
Progress of electroplating and electroless plating on magnesium alloy
WU Li-ping(伍立坪), ZHAO Jing-jing(赵京京), XIE Yong-ping(谢永平),YANG Zhong-dong(杨中东)
School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 23 September 2009; accepted 30 January 2010
Abstract:
The current research processes of electroplating and electroless Ni-P alloy plating on magnesium alloys were reviewed. Theoretically, the reason for difficulties in electroplating and electroless plating on magnesium alloys was given. The zinc immersion, copper immersion, direct electroless Ni-P alloy plating and electroplating and electroless plating on magnesium alloys prepared by chemical conversion coating were presented in detail. Especially, the research development of magnesium alloy AZ91 and AZ31 was discussed briefly. Based on the analysis, the existing problems and future research directions were then given.
Key words:
magnesium alloy; electroplating; electroless Ni-P alloy; chemical conversion coating; zinc immersion/copper; direct electroless Ni-P alloy plating;
1 Introduction
The magnesium alloys are the light materials among the metals used for practical applications. They have many excellent physical and mechanical properties, such as good electromagnetic shielding effectiveness, good electrical and thermal conductivity, as well as high specific strength, and excellent anti-shock resistance and vibration absorption. Additionally, they are easy to cut, form and recycle and are affordable at a low cost. Therefore, the magnesium alloys are ideal structural materials and are reputed to be green engineering materials and epoch metals for 21st century[1]. Presently, they are widely used in aerospace, defense, auto parts, e-communication, optical instruments and portable personal computers, etc[2-3].
However, an obstacle to their further applications lies in their high chemical and electrochemical reactivity which makes them prone to oxidation and corrosion in humid atmosphere, fresh water, seawater, most of organic acids and their salts, inorganic acids and their salts[4-5]. This makes a protective surface treatment an essential part before their applications. The typical surface treatments include anodizing[6-8], chemical conversion coatings[9-11], organic coatings[12], conversion film-organic coatings, electroplating[13], electroless deposition[14] and gas phase deposition[15]. Among them, the organic and conversion coatings are the most widely used and the most mature technology. The electroplating and electroless techniques are the most promising methods for common metals due to their excellent coating properties for corrosion as well as wear resistance, solderability and convenience of operation. However, the electroplating and electroless plating techniques on magnesium alloys are at developing stage and not mature enough. The difficulties and current research progress of the electroplating and electroless Ni-P alloy plating on magnesium alloys are reviewed in this work. And the application prospect of electroplating and electroless plating is analyzed.
2 Challenges
There are two main reasons for difficulties in electroplating and electroless plating on magnesium alloys[14, 16]. One is their extremely high chemical reactivity which means that a porous and non-uniformly covered oxide or hydroxide film can be easily developed on their surface. The Pourbaix (φ—pH) diagram[17] for the magnesium alloy is shown in Fig.1. This demonstrates that the film is unstable below pH of 10 and it dissolves to produce Mg2+. Moreover, the film dissolves more quickly in weak acid solution.
The processes of the electroplating and electroless plating and the quality of the obtained coatings will be influenced without special pre-treatments. The other is that the electroplating and electroless plating will be strongly influenced by the microstructure of magnesium alloys.
However, different magnesium alloys have the same microstructure, i.e., the α matrix phase and the secondary phase. For example, the secondary phase of Mg-Al series is β phase (intermetallic compounds Mg17Al12) with body-centered cubic crystal structure[17] as shown in Fig.2.
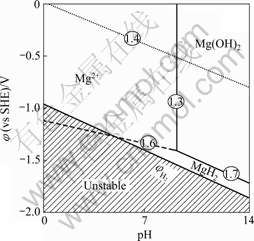
Fig.1 Pourbaix diagram of Mg-H2O system as developed by Perrault

Fig.2 Microstructure of die-cast AZ91D alloy
However, the electrochemical properties of the α and β phase differ. Taking AZ91D magnesium alloy as an example, the electrode potential of the α phase is -1.0 V, however, the electrode potential of the β phase is -1.73 V. Therefore, the substrate material is electrochemically heterogeneous and hence galvanic corrosion will have a destructive effect on the working pieces. This further makes the electroplating or electroless plating process complicated. Additionally, impurities and defects, particularly casting defects on magnesium alloys as shown in Fig.3 are unnegligible factors affecting the electroplating and electroless plating.

Fig.3 SEM image of casting flaws of die cast AZ91D magnesium alloy
Based on above-mentioned analysis, the substrate corrosion and coating plating occur simultaneously and compete with each other. If common treatments are used, the high quality of deposits is difficult to obtain or cannot be obtained. Therefore, it is necessary to apply unique pre-treatments according to the type of magnesium alloys to make their surface electrochemically homogeneous and suitable for subsequent procedures. And the substrate corrosion should be measured through electroplating or electroless plating. Besides, the electrode potential of the magnesium alloy is the lowest among that of structural materials, consequently, any metal layer on magnesium alloys functions as the cathode coating. Therefore, the electroplating and electroless deposits must be pore-free, otherwise, the effective protection cannot be ensured, and instead the substrate corrosion will be accelerated.
3 Electroplating
As above-mentioned, the corrosion happens easily on the surface of the magnesium alloys no matter in acid or alkaline electroplating solutions. Therefore, the key to the electroplating on magnesium alloys lies in the pre-treatment. Presently, two pre-treatments i.e., zinc immersion and direct electroless plating are mainly applied on magnesium alloys[18]. Recently, the copper immersion process is developed.
3.1 Zinc immersion
The zinc immersion pretreatment process has been used to remove the residual oxides and hydroxides and to produce a thin layer of zinc to prevent re-oxidation of the magnesium surface[19]. This process is first developed by Dow Chemical Company and hence called Dow process. Its sequence is as follows[20]: degrease→ cathodic cleaning→acid pickle→acid activation→ zincate→copper cyanide electroplating.
This process is complex, moreover, the obtained coating is non-adherent. A number of processes based on this pretreatment, such as the Norsk-Hydro process and the WCM canning process have been developed. The general pretreatment sequence for each of these is outlined below for comparison[21-22].
Norsk-Hydro process: degrease→acid pickle→ alkaline treatment→zincate→copper cyanide electro- plating.
WCM process: degrease→acid pickle→fluoride activation→zincate→copper cyanide electroplating.
Among the above-mentioned processes, the WCM process results in the most uniform zinc film and is the most successful in terms of adhesion, corrosion and decorative appearance. However, the disadvantages of three processes are as follows[23]. Adherent deposits will not be produced on magnesium alloys with aluminum content larger than 6%-7%. And preferential dissolution of rich α phase on the magnesium alloys occurs with all three processes, which limits the effectiveness of any of these pretreatment methods. The chromium trioxide and hydrofluoric acid are used in acid pickling and activation, respectively. Furthermore, the poisonous cyanide is frequently used through the electroplating to improve the adhesion strength of the copper deposits to the substrate surface. The electroplating poses additional challenges due to an uneven distribution of current density in the plating bath, which results in non-uniform plating of complex shapes, particularly in holes and recessed areas.
The precise control is required to ensure adequate adhesion in this process. The acid activation time has a significant effect on subsequent copper plating. If the acid activation time is too long, very little copper will be obtained. If the acid activation time is too short, non-adherent copper films will be produced. Therefore, the acid activation time is the key to success in plating on magnesium alloys.
This issue is well resolved in the patent by OLSEN et al[24]. Its flow chart is as follows: degreasing by organic solvents or alkaline cleaning baths→activation→ zinc immersion→zinc/copper electro- plating. The patent claimed that adherent copper deposits could be obtained on the zinc sub-layer, however, the acid activation solution tended to the corrode and open up any underlying porosity on the magnesium substrate. As a result, the quality of the copper depositing in terms of decorative appearance as well as corrosion was poor.
Consequently, a refined process was introduced by PEARSON et al[25]. The coatings were applied from a pyrophosphate-based zinc electrolyte solution containing a small quantity of fluoride ions. It was found that magnesium alloys containing a significant proportion of zinc can be processed for plating using an etch-free pre-treatment process, which eliminated the need for a pickling or activation stage in the plating process. It was claimed that zinc deposits with good adhesion strength to the substrate, excellent cosmetic appearance and good corrosion resistance could be obtained. Compared with conventional processes, this process had obvious commercial advantages. The basic sequence of this pretreatment is as follows: degreasing by alkaline cleaning baths→zinc immersion or zinc electro- deposition→copper electroplating→bright nickel electroplating.
In addition, the zinc immersion process was also refined by the work[26]. Reportedly, following degreasing, pickling in the mixture of phosphoric acid and nitric acid for 40 s and the surface activation in a potassium pyrophosphate containing solution, the electroplating nickel was plated onto AZ91D magnesium alloy. It was claimed that optimum time for zinc immersion varied from 30 s to 90 s. The nickel deposit with good adhesion strength to the substrate was obtained. The process flow is as follows: alkaline cleaning→acid pickling→activation→zinc immersion→ nickel electroplating.
In short, the zinc immersion pretreatment process is complex, as a result, copper immersion is developed.
3.2 Copper immersion
This pretreatment process is a new research direction. The process in the patent by LUAN and ELIZABETH[27] claiming that continuous and compact copper deposits could be obtained had many advantages: cost-competitive production, simple process, automatic stop effect in the copper immersion step and hence no need of precise control and ammonium bifluoride instead of harmful hydrofluoric acid used in activation step. However, the hydrofluoric acid was still used in copper sulfate containing immersion electrolyte. The process sequence is as follows: alkaline cleaning→acid activation→copper immersion
An orthogonal experiment adopted by YANG et al[28] optimizes the copper immersion process. The optimal experiment condition is identified as follows: etching solution using chromium oxide, activation by an alkaline solution containing potassium pyrophosphate, copper plate applied from hydrofluoric acid containing copper sulfate electrolyte accompanied with sonication. The process sequence is as follows: degreasing→ etching→activation→copper immersion.
Later, an alkaline immersion copper bath was introduced by LUAN and NAGATA[29] to substitute for the above-mentioned acid immersion copper electrolyte. Its composition includes potassium pyrophosphate, sodium fluoride, copper sulfate and sodium carbonate. And the etching solution contains potassium pyrophosphate. It was claimed that an compact and uniform copper coating was successively applied. Following copper immersion, the electroplating or electroless plating could be successively applied on magnesium alloys. Its process flow is as follows:
Cleaning with isopropanol by sonication→alkaline cleaning→chemical etching→copper immersion.
In addition, the effect of HF concentration and temperature on copper immersion on magnesium alloy was studied by YANG and LUAN[30]. Research achievements were systematically concluded in Ref.[31] and the processes of copper immersion and electroless plating were also analyzed. LUAN et al[32] claimed that the copper deposit was well applied on magnesium alloy from acid and alkali copper electrolyte followed by electroless nickel plating. The relationship between microstructure and electrochemical reaction on the substrate surface through the etching, activation and copper immersion was studied by GRAY-MUNRO et al[33]. The effect of sonication on copper immersion deposition on magnesium alloy was studied by YANG et al[34]. Reportedly, the sonication was found to effectively improve the immersion coating process and facilitate the process kinetics by the prevention or removal of fluoride containing film on the substrate surface. However, the disadvantage of all above- mentioned processes is that the hydrofluoric acid is used in the copper immersion step. Accordingly, an environment-friendly copper pyrophosphate containing treatment process is developed by LEE et al[35]. The pickling and activation step were not needed in this pretreatment and the electrolyte used for the copper immersion process was similar to that used for the copper electroplating. The process sequence is as follows: degreasing→copper immersion→copper electroplating.
3.3 Pre-electroless nickel plating
It is difficult to obtain the adherent deposit on magnesium alloys with high content of aluminum such as AZ91D Mg alloy. However, the process that following pre-electroless, the metal coating is electroplated onto the coated sample.
3.4 Alternative process
A novel pre-treatment for the electroplating is introduced by AAL[16]. Following zincate, the substrate surface was prepared by chemical conversion treatment, then the sample was immersed into a electrolyte to electrodeposit a Zn-13%Ni alloy consisting of single γ(Ni5Zn21) phase. Its process is outlined below: polishing→degreasing→pickling in phosphoric acid→ activation by HF→zincate→chemical conversion→ electrodeposition of Zn-Ni alloy.
It was said that local cells induced by interaction of different phases would not be present in this Zn-Ni alloy, therefore better corrosion resistance than that obtained from conventional preventive coatings was achieved. Moreover, a new electrolyte differing from the conventional zinc sulfate solution is used for zincate. It consists of the zinc oxide, sodium hydroxide, ferric chloride and potassium sodium tartrate. A phosphate- permanganate solution and zinc containing alkaline electrolyte were used to produce a chemical conversion coating and a Zn-Ni alloy on the coated sample, respectively. The cross-sectional microstructure of the AZ91 Mg alloy with electrodeposited Zn-13%Ni is shown in Fig.4.

Fig.4 Cross-sectional microstructure of AZ91 Mg alloy with electrodeposited Zn-13%Ni
An environment-friendly process was developed by NEUBERT et al[36-37]. Following degreasing and cleaning in flowing water, the sample was immediately transferred into a pyrophosphate bath to be anodically galvanostatic-etched for about several hundred seconds, then the copper deposit was electroplated in the same electrolyte as the etching solution. It was claimed that the quality of deposits obtained by this process in terms of uniformity, the compactness and adhesion strength was superior to that of deposits obtained by conventional methods as shown in Fig.5. Its process is outlined below: degreasing→galvanostatic etching→copper electro- plating.
An electroplating solution containing copper sulphate as the main salt and potassium sodium tartrate as the complexing agent was invented by HUANG et al[38]. The process flow is as follows: degreasing→ cleaning→ copper pre-electroplating→ copper electro- plating for thickening the copper coating layer→nickel electroplating.
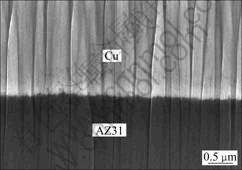
Fig.5 Cross-sectional SEM image of Cu deposited AZ31 Mg alloy
It was claimed that nickel plated magnesium alloys had excellent anti-corrosion and anti-wearing efficiency and varnish appearance. Moreover, the pre-treating solution contained no hydrofluoric acid and cyanide so that it was safe to human being through electroplating and its burden on environment after being discharged was lower.
Recently, a novel nickel source containing no ![]() and Cl- is developed in our laboratory. Following the pre-electroless nickel plating and bright copper electroplating, the bright nickel coating is electrodeposited on the magnesium alloy. The obtained deposits are uniform, gloss with good adhesion strength to the substrate surface. The corrosion resistance of the plated sample in salt spray test can reach 96 h when deposit thickness varies from 25 μm to 30 μm.
and Cl- is developed in our laboratory. Following the pre-electroless nickel plating and bright copper electroplating, the bright nickel coating is electrodeposited on the magnesium alloy. The obtained deposits are uniform, gloss with good adhesion strength to the substrate surface. The corrosion resistance of the plated sample in salt spray test can reach 96 h when deposit thickness varies from 25 μm to 30 μm.
Another possible pretreatment process for plating magnesium alloys with copper or noble metal or other coatings[39] involves an anodic treatment of the alloy, followed by coating the surface with an electrically- conductive resin film of 10-20 μm. The tin immersion[40] and direct silver electroplating[41] on magnesium alloys are also carried out.
4 Electroless plating
The electroless nickel plating method is widely used due to uniform coverage and extremely high corrosion resistance of obtained deposits. Because of unique chemical reactivity of magnesium alloys, the key to the electroless plating also lies in the pre-treatment.
4.1 Zincate and copper immersion
The flow process is as follows: magnesium alloys→zincate/copper immersion→process→copper electroplating→electroless nickel plating.
The zinc immersion or copper immersion step for the electroless plating is similar to the zinc immersion or copper immersion step for electroplating. Generally, the substrate surface will not be entirely covered by displacement films, so it is not suitable for the electroless plating on magnesium alloys immediately after the zincate or copper immersion and hence copper electroplating before electroless nickel plating is needed. A cyanide electrolyte or an alkaline pyrophosphate electrolyte is usually used for copper electroplating, however, an acid electrolyte is used for the electroless nickel plating.
4.2 Pre-electroless and direct electroless nickel plating
Adherent deposits will not be obtained on magnesium alloys with aluminum content greater than 6%-7% such as AZ91D by using the zinc immersion as the pretreatment. Then, a new technology, i.e. pre- electroless nickel plating, followed by electroless nickel plating to thicken the coating is developed by SAKATA[42] to resolve the problem. A uniformly covered, adherent deposit can be obtained by this process. The process flow is as follows: degreasing→alkaline etching→acid activation→ alkaline activation→alkaline electroless nickel strike→acid electroless nickel plating.
The nickel salt used for the pre-electroless nickel plating was basic nickel carbonate, which could avoid the corrosion of the magnesium substrate by ![]() and Cl-. The acid electroless nickel plating step was added due to expensive basic nickel carbonate. This process had been criticized for using an acid electroless nickel treatment that could result in the corrosion of the underlying magnesium if pores were present in the nickel strike layer. A simpler process has been developed by PMD (UK)[43]. The basic sequence of this pretreatment is as follows: alkaline clean→acid pickle→fluoride activation→electroless nickel plating.
and Cl-. The acid electroless nickel plating step was added due to expensive basic nickel carbonate. This process had been criticized for using an acid electroless nickel treatment that could result in the corrosion of the underlying magnesium if pores were present in the nickel strike layer. A simpler process has been developed by PMD (UK)[43]. The basic sequence of this pretreatment is as follows: alkaline clean→acid pickle→fluoride activation→electroless nickel plating.
The phosphorous content in the nickel layer varies from 4% to 5%. A bath containing basic nickel carbonate was used for the electroless plating. This process is widely used for reference.
However, its disadvantages are that the nickel source is expensive and the electroless plating solution is unstable. The acid etching, alkaline etching and activation step of the pre-electroless and direct electroless plating have a large influence on the adhesion strength of the deposit to the substrate surface. The chromium trioxide, hydrofluoric acid and a pyrophosphate containing solution are used for acid etching, acid activation and alkaline activation, respectively. They function to buffer and make the substrate surface uniform. Much attention is paid by researchers all over the world to the pre-electroless or direct electroless plating process due to its simplicity and easiness to operate[44-48]. However, its details are not introduced here.
4.3 Environment-friendly chemical conversion coatings based electroless plating
The characteristics of this process are outlined below: following degreasing, pickling and chemical conversion, the treated magnesium alloy surface is activated followed by electroless nickel plating. The process flow is as follows: degreasing→pickling→ conversion→activation→electroless nickel plating.
The so-called environmental-friendly chemical conversion is referred to the fact that a little or no hydrofluoric acid is used for conversion treatment of magnesium alloys. Stannate[11, 49], phosphate- permanganate[9, 50], molybdate[51] and other conversion coatings[52-54] are among the concrete examples. ELSENTRIECY and AZUMI[51] developed an environment-friendly chemical conversion solution. Following mechanically polishing, and pickling in the mixture of 0.25% HF and 0.25% HCl (mass fraction) solution, the sample was immersed into a solution of sodium molybdate dihydrate, the sodium nitrite and potassium nitrate to be coated with a conversion film.
Reportedly, the electroless plating layer was strongly anchored to the substrate, resulting in excellent adhesion strength (>18 MPa) (as shown in Fig.6).

Fig.6 Photo of Ni-P-plated samples after pull-off adhesion tests
But other people are cautious. According to SHARTAL and KIPOUROS[55], following conversion, the coated sample must be sealed to decrease the galvanic corrosion caused by the opening underlying Ni-P alloy as a result of crack in the conversion coating to ensure that the substrate surface is entirely and uniformly covered by the Ni-P alloy. Therefore, the sodium silicate solution is used to seal the converted sample. And the silver nitrate solution is used to activate the sealed sample, followed by electroless nickel plating due to the silicate layers produced on the surface being non-catalytic in nature. The phosphate-permanganate converted treatment is used as the pre-treatment for the electroless nickel plating. The process sequence is as follows: polishing→degreasing→pickling in phosphoric acid→activation by hydrofluoric acid→chemical conversion→sealing→surface activation→electroless nickel phosphorus plating.
Presently, using the chemical conversion treatment as the pre-treatment of the electroless plating is only an investigated research direction far from application.
5 Conclusions
More and more attention is paid to magnesium alloys from industries because of their unique performances. In particular, the AZ91D magnesium alloy is increasingly applied due to its good castability, high mechanical intensity and good ductility. However, presently, the magnesium alloys lag behind relatively in the research and development and application of surface treatment technologies. To date, no single coating technology has been developed which functions to adequately protect magnesium from corrosion in harsh service conditions. The organic coating and conversion- organic coating are more mature but far from meeting the requirement from industries. Compared with other surface finishing technologies, the electroplating and electroless plating on magnesium alloys need less investment and can obtain multi-functional, variously physiognomic deposits and can meet the requirement from all aspects. However, the waste disposal is a serious problem for electroless plating. Most of the electroless baths exibit short life service. As a result, the waste is easily generated. In addition, the use of toxic chemicals, such as chromium compounds, cyanide compounds and fluoride compounds, in the pretreatment and plating baths also necessitates further research into the development of ‘green’ plating technologies. However, for electroplating, it is difficult to obtain uniform coatings in complex shapes due to uneven throwing power of the current required for metal deposition.
The pre-treatment is crucial to high quality deposit due to unique electrochemical reaction activity of magnesium alloys which can make the substrate surface extremely difficult to plate. Many problems related to the zincate and pre-electroless nickel plating need to be resolved though these processes are applied to some extent. First one is the adhesion strength of the deposit. The electroplating or electroless plating process which can obtain better anti-corrosive and protective deposits should be developed in the precondition of good adhesion strength. The key to research and development lies in the pretreatment requirements which vary for different magnesium alloys. The practice shows that the proper processes are chosen according to the practical application conditions. Presently, the reported electroplating and electroless plating on magnesium alloys can only be applied in certain electronic components and auto parts and still have a long way to go before application, e.g. application in the wheel hub of automobiles. In addition, a large quantity of chromium trioxide, hydrofluoric acid, even violently poisonous cyanide which pollute the environment are used in the pre-treatment of magnesium alloys. The pitting can easily occur on the magnesium alloy surface in acid solutions, developing a kind of pre-treatment with no acidity, strong adaptability, long service life and low environmental load is a direction of future research.
References
[1] HEAKAL F E T, FEKRY A M, FATAYERJI M Z. Influence of halides on the dissolution and passivation behavior of AZ91D magnesium alloy in aqueous solutions [J]. Electrochimica Acta, 2009, 54(5): 1545-1557.
[2] ISHIHARA S, NOTOYA H, OKADA A, NAN Z Y, GOSHIMA T. Effect of electroless-Ni-plating on corrosion fatigue behavior of magnesium alloy [J]. Surface and Coatings Technology, 2008, 202(10): 2085-2092.
[3] ISHIZAKI T, SHIGEMATSU I, SAITO N. Anticorrosive magnesium phosphate coating on AZ31 magnesium alloy [J]. Surface and Coatings Technology, 2009, 203(16): 2288-2291.
[4] LIU Y, THOMPSON G E. Anodicilm growth on an Al-21at.%Mg alloy [J]. Corrosion Science, 2002, 44(5): 1133-1142.
[5] MORDIKE B L, EBERT T. Magnesium properties application potential [J]. Materials Sciences and Engineering, 2001, 302(1): 37-45.
[6] HIROMOTO S, SHISHIDO T, YAMAMOTO A, MARUYAMA N, SOMEKAWA H, MUKAI T. Precipitation control of calcium phosphate on pure magnesium by anodization [J]. Corrosion Science, 2008, 50(10): 2906-2913.
[7] BARBOSA D P, KN?RNSCHILD G. Anodization of Mg-alloy AZ91 in NaOH solutions [J]. Surface and Coatings Technology, 2009, 203(12): 1629-1636.
[8] KHASELEV O, WEISS D, YAHALOM J. Structure and composition of anodic films formed on binary Mg-Al alloys in KOH aluminate solutions under continuous sparking [J]. Corrosion Science, 2001, 43(7): 1295-1307.
[9] CHONG K Z, SHIH T S. Conversion-coating treatment for magnesium alloys by a permanganate-phosphate solution [J]. Mater Chem Phys, 2003, 80(1): 191-200.
[10] HAWKE D, ALBRIGHT D L. A phosphate-permanganate conversion coating for magnesium [J]. Metal Finishing, 1995, 93(10): 34-38.
[11] ZUCCHI F, FRIGNANI A, GRASSI V, TRABANELLI G, MONTICELLI C. Stannate and permanganate conversion coatings on AZ31 magnesium alloy [J]. Corrosion Science, 2007, 49(12): 4542-4552.
[12] SCHARNAGL N, BLAWERT C, DIETZEL W. Corrosion protection of magnesium alloy AZ31 by coating with polyetherimides(PEI) [J]. Surface and Coatings Technology, 2009, 203(10/11): 1423-1428.
[13] BAKKAR A, NEUBERT V. Electrodeposition onto magnesium in air and water stable ionic liquids: From corrosion to successful plating [J]. Electrochemistry Communications, 2007, 9(9): 2428-2435.
[14] ELMAHALLAWY N, BAKKAR A, SHOEIB M, PALKOWSKI H, NEUBERT V. Electroless Ni-P coating of different magnesium alloys [J]. Surface and Coatings Technology, 2008, 202(21): 5151-5157.
[15] RIE K T, WHOLE J. Plasma-CVD of TiCN and ZrCN films on light metals [J]. Surface and Coatings Technology, 1999, 112(1/3): 226-229.
[16] AAL A A. Protective coating for magnesium alloy [J]. Journal of Materials Science, 2008, 43(8): 2947-2954.
[17] SONG G, ATRENS A. Corrosion mechanisms of magnesium alloys [J]. Advanced Engineering Materials, 1999, 1(1): 11-33.
[18] HOLLSTEIN F, WIEDEMANN R, SCHOLZ J1. Characteristics of PVD-coatings on AZ31hp magnesium alloys [J]. Surface and Coatings Technology, 2003, 162(2/3): 261-268.
[19] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys—A critical review [J]. Journal of Alloys and Compunds, 2002, 336(1/2): 88-113.
[20] CHEN J, BRADHURST D H, DOU S X, LIU H K. The effect of chemical coating with Ni on the electrode properties of Mg2Ni alloy[J]. Journal of Alloys and Compounds, 1998, 280(1/2): 290-293.
[21] KATO J, URUSHIHARA W, NAKAYAMA T. Magnesium based alloys article and a method thereof. US 6068938 [P]. 2000-05-30.
[22] OLSEN, HALVORSEN S T. Method for the electrolytical metal coating of magnesium articles. US 4349390 [P]. 1982-09-14.
[23] UMEHARA H. Corrosion behavior of magnesium alloys and surface treated magnesium alloys [J]. Surface and Coatings Technology, 2006, 57(1): 42-50.
[24] OLSEN, ORKIDEVN A L. Chemical pretreatment for method for the electrolytical metal coating of magnesium articles. EP 0030305B1 [P]. 1986-09-03.
[25] PEARSON T, CORDERO-RANDO M D M. Pretreatment of magnesium substrates for electroplating. US 0039829A1 [P]. 2007-02-22.
[26] ZHANG Zi-ping, YU Gang, OUYANG Yue-jun, HE Xiao-mei, HU Bo-nian, ZHANG Jun, WU Zhen-jun. Studies on influence of zinc immersion and fluoride on nickel electroplating on magnesium alloy AZ91D [J]. Applied Surface Science, 2009, 255(17): 7773-7779.
[27] LUAN B L, ELIZABETH J. Acousto-immersion coating and process for magnesium and its alloy. US 6669997B2 [P]. 2003-12-30.
[28] YANG Lian-xi, LUAN B, CHEONG W J, JIANG Jia-ren. Optimization and performance analysis of copper immersion coating on AZ91 magnesium alloy [J]. Journal of Coatings Tech and Research, 2005, 2(6): 493-498.
[29] LUAN B, NAGATA J. Novel copper immersion coatings on magnesium alloy AZ91D in an alkaline bath [J]. Journal of Coatings Tech and Research, 2006, 3(3): 241-246.
[30] YANG Lian-xi, LUAN Ben. Copper immersion deposition on magnesium alloy: The effect of fluoride and temperature [J]. Journal of the Electrochemical Society, 2005, 152(7): 474-481.
[31] CHEONG W J. Development of a protective coating on the magnesium AZ91D alloy [D]. Ontario: The University of Western Ontario, 2005.
[32] LUAN Ben-li, GRAY J, YANG Lian-xi, CHEONG W J, SHOESMITH D. Surface modification of AZ91 magnesium alloy [J]. Materials Science Forum, 2007, 546/549(1): 513-518.
[33] GRAY-MUNRO J E, LUAN B, HUNTINGTON L. The influence of surface microchemistry in protective film formation on multi-phase magnesium alloys [J]. Applied Surface Science, 2008, 254(9): 2871-2877.
[34] YANG Lian-xi, LUAN Ben, CHEONG W J, SHOESMITH D. Sono-immersion deposition on magnesium alloy [J]. Journal of The Electrochemical Society, 2005, 152(3): 131-136.
[35] LEE J, KIM Y, KIM J, AHN Y, CHUNG W. Direct electroplating of copper on AZ31 magnesium alloy in pyrophosphate electrolyte [C]// 214th ECS Meeting. Hawaii, US. 2008.
[36] HUANG C A, WANG T H, WEIRICH T, NEUBERT V. Electro- deposition of a protective copper/nickel deposit on the magnesium alloy (AZ31) [J]. Corrosion Science, 2008, 50(5): 1385-1390.
[37] HUANG C A, WANGA T H, WEIRICH T, NEUBERT V. A pretreatment with galvanostatic etching for copper electrodeposition on pure magnesium and magnesium alloys in an alkaline copper- sulfate bath [J]. Electrochimica Acta, 2008, 53(24): 7235-7241.
[38] HUANG C A, WANG T H, CHEN K L. Cyanide-free pre-treating solution for electroplating copper coating layer on magnesium alloy surface and a pre-treating method thereof. US 20080156653A1 [P]. 2008-07-03.
[39] MASAO O. Plating method for magnesium alloy. JP 61276982 [P]. 1986-05-21.
[40] OSAMU M, MASAAKI O, ATARU Y. Coating method for article made of magnesium or magnesium base alloy. JP 55148774 [P]. 1980-04-23.
[41] NATWICK J W. Method of electrode plating silver on magnesium. US 3427232 [P]. 1969-02-11.
[42] SAKATA Y. Electroless nickel plating directly on magnesium alloy die castings [C]// The Proceedings of the 74th AESF. Annual Technical Conference. Orlando: AESF. 1987.
[43] LAWIRE B. UK company leads the way in magnesium plating [J]. Finishing, 1994, 18(11): 22-23.
[44] GEORGE R, VENKATACHALAM S, NINAN K N. Electrochemical impedance measurements on Ni-P coated magnesium alloy, chromated magnesium alloy, and anodised aluminium alloys in aqueous salt solutions [J]. British Corrosion Journal, 2002, 37(1): 37-42.
[45] AMBAT R, ZHOU W. Electroless nickel-plating on AZ91D magnesium alloy: Effect of substrate microstructure and plating parameters [J]. Surface and Coatings Technology, 2004, 179(2/3): 124-134.
[46] ANIK M, K?RPE E. Effect of alloy microstructure on electroless NiP deposition behavior on Alloy AZ91 [J]. Surface and Coatings Technology, 2007, 201(8): 4702-4710.
[47] LI J, SHAO Z, ZHANG X, TIAN Y. The electroless nickel-plating on magnesium alloy using NiSO4·6H2O as the main salt [J]. Surface and Coating Technology, 2006, 200(9): 3010-3015.
[48] HE Y D, FU H F, LI X G, GAO W. Microstructure and properties of mechanical attrition enhanced electroless Ni-P plating om magnesium alloy [J]. Scripta Materialia, 2008, 58(6): 504-507.
[49] ELSENTRIECY H H, AZUMI K, KONNO H. Effects of pH and temperature on the deposition properties of stannate chemical conversion coatings formed by the potentiostatic technique on AZ91D magnesium alloy [J]. Electrochimica Acta, 2008, 53(12): 4267-4275.
[50] ZHAO Ming, WU Shu-sen, LUO Ji-rong, FUKUDA Y, NAKAE H. A chromium-free conversion coating of magnesium alloy by a phosphate-permanganate solution [J]. Surface and Coatings Technology, 2006, 200(18/19): 5407-5412.
[51] ELSENTRIECY H H, AZUMI K. Electroless Ni-P deposition on AZ91 D magnesium alloy prepared by molybdate chemical conversion coatings [J]. Journal of the Electrochemical Society, 2009, 156(2): 70-77.
[52] MONTEMOR M F, SIMOES A M, CARMEZIM M J. Characterization of rare-earth conversion films formed on the AZ31 magnesium alloy and its relation with corrosion protection [J]. Applied Surface Science, 2007, 253(16): 6922-6931.
[53] LIN J K, HSIA C L, UAN J Y. Characterization of Mg, Al-hydrotalcite conversion film on Mg alloy and Cl- and CO32- anion-exchangeability of the film in a corrosive environment [J]. Scripta Materialia, 2007, 56(11): 927-930.
[54] ARDELEAN H, FRATEUR I, MARCUS P. Corrosion protection of magnesium alloys by cerium, zirconium and niobium-based conversion coatings [J]. Corrosion Science, 2008, 50(7): 1907-1918.
[55] SHARTAL K H M, KIPOUROS G J. Electroless nickel phosphorus plating on AZ31 [J]. Metallurgical and Materials Transactions B, 2009, 40(2): 208-222.
Corresponding author: YANG Zhong-dong; Tel: +86-24-83687727; E-mail: yangzd@smm.neu.edu.cn


