materialia, 2014, 77: 133-150.
[94] LIU, H, GAO Y, LIU J Z, ZHU Y M, WANG Y, NIE J F. A simulation study of the shape of β′ precipitates in Mg-Y and Mg-Gd alloys[J]. Acta Materialia, 2013, 61(2): 453-466.
[95] JANDER W. Reaktionsgeschwindigkeiten endotherm verlaufender Umsetzungen[J]. Zeitschrift für Anorganische und Allgemeine Chemie, 1927, 163(1): 1-30.
[96] GINSTLING A M, BROUNSHTEIN B I. On diffusion kinetics in chemical reactions taking place in spherical powder grains[J]. Zhurnal Priklad Khimii, 1950, 23: 1249-1259.
[97] JOHNSON W A, MEHL R. Reaction kinetics in processes of nucleation and growth[J]. Transactions of the Metallurgical Society of AIME, 1939, 135(8): 416-442.
[98] AVRAMI M. Kinetics of phase change. I General theory[J]. The Journal of Chemical Physics, 1939, 7(12): 1103-1112.
[99] AVRAMI M. Kinetics of phase change. II transformation- time relations for random distribution of nuclei[J]. The Journal of Chemical Physics, 1940, 8(2): 212-224.
[100] AVRAMI M. Granulation, phase change, and microstructure kinetics of phase change. III[J]. The Journal of Chemical Physics, 1941, 9(2): 177-184.
[101] KOLMOGOROV A N. On the statistical theory of the crystallization of metals[J]. Bulletin of the Academy of Science of the USSR, 1937, 1(3): 355-359.
[102] LIU F, SOMMER F, BOS C. Analysis of solid state phase transformation kinetics: Models and recipes[J]. International Materials Reviews, 2007, 52(4): 193-212.
[103] SONG S J, LIU F, ZHANG Z H. Analysis of elastic-plastic accommodation due to volume misfit upon solid-state phase transformation[J]. Acta Materialia, 2014, 64: 266-281.
[104] PENG H R, GONG M M, CHEN Y Z, LIU F. Thermal stability of nanocrystalline materials: Thermodynamics and kinetics[J]. International Materials Reviews, 2017, 62(6): 303-333.
[105] CHEN Y Z, HERZ A, LI Y J, BORCHERS C, CHOI P, RAABE D, KIRCHHEIM R. Nanocrystalline Fe-C alloys produced by ball milling of iron and graphite[J]. Acta Materialia, 2013, 61(9): 3172-3185.
[106] LIU F, YANG G. Effects of anisotropic growth on the deviations from Johnson-Mehl-Avrami kinetics[J]. Acta Materialia, 2007, 55(5): 1629-1639.
[107] SONG S J, LIU F, JIANG Y H, WANG H F. Kinetics of solid-state transformation subjected to anisotropic effect: model and application[J]. Acta Materialia, 2011, 59(8): 3276-3286.
[108] JIANG Y H, LIU F, SONG S J, SUN B. Effect of thermodynamics on transformation kinetics; analysis of recipes[J]. Journal of Non-Crystalline Solids, 2013, 378: 110-114.
[109] WANG D, LIU Y, GAO Z, ZHANG Y. A multi-peak transformation kinetics model and its application to the isothermal crystallization of Mg65Cu25Y10 amorphous alloy[J]. Journal of Non-Crystalline Solids, 2008, 354(33): 3990-3999.
[110] LIU Y, WANG D, SOMMER F, MITTEMEIJER E J. Isothermal austenite-ferrite transformation of Fe-0.04 at.% C alloy: Dilatometric measurement and kinetic analysis[J]. Acta Materialia, 2008, 56(15): 3833-3842.
[111] LIU Y, LIU C, SOMMER F, MITTEMEIJER E J. Martensite formation kinetics of substitutional Fe-0.7 at.% Al alloy under uniaxial compressive stress[J]. Acta Materialia, 2015, 98: 164-174.
[112] LIU Y C, SOMMER F, MITTEMEIJER E J. Austenite-ferrite transformation kinetics under uniaxial compressive stress in Fe-2.96 at.% Ni alloy[J]. Acta Materialia, 2009, 57(9): 2858-2868.
[113] ZHANG C, CAO W, CHEN S L, ZHU J, ZHANG F, LUO A A, SCHMID-FETZER R. Precipitation simulation of AZ91 alloy[J]. JOM, 2014, 66(3): 389-396.
[114] CAO W, CHEN S L, ZHANG F, WU K, YANG Y, CHANG Y A, OATES W A. PANDAT software with PanEngine, PanOptimizer and PanPrecipitation for multi-component phase diagram calculation and materials property simulation[J]. Calphad, 2009, 33(2): 328-342.
[115] PANG Y, SUN D, GU Q, CHOU K C, WANG X, LI Q. Comprehensive determination of kinetic parameters in solid-state phase transitions: An extended Jonhson-Mehl- Avrami-Kolomogorov model with analytical solutions[J]. Crystal Growth & Design, 2016, 16(4): 2404-2415.
[116] PANG Y, LI Q. A review on kinetic models and corresponding analysis methods for hydrogen storage materials[J]. International Journal of Hydrogen Energy, 2016, 41(40): 18072-18087.
[117] CHOU K C, XU K. A new model for hydriding and dehydriding reactions in intermetallics[J]. Intermetallics, 2007, 15(5/6): 767-777.
[118] CHOU K C, LI Q, LIN Q, JIANG L J, XU K D. Kinetics of absorption and desorption of hydrogen in alloy powder[J]. International Journal of Hydrogen Energy, 2005, 30(3): 301-309.
[119] CHOU K C, LUO Q, LI Q, ZHANG J Y. Influence of the density of oxide on oxidation kinetics[J]. Intermetallics, 2014, 47: 17-22.
[120] LI Q, PAN Y, LENG H, CHOU K C. Structures and properties of Mg-La-Ni ternary hydrogen storage alloys by microwave-assisted activation synthesis[J]. Internal Journal of Hydrogen Energy, 2014, 39(26): 14247-14254.
[121] PANG Y, LI Q, LI Q, LUO Q, CHOU K C. Kinetic mechanisms of hydriding and dehydriding reactions in La-Mg-Ni alloys investigated by the modified Chou model[J]. International Journal of Hydrogen Energy, 2016, 41(21): 9183-9190.
[122] ZHOU B, WANG L, CHEN B, JIA Y, WEN W, LI D, DING W J. Study of age hardening in a Mg-2.2 wt% Nd alloy by in situ synchrotron X-ray diffraction and mechanical tests[J]. Materials Science and Engineering A, 2017, 708: 319-328.
[123] PIKE T J, NOBLE B. The formation and structure of precipitates in a dilute magnesium-neodymium alloy[J]. Journal of the Less Common Metals, 1973, 30(1): 63-74.
[124] YAMASAKI M, SASAKI M, NISHIJIMA M, HIRAGA K, KAWAMURA Y. Formation of 14H long period stacking ordered structure and profuse stacking faults in Mg-Zn-Gd alloys during isothermal aging at high temperature[J]. Acta Materialia, 2007, 55(20): 6798-6805.
[125] XIA X, SUN W, LUO A A, STONE D S. Precipitation evolution and hardening in MgSmZnZr alloys[J]. Acta Materialia, 2016, 111: 335-347.
[126] LI Q, PAN Y, LENG H, CHOU K C. Structures and properties of Mg-La-Ni ternary hydrogen storage alloys by microwave-assisted activation synthesis[J]. Internal Journal of Hydrogen Energy, 2014, 39(26): 14247-14254.
[127] LI, T X, LI Q, LONG H Y, CHOU K C, LUO Q. Interpretation of negative temperature dependence of hydriding reaction in LaNi5-Mg alloys by modified Chou model[J]. Catalysis Today, 2018, 318: 97-102.
[128] LUO Q, LI J, LI B, LIU B, SHAO H, LI Q. Kinetics in Mg-based hydrogen storage materials: Enhancement and mechanism[J]. Journal of Magnesium and Alloys, 2019, 7(1): 58-71.
[129] LUO Q, CHEN S L, ZHANG J Y, LI L, CHOU K C, LI Q. Experimental investigation and thermodynamic assessment of Nd-H and Nd-Ni-H systems[J]. Calphad, 2015, 51: 282-291.
[130] LUO Q, GU Q F, ZHANG J Y, CHEN S L, CHOUK C, LI Q. Phase equilibria, crystal structure and hydriding/dehydriding mechanism of Nd4Mg80Ni8 compound[J]. Scientific Reports, 2015, 5: 15385.
[131] PENG C, LI D, ZENG X, DING W J. First principles investigation of β′-short and β′-long in Mg-Gd alloy[J]. Journal of Alloys and Compounds, 2016, 671: 177-183.
[132] JI Y Z, ISSA A, HEO T W. Predicting β′ precipitate morphology and evolution in Mg-RE alloys using a combination of first-principles calculations and phase-field modeling[J]. Acta Materialia, 2014, 76: 259-271.
[133] CAO L J, WU Y J, PENG L M, WANG Q D, DING W J. Microstructure and tribological behavior of Mg-Gd-Zn-Zr alloy with LPSO structure[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(12): 3785-3791.
[134] 汤伊金, 章桢彦, 靳 丽, 董 杰, 丁文江. Mg-Gd系合金时效析出研究进展[J]. 中国有色金属学报, 2014, 24(1): 8-24.
TANG Yi-jin, ZHANG Zhen-yan, JIN Li, DONG Jie, DING Wen-jiang. Research progress on ageing precipitation of Mg-Gd alloys[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(1): 8-24
[135] 曾小勤, 朱庆春, 李扬欣, 丁文江. 镁合金中的第二相颗粒强化[J]. 中国材料进展, 2019, 38(3): 193-204.
ZENG Xiao-qin, ZHU Qing-chun, LI Yang-xin, DING Wen-jiang. Second phase particle strengthening in magnesium alloys[J]. Materials China, 2019, 38(3): 193-204.
[136] WANG Q, LI Z, PANG S, LI X, DONG C, LIAW P. Coherent precipitation and strengthening in compositionally complex alloys: A review[J]. Entropy, 2018, 20(11): 878.
[137] WANG D, AMSLER M, HEGDE V I, SAAL J E, ISSA A, ZHOU B C. WOLVERTON C. Crystal structure, energetics, and phase stability of strengthening precipitates in Mg alloys: A first-principles study[J]. Acta Materialia, 2018, 158: 65-78.
[138] NIE J F. Effects of precipitate shape and orientation on dispersion strengthening in magnesium alloys[J]. Scripta Materialia, 2003, 48(8): 1009-1015.
[139] WANG F, BHATTACHARYYA J J, AGNEW S R. Effect of precipitate shape and orientation on Orowan strengthening of non-basal slip modes in hexagonal crystals, application to magnesium alloys[J]. Materials Science and Engineering A, 2016, 666: 114-122.
[140] ESMAILY M, SVENSSON J E, FAJARDO S, BIRBILIS N, FRANKEL G S, VIRTANEN S, JOHANSSON L G. Fundamentals and advances in magnesium alloy corrosion[J]. Progress in Materials Science, 2017, 89: 92-193.
[141] BIRBILIS N, EASTON M A, SUDHOLZ A D. On the corrosion of binary magnesium-rare earth alloys[J]. Corrosion Science, 2009, 51(3): 683-689.
[142] SUDHOLZ A D, GUSIEVA K, CHEN X B, MUDDLE B C, GIBSON M A, BIRBILIS N. Electrochemical behaviour and corrosion of Mg-Y alloys[J]. Corrosion Science, 2011, 53(6): 2277-2282.
[143] TAKENAKA T, ONO T, NARAZAKI Y, NAKA Y, KAWAKAMI M. Improvement of corrosion resistance of magnesium metal by rare earth elements[J]. Electrochimica Acta, 2007, 53(1): 117-121.
[144] QIN H, ZHAO Y, AN Z, CHENG M, WANG Q, CHENG T, YUAN G Y. Enhanced antibacterial properties, biocompatibility, and corrosion resistance of degradable Mg-Nd-Zn-Zr alloy[J]. Biomaterials, 2015, 53: 211-220.
[145] MIAO H, HUANG H, SHI Y, ZHANG H, PEI J, YUAN G. Effects of solution treatment before extrusion on the microstructure, mechanical properties and corrosion of Mg-Zn-Gd alloy in vitro[J]. Corrosion Science, 2017, 122: 90-99.
[146] ATRENS A, SONG G L, LIU M, SHI Z, CAO F, DARGUSCH M S. Review of recent developments in the field of magnesium corrosion[J]. Advanced Engineering Materials, 2015, 17(4): 400-453.
[147] CHANG J W, GUO X W, FU P H, PENG L M, DING W J. Effect of heat treatment on corrosion and electrochemical behaviour of Mg-3Nd-0.2Zn-0.4Zr (wt.%) alloy[J]. Electrochimica Acta, 2007, 52(9): 3160-3167.
[148] LIANG S, GUAN D, TAN X. The relation between heat treatment and corrosion behavior of Mg-Gd-Y-Zr alloy[J]. Materials & Design, 2011, 32(3): 1194-1199.
[149] KUBASEK J, VOJTECH D. Structural and corrosion characterization of biodegradable Mg-RE (RE=Gd, Y, Nd) alloys[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1215-1225.
[150] ZHU W F, LUO Q, ZHANG J Y, LI Q. Phase equilibria of Mg-La-Zr system and thermal conductivity of selected alloys[J]. Journal of Alloys and Compounds, 2018, 731: 784-795.
[151] ZHU Z, PELTON A D. Thermodynamic modeling of the La-Mg-Zn, Pr-Mg-Zn and Sm-Mg-Zn system[J]. Journal of Alloys and Compounds, 2015, 652: 415-425.
[152] ZHENG X, DONG J, XIANG Y, CHANG J, WANG F, JIN L, DING W J. Formability, mechanical and corrosive properties of Mg-Nd-Zn-Zr magnesium alloy seamless tubes[J]. Materials & Design, 2010, 31(3): 1417-1422.
[153] LIU W, CAO F, CHANG L, ZHANG Z, ZHANG J. Effect of rare earth element Ce and La on corrosion behavior of AM60 magnesium alloy[J]. Corrosion Science, 2009, 51(6): 1334-1343.
[154] LIU M, SCHMUTZ P, UGGOWITZER P J. The influence of yttrium (Y) on the corrosion of Mg-Y binary alloys[J]. Corrosion Science, 2010, 52(11): 3687-3701.
[155] BEN-HAROUSH M, BEN-HAMU G, ELIEZER D, WAGNER, L. The relation between microstructure and corrosion behavior of AZ80 Mg alloy following different extrusion temperatures[J]. Corrosion Science, 2008, 50(6): 1766-1778.
[156] SüDHOLZ A D, KIRKLAND N T, BUCHHEIT R G, BIRBILIS N. Electrochemical properties of intermetallic phases and common impurity elements in magnesium alloys[J]. Electrochemical and Solid-State Letters, 2011, 14(2): C5-C7.
[157] FAJARDO S, FRANKEL G S. Effect of impurities on the enhanced catalytic activity for hydrogen evolution in high purity magnesium[J]. Electrochimica Acta, 2015, 165: 255-267.
[158] SHI P, NG W F, WONG M H, CHENG F T. Improvement of corrosion resistance of pure magnesium in Hanks’ solution by microarc oxidation with sol-gel TiO2 sealing[J]. Journal of Alloys and Compounds, 2009, 469(1-2): 286-292.
[159] WANG H, SHI Z. In vitro biodegradation behavior of magnesium and magnesium alloy[J]. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2011, 98(2): 203-209.
[160] ZONG Y, YUAN G, ZHANG X, MAO L, NIU J, DING W. Comparison of biodegradable behaviors of AZ31 and Mg-Nd-Zn-Zr alloys in Hank's physiological solution[J]. Materials Science and Engineering B, 2012, 177(5): 395-401.
[161] GU X N, ZHENG Y F, CHEN L J. Influence of artificial biological fluid composition on the biocorrosion of potential orthopedic Mg-Ca, AZ31, AZ91 alloys[J]. Biomedical Materials, 2009, 4(6): 065011.
[162] HE W, ZHANG E, YANG K. Effect of Y on the bio-corrosion behavior of extruded Mg-Zn-Mn alloy in Hank’s solution[J]. Materials Science and Engineering C, 2010, 30(1): 167-174.
[163] PENG Q, ZHAO H, LI H, MA N, TIAN Y. High pressure solidification: an effective approach to improve the corrosion properties of Mg-Y based implants[J]. International Journal Electrochemical Science, 2012, 7(6): 5581-5595.
[164] LIU X, SHAN D, SONG, Y, HAN E H. Influence of yttrium element on the corrosion behaviors of Mg-Y binary magnesium alloy[J]. Journal of Magnesium and Alloys, 2017, 5(1): 26-34.
[165] SHI Z, CAO F, SONG G L, LIU M, ATRENS A. Corrosion behaviour in salt spray and in 3.5% NaCl solution saturated with Mg (OH)2 of as-cast and solution heat-treated binary Mg-RE alloys: RE= Ce, La, Nd, Y, Gd[J]. Corrosion Science, 2013, 76: 98-118.
[166] ZENG Z, STANFORD N, DAVIES C H J, NIE J F, BIRBILIS N. Magnesium extrusion alloys: a review of developments and prospects[J]. International Materials Reviews, 2019, 64(1): 27-62.
[167] GUI Z, KANG Z, LI Y. Mechanical and corrosion properties of Mg-Gd-Zn-Zr-Mn biodegradable alloy by hot extrusion[J]. Journal of Alloys and Compounds, 2016, 685: 222-230.
[168] LIU J, SONG Y, SHAN D, HAN E H. Different microgalvanic corrosion behavior of cast and extruded EW75 Mg alloys[J]. Journal of the Electrochemical Society, 2016, 163(14): C856-C863.
[169] ZHANG X, WANG Z, YUAN G, XUE Y. Improvement of mechanical properties and corrosion resistance of biodegradable Mg-Nd-Zn-Zr alloys by double extrusion[J]. Materials Science and Engineering B, 2012, 177(13): 1113-1119.
[170] COUILLAUD S, GAUDIN E, BOBET J L. Rich magnesium ternary compound so-called LaCuMg8 derived from La2Mg17. Structure and hydrogenation behavior[J]. Intermetallics, 2011, 19(3): 336-341.
[171] LIU J W, ZOU C C, WANG H, OUYANG L Z, ZHU M. Facilitating de/hydrogenation by long-period stacking ordered structure in Mg based alloys[J]. International Journal of Hydrogen Energy, 2013, 38(25): 10438-10445.
[172] ZHANG Q A, LIU D D, WANG Q Q, FANG F, SUN D L, OUYANG L Z, ZHU M. Superior hydrogen storage kinetics of Mg12YNi alloy with a long-period stacking ordered phase[J]. Scripta Materialia, 2011, 65(3): 233-236.
[173] LI Q, LUO Q, GU Q F. Insights into the composition exploration of novel hydrogen storage alloys: Evaluation of the Mg-Ni-Nd-H phase diagram[J]. Journal of Materials Chemistry A, 2017, 5(8): 3848-3864.
[174] LI Y, GU Q, LI Q, ZHANG T. In-situ synchrotron X-ray diffraction investigation on hydrogen-induced decomposition of long period stacking ordered structure in Mg-Ni-Y system[J]. Scripta Materialia, 2017, 127: 102-107.
Relationships between key phases and their interfaces with properties in rare earth-magnesium alloys
LI Qian1, 2, 3, CHOU Kuo-chih1, 2
(1. School of Materials Science and Engineering, Shanghai University, Shanghai 200444, China;
2. State Key Laboratory of Advanced Special Steel, Shanghai University, Shanghai 200444, China;
3. Materials Genome Institute, Shanghai University, Shanghai University, Shanghai 200444, China)
Abstract: Magnesium alloys are one of the important materials to realize the lightweight of aerospace, transportation, civil construction, etc, and to alleviate the increasingly serious energy problems. In this paper, the rare earth-magnesium (RE-Mg) alloys were taken as the object. Focusing on the effect of key phases on the properties in multicomponent alloys, the intermetallic compounds and their stability in the RE-Mg system were thermodynamically analyzed, and then the recent phase transformation kinetic models as well as their applications in phase transformation mechanism analysis of RE-Mg alloys were reviewed. It discusses the effects of phase crystal structure and interface on the mechanical properties, corrosion resistance and hydrogen storage properties, and looks forward to the prospect and development directions of new type RE-Mg alloys designed by controlling the phase transformation of key phases and second phases.
Key words: rare earth-magnesium alloys; intermetallic compounds; phase interfaces; thermodynamics; kinetics
Foundation item: Project(51671118) supported by the National Natural Science Foundation of China; Project (19010500400) supported by the Science and Technology Committee of Shanghai, China
Received date: 2019-07-10; Accepted date: 2019-08-22
Corresponding author: CHOU Kuo-chih; Tel: +86-21-66136572; E-mail: kcc126@126.com
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51671118);上海市科学技术委员会科研计划项目(19010500400)
收稿日期:2019-07-10;修订日期:2019-08-22
通信作者:周国治,教授;电话:021-66136572;E-mail: kcc126@126.com
摘 要:镁合金是实现航空航天、交通运输、民用建筑等轻量化、缓解日益严重的能源问题的重要材料之一。本文以稀土镁合金为对象,聚焦多元体系中关键相对合金性能的作用机制,从热力学上分析稀土镁合金中的金属间化合物种类及其物相稳定性,进一步总结相变动力学模型及其在稀土镁合金相转变机理分析中的应用。讨论相结构和界面对稀土镁合金力学性能、耐蚀性能和储氢性能的影响规律,展望通过调控关键相和第二相转变设计新型稀土镁合金的前景和发展方向。
[1] 冯 艳, 陈 超, 彭超群, 王日初. 镁基复合材料的研究进展[J]. 中国有色金属学报, 2017, 27(12): 2385-2407.
[2] 左铁镛. 21世纪的轻质结构材料 —— 镁及镁合金发展[J]. 新材料产业, 2007(12): 22-26.
[3] 丁文江, 吴玉娟, 彭立明, 曾小勤, 林栋樑, 陈 彬. 高性能镁合金研究及应用的新进展[J]. 中国材料进展, 2010, 29(8): 37-45.
[7] 丁文江. 镁合金科学与技术[M]. 北京: 科学出版社, 2007.
DING Wen-jiang. Magnesium science and technology[M]. Beijing: Science Press, 2007.
[9] 周丽萍, 曾小勤, 李德江, 杨春明. Mg-12Gd合金的时效析出行为[J]. 中国有色金属学报, 2015, 25(6): 1409-1416.
[10] 周海涛, 曾小勤, 刘文法, 丁文江, 朱燕萍. 稀土铈对AZ61变形镁合金组织和力学性能的影响[J]. 中国有色金属学报, 2004, 14(1): 99-104.
[15] 黄 伦, 黄光胜, 邓钱元, 汤爱涛, 蒋 斌, 潘复生. 微量Ce和Ca对AZ31组织演变及成形性能的影响[J].中国有色金属学报, 2019, 29(3): 429-438.
[18] 黄晓锋, 周 宏, 何镇明. 富铈稀土对镁合金起燃温度的影响[J]. 中国有色金属学报, 2001, 11(4): 638-641.
[35] 刘楚明, 朱秀荣, 周海涛. 镁合金相图集[M]. 长沙: 中南大学出版社, 2006.
[73] 吴菊英, 单丽梅, 汤爱涛, 潘复生, 杨明波, 吴 璐. 含锶Mg-Al系镁合金中第二相研究述评[J]. 中国有色金属学报, 2017, 27(9):1757-1767.
[134] 汤伊金, 章桢彦, 靳 丽, 董 杰, 丁文江. Mg-Gd系合金时效析出研究进展[J]. 中国有色金属学报, 2014, 24(1): 8-24.
[135] 曾小勤, 朱庆春, 李扬欣, 丁文江. 镁合金中的第二相颗粒强化[J]. 中国材料进展, 2019, 38(3): 193-204.


