J. Cent. South Univ. Technol. (2007)02-0157-06
DOI: 10.1007/s11771-007-0032-1 ![]()
Biosorption mechanism of Cr (Ⅵ) onto cells of Synechococcus sp.
SHEN Li(申 丽), XIA Jin-lan(夏金兰), HE Huan(何 环), NIE Zhen-yuan(聂珍媛), QIU Guan-zhou(邱冠周)
(Key Laboratory of Biometallurgy of Ministry of Education, School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract:
The biosorption mechanism of Cr (Ⅵ) ions on Synechococcus sp. biosorbent was studied by analyzing the biosorption kinetics as well as speciation change and bond formation during the biosorption process. The kinetics study shows that the adsorption process of Cr (Ⅵ) consists of a very fast stage in the first several minutes, in which more than half of the saturation adsorption is attained, and a slower stage that approximately follows the first order kinetic model, basically Freundlich isotherm models were observed. Comparative studies of FT-IR spectra of K2Cr2O7, free cells of Synechococcus sp., and Cr-bound cells of Synechococcus sp. show that the speciation of chromium that binds to the cells of Synechococcus sp. is Cr (Ⅲ), instead of Cr (Ⅵ), and the carboxylic, alcoholic, amido and amino groups may be involved in the binding of Cr (Ⅲ). Integrative analyses of the surface electric potential, the effect of pH value on adsorption behavior of Cr (Ⅵ), and the results of FT-IR show that the biosorption of Cr (Ⅵ) follows two subsequent steps, biosorption of Cr2O72- by electrostatical force at the protonated active sites and reduction of Cr2O72- to Cr3+ by the reductive groups on the surface of the biosorbents.
Key words:
biosorption; Synechococcus sp.; Cr (Ⅵ); biosorption mechanism; biosorption kinetics;
1 Introduction
Water polluted with toxic Cr(Ⅵ) ions discharged from industries and inhabited areas is seriously harmful to the health of human beings and the security of the eco-system, water treatment is thus significant. Traditional wastewater treatment methods including sludge separation, chemical precipitation, electrochemical process, membrane separation, reverse osmotic treatment, ion-exchange and solvent extraction, were often expensive and impractical when they were used to treat the wastewater lower than 100 mg/L of heavy metal ions, to which, biosorption, however, can offer cost-effective treatment[1-3]. Limited essays on the removal of heavy metals from wastewater[4-7] by cyanobacterium Dunaliella, Spirulina (Arthrospira), Nostoc, anabaena or Synechococcus show cyanobacteria is one of promising biosorbents for removing Cr(Ⅵ).
Cells of Synechococcus sp., typically 2-3 μm, secrete enough viscous extracellular polysacchardes at the early stationary phase of growth while maintaining single or dicoccus, instead of Synechococcus at the later stationary phase and death phase, suggesting that the biomass of Synechococcus harvested at that time may be effective for the enrichment of metal ions due to their enough reactive polysaccharides and higher apparent surface area. GARDEA-TORRESDEY et al studied the removal of Cu (Ⅱ), Pb (Ⅱ), Ni (Ⅱ), Cd (Ⅱ) and Cr (Ⅲ) by Synechococcus[8-9], but the adsorption mechanism of Cr(Ⅵ) by Synechococcus sp. was not yet well elucidated. Many researchers studied the biosorption of Cr (Ⅵ) on various kinds of adsorbents, but very few of them stressed on studying the adsorption mechanism related to the binding and speciation state of the chromium[10-15]. In this paper, cells of Synechococcus sp. harvested at the early stage of the stationary phase were used as adsorbents, and the biosorption kinetics was studied by analyzing the effect of pH value, temperature, biosorption process characteristics and the biosorption mechanism of Cr(Ⅵ).
2 Materials and methods
2.1 Culture and harvest of cells of Synechococcus sp.
A pure strain of Synechococcus sp. obtained from the Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan city, China, was cultured in the HGZ medium(NaNO3 496 mg/L, K2HPO4 39 mg/L, CaCl2?2H2O 36 mg/L, MgSO4·7H2O 75 mg/L, Na2SiO3·9H2O 58 mg/L, PⅡ metals solution 3 mL/L, soil extract 3 mL/L) in a home-made rectangular bioreactor composed by 5 sub-rectangular compartments (each size 5 cm×5 cm×70 cm) at 25 ℃ and under continuous illumination (2 klx) and air-lift mixing through the outside-columns of compartments. The growth curve was monitored by measuring the change in turbidity of the medium with spectrophotometry at 540 nm (UV3000, Shimadzu, Japan). The cells were harvested at the early stage of the stationary phase, and filtrated via membranes with pore size of 0.45 μm. The harvested cells were washed several times with deionized distilled water, and then dried by lyophilization and stored at room temperature.
2.2 Measurement of Zeta potentials of free cells
The Zeta potentials of the free cells of Synechococcus sp. in solutions with pH values 2-12 were measured by a Zeta potential analyzer (Delsa 440SX, Coulter, USA) at 25 ℃, where the range of pH values for the measurements was defined by the analyzer.
2.3 Preparation of Cr (Ⅵ) solution
1 g/L of stock solution of Cr (Ⅵ) was prepared by dissolving quantitative K2Cr2O7 (AR) into deionized distilled water. Suitable concentrations of Cr (Ⅵ) for biosorption experiments were prepared by diluting such stock solutions with deionized distilled water.
2.4 Determination of concentrations of Cr (Ⅵ) ion
The concentration of Cr(Ⅵ) of the sample was determined by spectrophotometry (UV-3000, Shimadzu) with 1, 5 diphenylcarbazide acid solution at 540 nm[16], in which the reagent-blank was taken as the reference.
2.5 Biosorption studies
Cr (Ⅵ) biosorption experiments to account for the influences of pH value, temperature and initial concentration of Cr(Ⅵ) were carried out in 150 mL conical flasks containing 50 mL of metal ion solution and 45 mg dry biomass of Synechococcus sp. The flasks were agitated for 2 h at 70 r/min. The incubation time of biosorption was studied at definite time intervals (5 min, 10 min, 15 min, 20 min, 25 min, 30 min, 1 h, 2 h, and 3 h). 1 mL of metal ion solution taken at definite intervals or equilibrium time was centrifuged at 4 000 r/min for 25 min, and Cr(Ⅵ) ions in the supernatants were analyzed by spectrophotometer as described above.
The amount of metal ions adsorbed per unit gram of free cells biosorbents (mg metal ions /g dry biosorbents) at equilibrium and any time of biosorption were calculated respectively by the following equations[22]:
qe=0.001(ci-ce)V/m (1)
qt=0.001(ci-ct)V/m (2)
where qe, qt are the amount of metal ions adsorbed onto
the unit amount of the biosorbents (mg/g) at equilibrium and any biosorption time, respectively; ci, ce and ct are the concentrations of metal ions in the bulk solutions at the initial, equilibrium as well as any biosorption time, respectively; V is the volume of bulk solution, and m is the mass of biosorbent.
2.6 FT-IR measurements
FT-IR spectra for free cells of biosorbents before and after biosorption of Cr (Ⅵ) as well as for K2Cr2O7 were obtained by use of the KBr pellet method operated at the FT-IR analyzer Nexus 670, Nicolet, USA.
3 Results and discussion
3.1 Zeta potentials of free cells of Synechococcus sp.
The Zeta potentials of Synechococcus sp. cells in different pH media (pH value in range of 2-12) are given in Fig.1, which shows that cells of Synechococcus sp. have an isoelectric point (IEP) of about 3.0, and the cells surface is electro-negative at pH>3, and electro-positive at pH <3. This is in accordance with other kinds of algal cells[4, 13, 17]. It could be expected that the acidic value of IEP is due to the reciprocity between the numerous acidic units, e.g. uronic acidic and carboxylic groups, and few alkaline units, e.g. NH2 and NH3+ groups, on the cell surface, suggesting that at pH>3 electrostatic biosorption of positive metal ions is carried out on the cell surface because of dissociation of acidic units, while at pH<3, on the contrary, it benefits to the biosorption of negative metal ions for the protonation of alkaline units while numerous acidic units sustain un-dissociated.
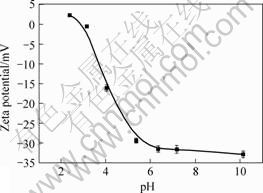
Fig. 1 Zeta potential curve of cells of Synechococcus sp.
3.2 Effects of pH value and temperature on biosorption of Cr (Ⅵ)
The effect of pH value on the biosorption of Cr (Ⅵ) is given in Fig.2, which shows that an apparent transit in biosorption of Cr (Ⅵ) on the cells surface of Synechococcus sp. occurrs at pH value of about 3, the same point as that of IEP discussed above, suggesting an electrostatic biosorption of Cr (Ⅵ) ions to the cell surface. Cr (Ⅵ) ions in acidic solution are probably diverse species [HCrO4] -, [Cr2O7] 2-, [Cr4O13] 2- and [Cr3O10] 2-, and are prone to adsorb on the protonated active sites of the biosorbent primarily [18]. Before the IEP, more protonated active sites will appear on the cells surface of Synechococcus sp., which results in the increase in biosorption of Cr (Ⅵ) ions.
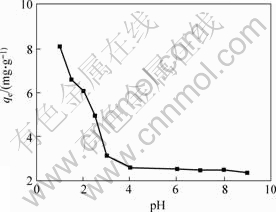
Fig.2 Effect of pH value on adsorption of Cr (Ⅵ) ions (30 ℃, initial concentration of Cr (Ⅵ) 10 mg/L, mass of adsorbents 45 mg)
Taking into account the pH stability and IEP of the biosorbents, pH 1-2 may be preferable for the removal of Cr(Ⅵ) by cells of Synechococcus. The optimum biosorptive removal of Cr (Ⅵ) at pH 2.0 was reported for biosorbents of Dunaliella sp.[4], Bacillus sp.[19] and Rhizopus nigricans[20].
The effect of temperature on the biosorption of Cr (Ⅵ) is given in Fig.3, which shows that contrary to the effect of pH value, the effect of temperature on biosorption of Cr(Ⅵ) seems much weaker in the tested temperature range of 10-50 ℃.
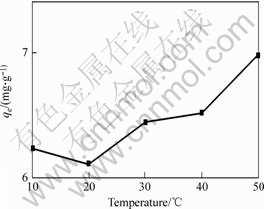
Fig.3 Effect of temperature on adsorption of Cr (Ⅵ) ions (initial concentration of Cr (Ⅵ) 10 mg/L, mass of adsorbents 45 mg, initial pH value 1.5)
3.3 Biosorption process characteristics
The biosorption process curve of Cr (Ⅵ) by cells of Synechococcus sp. at the preferable pH value and ambient temperature is shown in Fig.4, which shows that the biosorption of Cr (Ⅵ) is very fast in the very early stage of biosorption, in which the biosorption quantities attain more than half of the saturation values, and then goes into a relatively slow stage until the arrival of saturation biosorption values of Cr (Ⅵ) in about 120 min.
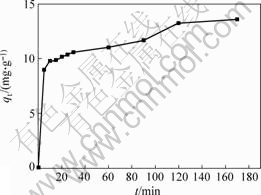
Fig.4 Adsorption curve of Cr (Ⅵ) ions under conditions of 30 ℃ and initial concentration of Cr (Ⅵ) 10 mg/L, mass of adsorbents 45 mg, and initial pH value 1.5
The fastest stage of biosorption may be principally dependent on the surface nature of the cells, which is substantially related to the composition of the proteins and carbohydrate and the charge density on the cells surface. The relatively slow stage can be approximately described by the first order kinetic model, i.e. a linear relationship between ln ct and biosorption time t (as seen in Fig.5), suggesting that this stage of the process may depend on the diffusion rate of the ions through the cells skeleton, in which the topography and surface structure of the cells may be the major effective factors. Taking into account the features of the biosorption process and the much weakly effect of temperature on the biosorption, it can be derived that the biosorbent has promising potential to be applied as a fast treatment of large volumes of industrial wastewater containing Cr(Ⅵ), under normal temperature and atmosphere conditions.
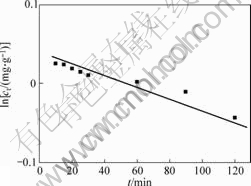
Fig.5 Modulation of relatively slow stage of adsorption curve of Cr (Ⅵ) ions in Fig.4 by the first order kinetics model
3.4 Effect of initial concentration of Cr(Ⅵ) and modeling of biosorption of Cr(Ⅵ)
The effect of initial concentration of Cr (Ⅵ) is shown in Fig.6, which shows that along with the increase in concentration of Cr (Ⅵ), the time to get adsorption equilibrium is longer and the adsorption capacity is also increased. For instance, when the initial concentration of Cr (VI) is 10 mg/L, it only needs 50 min to get equilibrium. But when the concentration is 1 000 mg/L, it needs about 360 min. The result is similar to PALMIERI[21] and CHANG Xiu-lian’s[22] research.
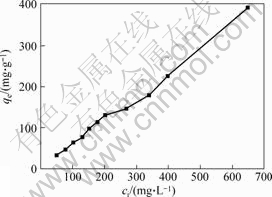
Fig.6 Equilibrium adsorption isotherms of Cr (Ⅵ) (mass of adsorbents 45 mg, pH 1.5,50 ℃)
Langmuir and Freundlich models are adopted to model the biosorptions of Cr(Ⅵ). The Langmuir equation is valid for monolayer biosorption onto a surface with a finite number of identical sites and the model is described by the following equation:
![]() (3)
(3)
or linear equation:
![]() (4)
(4)
where ce and qe are the metal ion concentration in the bulk phase and the amount of metal ions adsorbed on the adsorbent at equilibrium, respectively; qm is the maximal biosorption capacity; kd is the Langmuir constant of the system. In this model, ce/qe is linearly related to ce[17].
However, when there are interactions between adsorbed molecules, a phenomenon referred to as cooperativity occurs, that is a molecule attached to a surface may make it difficult for another molecule to become attached to a neighboring site and this would lead to a deviation from the Langmuir biosorption equation. In this case, Freundlish model may be suitable, which can be expressed by equation:
![]() (5)
(5)
or the linear equation in the form of logarithm:
![]() (6)
(6)
where KF and n are Freundlich constants. This model shows that lg qe is linearly related to lg ce.
The modeling results by Langmuir and Freundlich models are respectively shown in Figs.7(a) and (b), which indicate that the biosorption of Cr (Ⅵ) only obeys Freundlich model.
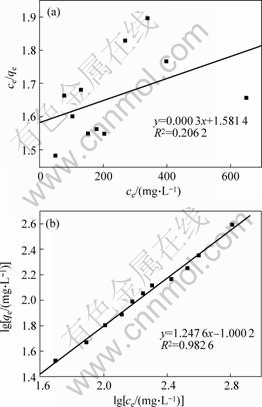
Fig.7 Modeling of Cr (Ⅵ) biosorption isotherm by Langmuir and Freundlich models(a) Langmuir model; (b) Freundlich model
3.5 Biosorption mechanisms of Cr (Ⅵ)
FT-IR spectra are useful to judge the binding states of function groups and metal ions. The comparison in IR spectra of K2Cr2O7, free cells, and Cr2O72--bound cells of Synechococcus sp. is shown in Fig.8. The frequencies of the spectra bands and their assignments are listed in Table 1. It can be derived that: 1) the larger broad bands in the range 3 000-3 500 cm-1 are attributed to the stretching of O—H, N—H of the secondary amides and NH3+ were shifted and increased in intensity after biosorption of chromium, indicating the involvements of these groups in the Cr (Ⅵ) biosorption; 2) the stronger bands 1 659, 1 546, 1 079, and 1 042 cm-1 in the spectra of free cells, which are assigned to C== O stretching in carboxyl or amide groups (amide I band), N—H bending amide II and C—N stretching in —CO—NH—, C—OH stretching, and COO- symmetric stretching, respectively, are red-shifted and intensity-increased in the spectra of Cr-bound cells, indicating the carbonyl of carboxylic and amido groups, oxygenated forms and nitrogenated forms of carbon are involved in the biosorption; 3) the larger bands in the region 500-800 cm-1 that are assigned to waggle N—H bending for hydrogen-bonded amides are apparently elongated and increased in intensity in the spectra of Cr-bound cells, further indicating the involvement of amide nitrogen in the chromium biosorption; 4) the specific bands in the region 700-980 cm-1 for K2Cr2O7 do not occur in Cr-bound cells, which, however, shows a broad band in the region of 500-700 cm-1 that may be of the waggle N—H bending for hydrogen-bonded amides. Cr2O72- cannot coordinate to the carboxyl or amide groups, so it seems that Cr2O72- must have been reduced into Cr3+.
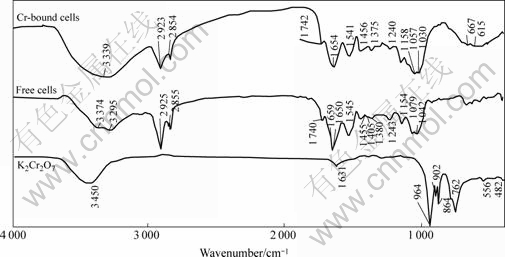
Fig.8 Comparison in FT-IR spectra of K2Cr2O7 and free cells of Synechococcus sp. and chromium-bound cells of Synechococcus sp.
Table 1 Frequency (cm-1) and assignments of FT-IR bands of free cells, Cr-bound free cells
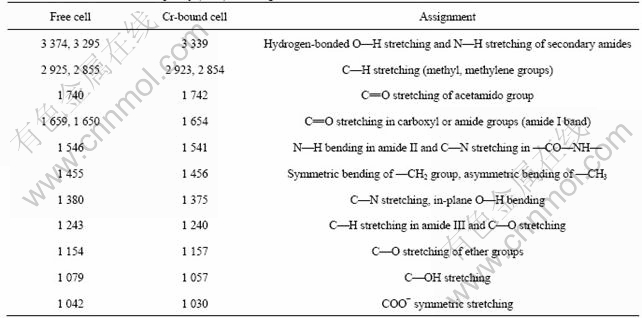
Taking into account the change in biosorption character at the IEP of the biosorbents (Figs.1 and 2), it may be deduced that the biosorption of Cr(Ⅵ) follows two subsequent steps, a step of biosorption of Cr2O72- by electrostatical force at the protonated active sites and another step of reduction of Cr2O72- to Cr3+ by the reductive groups on the surface of the biosorbents.
4 Conclusions
1) The kinetic adsorption process of Cr(Ⅵ) consists of a very fast stage in the first several minutes, in which more than half of saturation adsorption is attained, and a slower stage that approximately obeys the first order kinetic model, basically only obeys Freundlich isotherm models.
2) The biosorption of Cr (Ⅵ) follows two subsequent steps, the biosorption of Cr2O72- by electrostatical force at the protonated active sites followed by another step of reduction of Cr2O72- to Cr3+ by the reductive groups on the surface of the biosorbents.
References
[1] BAILEY S E, OLIN T J, BRICKA R M, et al. A review of potentially low-cost sorbents for heavy metals[J]. Water Res, 1999, 33(1): 2469-2479.
[2] ECCLES H. Removal of heavy metals from effluent streams-Why select a biological process[J]. Int Biodeterior Biodegrad, 1995, 35: 5-16.
[3] VOLESKY B. Advances in biosorption of metals: Selection of biomass types[J]. FEMS Microbiol Rev, 1994, 14(2/3): 291-302.
[4] DONMEZ G, AKSU Z. Removal of chromium (VI) from saline wastewaters by Dunaliella species[J]. Process Biochem, 2002, 38(5): 751-762.
[5] CHOJNACKA K, CHOJNACKI A, G?RECKA. Trace element removal by Spirulina sp. from copper smelter and refinery effluents[J]. Hydrometallurgy, 2004, 73(1/2): 137-153.
[6] EL-SHEEKH M M, EL-SHOUNY W A, OSMAN M E H, et al. Growth and heavy metals removal efficiency of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial wastewater effluents[J]. Environ Toxicol Pharmacol, 2005, 19: 357-365.
[7] GUPTA V K, SHRIVASTAVA A K, JAIN N. Biosorption of chromium (VI) from aqueous solution by green algae Spirogyra species[J]. Biotechnol Bioeng, 2001, 35(17): 4079-4085.
[8] GARDEA-TORRESDEY J L, ARENAS J L, WEBB R, et al. Ability of immobilized cyanobacteria to remove metal ions from solution and demonstration of metallothionin genes in variuos strains[J]. J Hazard Substance Res, 1997, 1(3): 1-18.
[9] GARDEA-TORRESDEY J L, ARENAS J L, WEBB R, et al. Determination of the ability of inactivated and immobilized cells of Synechococcus PCC 7942 (Cyanobacteria) to uptake metal ions from solution[C]// ERICKSON L E, RANKIN M M, GRANT S C, MCDONALD J P. Proceedings of the 12th Annual Conference on Hazardous Waste Research. Manhattan; KS, Building Partnerships for Innovative Technologies, Kansas State Univ, 1997: 16-32
[10] YU Xia, CHAI Li-yuan, MIN Xiao-bo. Removal of lead in wastewater by immobilized inactivated cells of Rhizopus oligosporus[J]. J Cent South Univ Technol, 2003, 10(4): 313-317.
[11] XIA Jin-lan, SHEN Li, et al. Camparative study of biosorption of Cr(Ⅵ) onto free and immobilized cells of symechococcus sp[J]. J Cent South Univ Technol, 2006, 37(2): 241-246.(in Chinese)
[12] CARMONA M E R, da SILVA M A P, LEITE S G F. Biosorption of chromium using factorial experimental design[J]. Process Biochem, 2005, 40(2): 779-788.
[13] TOBIN J M, ROUX J C. Mucor biosorbent for chromium removal from tanning effluent[J]. Water Res, 1998, 32(5): 1407-1416.
[14] SAG Y, KUTSAL T. The simultaneous biosorption of Cr(VI), Fe(III) and Cu(II) on Rhizopus arrhizus[J]. Process Biochem, 1998, 33(5): 571-579.
[15] D?NMEZ G ?, AKSU Z, ?ZT?RK A, KUTSAL T. A comparative study on heavy metal biosorption characteristics of some algae[J]. Process Biochem, 1999, 34(9): 885-892.
[16] EATON A D, CLESCERI L S, GREENBERG A E. Standard methods for the examination of water and waste water[S]. American Public Health Association (APHA), AWWA, WPCF: Washington, DC, 1995: 4-23.
[17] CRIST H R, OBERHOLSER K, SHANK N, et al. Nature of bonding between metallic ions and algal cell walls[J]. Environ Sci Technol, 1981, 15(10): 1212-1217.
[18] ?ZER A, ?ZER D. Comparative study of the biosorption of Pb (II), Ni (II) and Cr (VI) ions onto S. cerevisiae: Determination of biosorption heats[J]. J Hazard Mater, 2003, 100(1/3): 219-229.
[19] NURBA M? NOURBAKHSH S, KILI?ARSLAN S. Biosorption of Cr6+, Pb2+ and Cu2+ ions in industrial waste water on Bacillus sp.[J]. Chem Eng, 2002, 85(2/3): 351-355.
[20] SUDHA S R, ABRAHAM T E. Biosorption of Cr(VI) from aqueous solution by Rhizopus nigricans[J]. Bioresour Technol, 2001, 79(1): 73-81.
[21] PALMIERI M C, GARCIA JR O, MELNIKOV P. Neodymium biosorption from acidic solutions in batch system[J]. Process Biochem, 2000, 36(5): 441-444.
[22] CHANG Xiu-lian, WANG Wen-hua, WEN Shao-hong. Investigation of cadmium(II) biosorption on shrimp shell[J]. Ion Exchange and Adsorption, 2002, 18(3): 241-248. (in Chinese)
Foundation item: Project(50321402) supported by the National Natural Science Foundation of China
Received date: 2006-04-05; Accepted date: 2006-06-12
Corresponding author: XIA Jin-lan, PhD, Professor;Tel: +86-731-8836944; E-mail: jlxia@mail.csu.edu.cn
(Edited by YUAN Sai-qian)
Abstract: The biosorption mechanism of Cr (Ⅵ) ions on Synechococcus sp. biosorbent was studied by analyzing the biosorption kinetics as well as speciation change and bond formation during the biosorption process. The kinetics study shows that the adsorption process of Cr (Ⅵ) consists of a very fast stage in the first several minutes, in which more than half of the saturation adsorption is attained, and a slower stage that approximately follows the first order kinetic model, basically Freundlich isotherm models were observed. Comparative studies of FT-IR spectra of K2Cr2O7, free cells of Synechococcus sp., and Cr-bound cells of Synechococcus sp. show that the speciation of chromium that binds to the cells of Synechococcus sp. is Cr (Ⅲ), instead of Cr (Ⅵ), and the carboxylic, alcoholic, amido and amino groups may be involved in the binding of Cr (Ⅲ). Integrative analyses of the surface electric potential, the effect of pH value on adsorption behavior of Cr (Ⅵ), and the results of FT-IR show that the biosorption of Cr (Ⅵ) follows two subsequent steps, biosorption of Cr2O72- by electrostatical force at the protonated active sites and reduction of Cr2O72- to Cr3+ by the reductive groups on the surface of the biosorbents.


