J. Cent. South Univ. (2016) 23: 2784-2791
DOI: 10.1007/s11771-016-3341-4
Synthesis of size-controllable Fe3O4 magnetic submicroparticles and its biocompatible evaluation in vitro
TIAN Qing-hua(田庆华)1, NING Wen-bo(宁文博)1, WANG Wei-jia(王惟嘉)1,
YUAN Xiu-hong(袁秀洪)1, BAI Zhi-ming(白志明)2
1. School of Metallurgy and Enviroment, Cental South University, Changsha 410083, China;
2. Haikou Municipal People’s Hospital, Haikou 570100, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
Large scaled uniform and size-controllable magnetic submicroparticles (MSPs) were synthesized via solvothermal method with ferric chloride as iron source and sodium acetate as trapping agent. The influence of Fe3+ and NaAc contents on the size distribution of MSPs was investigated. The structural and morphological properties of the synthesized particles were studied by scanning electron microscopy (SEM), X-ray power diffraction (XRD) and vibrating sample magnetometer (VSM). The well-dispersed MSPs with size of 100-1000 nm were obtained by simply adjusting the contents of Fe3+ and NaAc. In addition, the hemolysis and cytotoxicity of Fe3O4 MSPs, and their ability to case arrest in cell life-cycles were studied. The results indicate that larger size could lead to lower hemolysis. From MTT(3-(4,5-dimethylthuazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, the interactions between MSPs and adhesive mouse fibroblast cell line(L929) were probed. Larger size of Fe3O4 MSPs demonstrates lower cell viability following an exposure to the cells.
Key words:
magnetite; submicroparticles; biocompatibility; hemolysis; cytotoxicity;
1 Introduction
During the past decade, considerable efforts have been made on magnetic particles due to their potential applications such as magnetic drug targeting, magnetic resonance imaging for clinical diagnosis, recording material and catalyst [1-3]. To prepare size-controllable and well-dispersed magnetic particles is the key to realize these applications [4]. It is known that there are various ways to synthesize Fe3O4 particles, such as co- precipitation method, sol-gel method, and solvothermal method [5-7]. However, well-dispersed and size- controlled Fe3O4 magnetic particles have met with limited success by those methods because of their complex process condition control such as reaction temperature, solution pH, solution concentration, stirring rate, and surfactant concentration [8-10]. On the other hand, Fe3O4 MSPs are expected to have novel physicochemical properties by adjusting their size. For example, they can penetrate into body with small size [11]. This novel property raises risks or safety concerns for biological system. Some recent studies have suggested that nanoparticles or microparticles have potential toxicity and affect biological behavior [12-15]. However, there are seldom studies on the size effect of Fe3O4 MSPs on their biocompatibility.
In this work, a simple and efficient solvothermal method to produce well-dispersed and size-controlled Fe3O4 MSPs with size of 100-1000 nm simply controlling the contents of Fe3+ and NaAc was proposed. While the size of those Fe3O4 MSPs is comparable to the dimensions of some cells(10-100 μm), viruses(20-450 nm), proteins (5-50 nm), or genes (2 nm wide and 10-100 nm long)[16-18], and the size of the magnetic particles is one of the important parameters influencing the hemolytic level and cytotoxic effects [19-20]. The hemolytic levels and cytotoxic effects on cultured fibroblast cells were measured to investigate possible relationships between the Fe3O4 MSPs with different sizes among different concentrations. The results show that Fe3O4 MSPs with larger size lead to lower hemolysis rate. And Fe3O4 MSPs of larger size demonstrate lower cell viability following an exposure to the cells.
2 Experimental
2.1 Materials and reagents
Ferric chloride hexahydrate (FeCl3·6H2O), anhydrous sodium acetate (CH3COONa, NaAc), ethylene glycol (EG) and poly ethylene glycol 200 (PEG 200) were obtained from the Sinopharm Group Chemical Reagent Co., Ltd., Beijing, China, MTT [3-(4,5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide], dimethyl sulfoxide (DMSO) and ethanol of analytical reagent grade, were purchased from Aladdin Industrial Corporation (Shanghai, China). DMEM medium, fetal bovine serum (FBS), PBS, penicillin and streptomycin were from Dawnrays (Guangdong, China). All reagents were used without further treatment. Ultrapure water with a resistivity of 18.2 MΩ/cm, produced with a Milli-Q apparatus (Millipore), was used throughout all of the experiments. Mouse fibroblast cells(L929) were provided by the China Infrastructure of Cell Line Resources (Shanghai, China). Fresh human blood was provided by Haikou People’s Hospital (Hainan, China).
2.2 Synthesis of Fe3O4 magnetic submicroparticles
Typical synthesis route of Fe3O4 magnetic submicroparticles (MSPs) is as the following [21-22]: 1.35 g FeCl3·6H2O (5 mmol) was dissolved in ethylene glycol (30 mL) to form a clear solution, followed by the addition of 3.6 g NaAc (43.7 mmol) and polyethylene glycol (2 mL). The mixture was stirred vigorously for 30 min and then sealed in a teflon-lined stainless-steel autoclave (50 mL capacity). The autoclave was heated to and maintained at 200 °C for 10 h, and allowed to cool to room temperature. The black products were washed several times with ethanol and dried at 60 °C for 6 h. The specific experimental conditions with different contents of Fe3+ are 1, 2, 4, 6 and 8 mmol, and different conditions of NaAc are 6.10, 12.20, 24.44, 36.57 and 48.76 mmol.
2.3 Characterization
2.3.1 Morphology and phase characterization of MSPs
Scanning electron microscopy (SEM, JSM-6360) was used to characterize the morphology of the nanoparticles. The X-ray diffraction (XRD) patterns of the Fe3O4 were obtained using Rigaku D/Max-RB diffractometer with Cu Kα radiation (λ=0.15406 nm,35 kV, 40 mA). Magnetic properties of the product were investigated using a vibrating sample magnetometer (VSM, EV7, ADE) with an applied field between -48 and 48 kA/m at room temperature.
2.3.2 Hemolysis test of Fe3O4 MSPs
Blood, anti-coagulated with sodium citrate, was obtained from healthy volunteers, and diluted with 0.9% saline at the volume ratio of 8:10. The dried Fe3O4 MSPs were rinsed three times with 0.9% saline, with a final concentration reached of 10 mg/mL. 0.9% saline and distilled water were used as negative control (0% hemolysis) and the positive control (100% hemolysis). The Fe3O4 MSPs with different sizes were divided into five concentration groups: 0.0625, 0.25, 5, 1 and 10 mg/mL. Each group contained three test tubes, each of which contained either 2 mL liquor of the Fe3O4 MSPs, 0.9% saline, or distilled water. Then, 25 μL of diluted blood was added to each tube preheated for 30 min at 37 °C. After incubation for 120 min at 37 °C in water bath shaker with moderate shaking, the tubes were centrifugated at 2500 r/min for 5 min. Finally, the supernatant fluid was assembled, and optical density (OD, Do), values were measured at 540 nm by ELISA. The hemolysis rate (HR) was calculated using the mean OD value for each group as follows [23]:
Rh=(Dt-Dnc)/(Dpc-Dnc)×100%
where Dt is the absorbance of the testing sample; Dpc and Dnc are the absorbance of the positive control and the negative control, respectively [23-24].
2.3.3 Cytotoxic evaluation of MSPs
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylte- trazolim bromide) was performed on the L929 cells in vitro to evaluate the cytotoxicity [25-26]. Cells with a density of 1×104 cells per well were seeded in a 96-well cell culture plate, and cultured in 5% CO2 at 37 °C for 24 h at first. Then the wells were divided into six groups (four wells per group). 50 uL Fe3O4 MSPs suspensions with various concentrations, as experimental groups, were added to replace equal volume of the medium with the final concentrations of 1.0000, 0.2500, 0.0625, 0.0156 mg/mL. 0.05 mL of PBS(phosphate buffered saline) and DMSO(dimethylsulfoxide) was added into the negative group and the positive group. After 48 h of incubation, 0.05 mL of MTT solution was added to each well and incubated for further 4 h. Since L929 cells metabolized the MTT in their mitochondria and formed blue formazan crystals, 0.15 mL of DMSO was added into each well to dissolve the formed crystals after incubation and discarding the suspension. The ELISA plate reader was used to read the wells at 570 nm to get the OD. The cell relative growth rate (RGR, Rrg) was calculated as follows [27]:
Rrg=Doeg /Donce
where Doeg is the optical density of experimental group and Doncg is the optical density of negative control group.
The toxicity grade is precise when the experimental results show grade 0 and grade 1. If the results show grade 2, the toxicity should be evaluated by both RGR and the morphological changes of cultured cells [27]. The toxicity is uncertain when the results belong to other grades (Table 1).
Table 1 RGR and toxicity grade conversion

3 Results and discussion
3.1 Influence of Fe3+ content on size of Fe3O4 MSPs
The structure of as-prepared Fe3O4 was measured by XRD, as shown in Fig. 1. All the diffraction peaks at 18.32°, 30.10°, 35.48°, 43.10°, 53.40°, 57.02° and 62.58° can be indexed to the indices (111), (220), (311), (400), (422), (511), and (440) of Fe3O4.
The SEM images and size distribution of the Fe3O4 MSPs are presented in Figs. 2(a)-(e) and (f), respectively. Particle size distribution and the dependence of Fe3O4 MSPs particle size on Fe3+ contents of 1, 2, 4, 6 and 8 mmol are shown in Fig. 2(f), respectively. As seen, Figs. 2(a)-(e) and (f) show that the size of mono- dispersed particles monotonously increases from 186 nm (Fig. 2(a)) to 1021 nm (Fig. 2(e)) with the increasing Fe3+ content from 1 mmol to 6 mmol, and then the particle size decreases to 759 nm when Fe3+ content is 8 mmol. Briefly speaking, the SEM images, XRD pattern and dependent curve of particle size to Fe3+ content indicate that controlling the Fe3+ content is an effective way to achieve Fe3O4 synthesis strategy with tunable size.
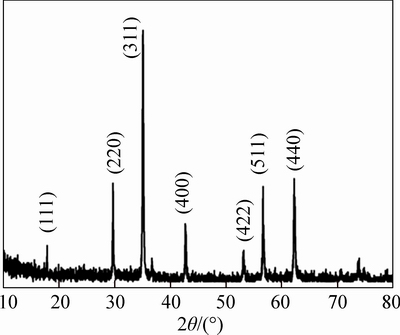
Fig. 1 XRD pattern of as-prepared Fe3O4
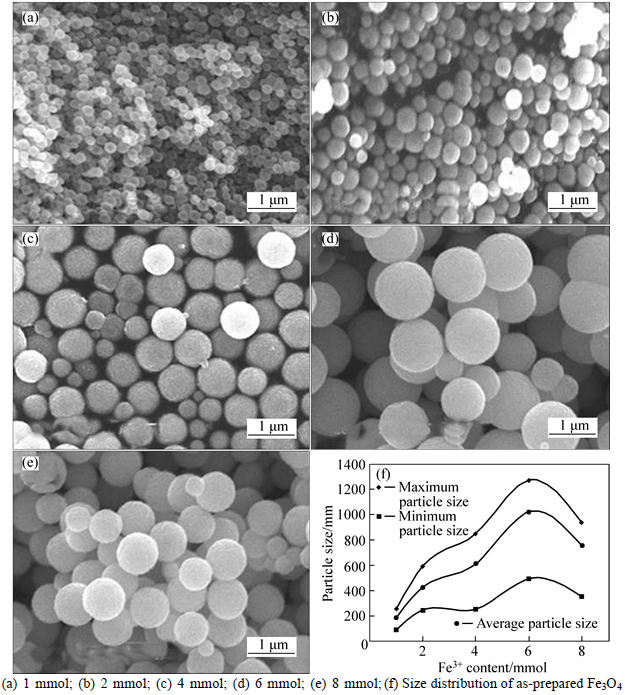
Fig. 2 SEM images of Fe3O4 MSPs with different Fe3+ contents:
3.2 Influence of NaAc content on size of Fe3O4 MSPs
The structure of as-prepared Fe3O4 was measured by XRD, as shown in Fig. 3. All the diffraction peaks at 18.41°, 30.32°, 35.50°, 43.17°, 53.56°, 57.22° and 62.68° can be indexed to the indices (111), (220), (311), (400), (422), (511) and (440) of Fe3O4.
The SEM images and size distribution of the Fe3O4 MSPs are presented in Figs. 4(a)-(e) and (f), respectively. Particle size distribution and the dependence of Fe3O4 MSPs particle size on NaAc content of 6.10, 12.20,24.44, 36.57 and 48.76 mmol are shown in Fig. 4. As seen, Figs. 4(a)-(e) and (f) show that the size of mono- dispersed particles monotonously increases from 254 nm (Fig. 3(a)) to 414 nm(Fig. 3(e)) with the increasing NaAc contents from 6.10 mmol to 24.44 mmol, and then the particle size decreases to 86 nm when NaAc content increases to 48.76 mmol. According to the SEM images, XRD pattern and dependence of particle size on NaAc content, the other way to tune the particle size has been found, which indicates that the NaAc content is also the key factor to control the particle size.

Fig. 3 XRD pattern of as-prepared Fe3O4
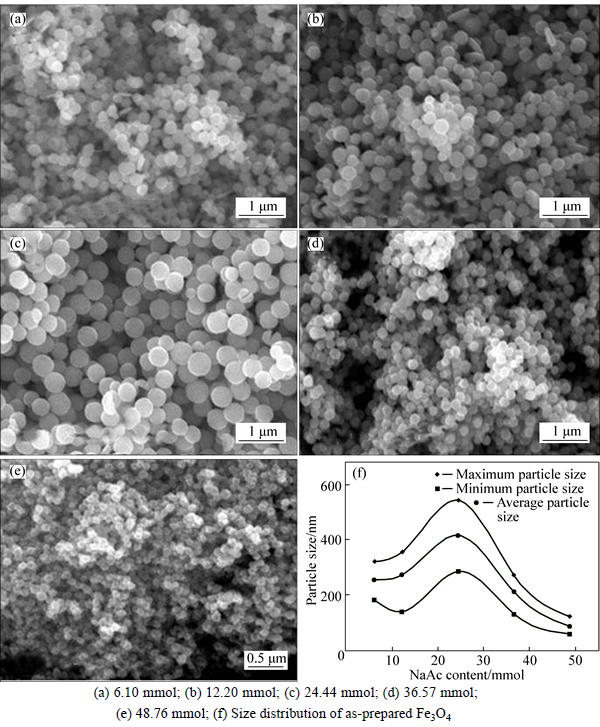
Fig. 4 Response surface of particle size to Fe3+ and NaAc contents:
3.3 Collaborative influence of Fe3+ and NaAc contents on size of Fe3O4 MSPs
According to the single-factor studies of Fe3+ and NaAc content on synthesis of size tunable Fe3O4 MSPs, it is found that both two factors could affect the particle size. These results encourage us to carry out further study to obtain specific size Fe3O4 MSPs. Based on the former research, a response surface is proposed which is constituted with Fe3+ content as x axis, NaAc content as y axis and particle size as z axis. The response surface is shown in Fig. 5.
As shown in Figs. 6(a)-(e), different sizes of Fe3O4 MSPs were obtained from 100 to 1000 nm by varying Fe3+ and NaAc content. This result is well matched with the response surface proposed, which indicates that the tunable size synthesis strategy of Fe3O4 is feasible.
The magnetic properties of the as-prepared magnetite submicroparticles are investigated with VSM at room temperature. Figure 7 shows a typical magnetic hysteresis curve for the submicropartcicles. The saturation magnetization of the submicrosparticles exhibits a high saturation magnetization (Ms) of about 93.56, 99.619, 101.06, 135.67 and 128.94 A·m2/kg with size of 100, 300, 500, 800 and 1000 nm, respectively. It is obvious that ferromagnetic submicroparticles obtain higher Ms with larger size.
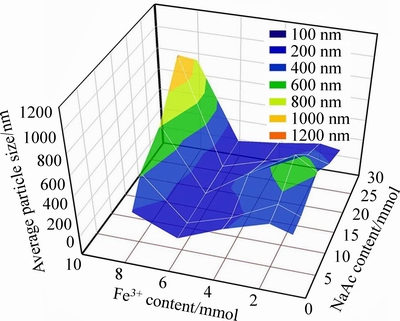
Fig. 5 Response surface of particle size to Fe3+ and NaAc contents
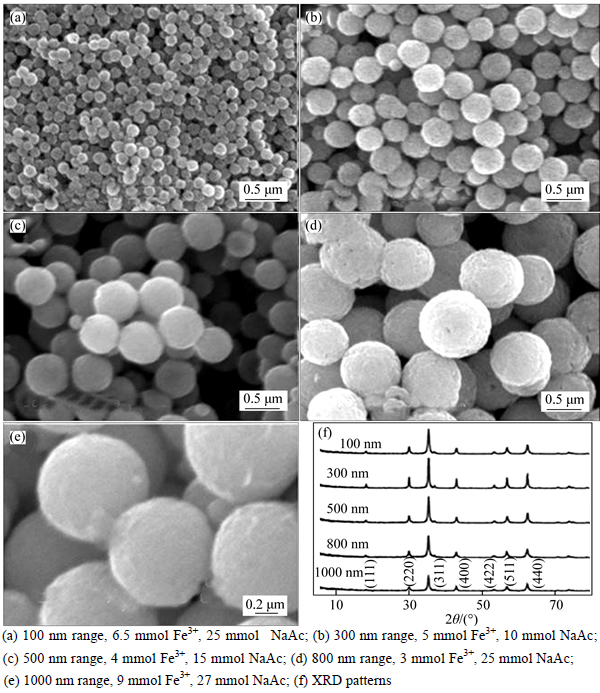
Fig. 6 SEM images and XRD pattern of Fe3O4 with different Fe3+ and NaAc contents:
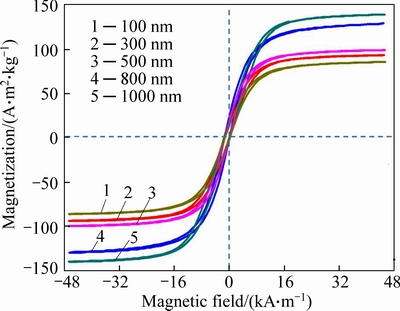
Fig. 7 Magnetization curve of Fe3O4 MSPs at room temperature
3.4 Hemolysis evaluation of Fe3O4 MSPs with different sizes
For in vivo bio-applications, the materials must have excellent blood compatibility, such as very low hemolysis effects when they are administrated by vein injection. A hemolysis assay was conducted to evaluate the Fe3O4 MSPs with different sizes according to previous report [27].
Figure 8 shows the hemolysis rate (HR) of submicroparticles varying with the Fe3O4 concentration and size.
It is indicated that smaller submicroparticles lead to higher hemolysis rate with increasing submicroparticles concentration in blood. The HR of Fe3O4 MSPs increases with Fe3O4 concentration, the HR of Fe3O4 MSPs with size of 300 nm and 1000 nm increases from 0.37% and 0.3% to as high as 35.27% and 24.93% with the concentration varying from 0.0625 mg/mL to 10 mg/mL, respectively. Also, it is obvious that smaller Fe3O4 MSPs enjoy greater effect on blood, the HR of blood exposed to Fe3O4 MSPs at the size of 100 nm is 6.5% at 2 mg/mL, which exceeds 5% compared with Fe3O4 MSPs at other size whose HR are 4.03%, 3.54% and 3.25%, and then climbed as high as 40.07% at 10 mg/mL, while the HR of Fe3O4 MSPs at the size of 1000 nm is only 24.93%. When the concentration is 3 mg/mL, only the HR of Fe3O4 MSPs at the size of 1000 nm is 3.88% lower than 5%.
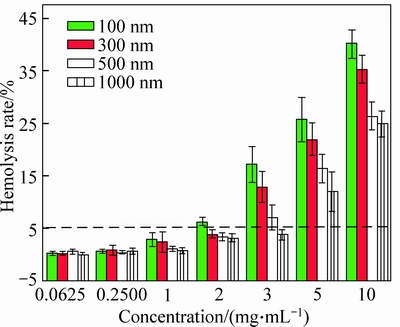
Fig. 8 Hemolysis test of Fe3O4 magnetic submicropaticles
3.5 Cytotoxicity analysis of Fe3O4 MSPs with different size on L929 cells
The cell RGR of L929 cells varies with the particles size and concentration of Fe3O4 MSPs. As shown in Fig. 9, the cytotoxicity of submicroparticles increases with particle size of Fe3O4 MSPs. At the group with the concentration of 0.25 mg/mL, the RGR decreases from 82.04% and 75.97% (no cytotoxicity, see Table 2) to 68.19% and 63.72% (with cytotoxicity, toxicity grade 2) as the particle size increasing from 100 nm to 1000 nm. Cells exposed to particles with larger size show increasing DNA damage [28]. When L929 cells are exposed to Fe3O4 MSPs for 48 h, there is a significant increase in DNA damage from particles with lager size, and that leads to higher cytotoxicity which is in agreement with our results. L929 cells exposed to submicroparticles with higher concentration (>0.0625 mg/mL) show a higher content of non-viable cells, the RGR of Fe3O4 MSPs with size of 100 nm and 500 nm decreases from 91.35% and 83.44% to 72.78% and 59.12% with the concentration changes from 0.0156 mg/mL to 1.0000 mg/mL, which means that the toxicity grade changes from grade 1 to 2. All those are in agreement with the findings from inverted microscopy in Fig. 10.
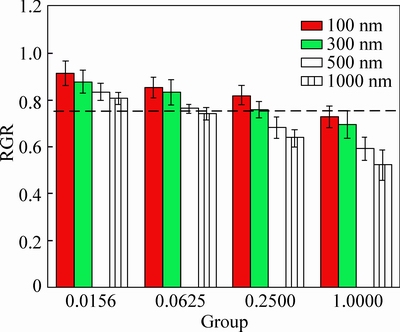
Fig. 9 MTT results of L929 cells after 48 h exposure to Fe3O4 MSPs

Fig. 10 Inverted microscopic pictures of L929 cells after 48 h exposure to Fe3O4 MSPs
4 Conclusions
A size-controlled map has gained and succeeded in synthesizing monodispersed Fe3O4 MSPs with the size from 100 nm to 1000 nm by simply controlling the content of Fe3+ and NaAc via solvothermal route. XRD and SEM studies reveal that the Fe3O4 MSPs have well-distribution size. The saturation magnetization of Fe3O4 MSPs increases from 99.619 to 135.67 A·m2/kg with the size varying from 100 nm to 1000 nm. Cytotoxicity analysis shows that Fe3O4 MSPs with larger size show higher cytotoxicity, which demonstrates lower cell viability following an exposure to the cells after 48 h. Hemolysis evaluation of Fe3O4 MSPs shows that larger size leads to lower hemolysis rate, and all those Fe3O4 MSPs show well blood compatibility at low Fe3O4 MSPs concentration. The studies pave a new way for size-controlled preparation of Fe3O4 MSPs and afford significant evaluation data of toxicity and hemolysis for further application in biomedical area.
References
[1]  W,TELLER J, ZBOROWSKI M.Scientific and clinical applications of magnetic drug carriers [M]. Springer Science & Business Media, 1997.
W,TELLER J, ZBOROWSKI M.Scientific and clinical applications of magnetic drug carriers [M]. Springer Science & Business Media, 1997.
[2] LIAN Suo-yuan, WANG En-bo, KANG Zhen-hui, BAI Yun-peng, GAO Lei, JIANG Min, HU Chang-wen, XU Lin. Synthesis of magnetite nanorods and porous hematite nanorods [J]. Solid State Commun, 2004, 129: 485-490.
[3] ZAITSEV V S, FILIMONOV D S, PRESNYAKOV I A, GAMBINO R J, CHU B. Physical and chemical properties of magnetite and magnetitepolymer nanoparticles and their colloidal dispersions [J]. Journal of Colloid and Interface Science, 1999, 212: 49-57.
[4] RAO C N R, KULKARNI G U, THOMAS P J, EDWARDS P P. Metal nanoparticles and their assemblies [J]. Chemical Society Reviews, 2000, 29(1): 27-35.
[5] WU K T, KUO P C, YAO Y D. Magnetic and optical properties of Fe3O4 nanoparticle ferrofluids prepared by coprecipitation technique [J]. IEEE Transactions on Magnetics, 2001, 37(4): 2651-2653.
[6] XU Jing, YANG Hai-bin, FU Wu-you, DU Kai, SUI Yong-ming, CHEN Jiu-ju, ZENG Yi, LI Ming-hui, ZOU Guang-tian. Preparation and magnetic properties of magnetite nanoparticles by sol–gel method [J]. Journal of Magnetism and Magnetic Materials, 2007, 309(2): 307-311.
[7] GAO Guan-hua, LIU Xiao-he, SHI Rong-rong, ZHOU Ke-chao, SHI You-guo, MA Ren-zhi. Shape-controlled synthesis and magnetic properties of monodisperse Fe3O4 nanocubes [J]. Crystal Growth & Design, 2010, 10(7): 2888-2894.
[8] HYEON T, LEE S S, PARK J, CHUNG Y, NA H B. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process [J]. Journal of the American Chemical Society, 2001, 123(51): 12798-12801.
[9] CARUSO F, CARUSO R A,  H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating [J]. Science, 1998, 282(5391): 1111-1114.
H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating [J]. Science, 1998, 282(5391): 1111-1114.
[10] CARUSO F, SPASOVA M,  -MACEIRA V, LIZ-
-MACEIRA V, LIZ-  L M. Multilayer assemblies of silica-encapsulated gold nanoparticles on decomposable colloid templates [J]. Advanced Materials, 2001, 13(14): 1090-1094.
L M. Multilayer assemblies of silica-encapsulated gold nanoparticles on decomposable colloid templates [J]. Advanced Materials, 2001, 13(14): 1090-1094.
[11] NEL A, XIA T,  L. Toxic potential of materials at the nanolevel [J]. Science, 2006, 311(5761): 622-627.
L. Toxic potential of materials at the nanolevel [J]. Science, 2006, 311(5761): 622-627.
[12] SERVICE R F. Nanotechnology grows up [J]. Science, 2004, 304(5678): 1732-1734.
[13]  G,
G,  E,
E,  J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles [J]. Environmental Health Perspectives, 2005, 113(7): 823-839.
J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles [J]. Environmental Health Perspectives, 2005, 113(7): 823-839.
[14] POLAND C A, DUFFIN R, KINLOCH I, MAYNARD A, WALLACE W A, SEATON A, STONE V, BROWN S, MACNEE W, DONALDSON K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study [J]. Nature Nanotechnology, 2008, 3(7): 423-428.
[15] TAKAGI A, HIROSE A, NISHIMURA T, FUKUMORI N, OGATA A, OHASHI N, KITAJIMA S, KANNO J. Induction of mesothelioma in p53+/-mouse by intraperitoneal application of multi-wall carbon nanotube [J]. The Journal of Toxicological Sciences, 2008, 33(1): 105-116.
[16] YANG J, PARK S B, YOON H G, HUH Y M, HAAM S. Preparation of poly ε-caprolactone nanoparticles containing magnetite for magnetic drug carrier [J]. International Journal of Pharmaceutics, 2006, 324(2): 185-190.
[17] PANKHURST Q A, CONNOLLY J, JONES S K, DOBSON J. Applications of magnetic nanoparticles in biomedicine [J]. Journal of Physics D: Applied Physics, 2003, 36(13): R167.
[18] 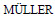 R H, MAAΒEN S, WEYHERS H, SPECHTB F, LUCKSB J S. Cytotoxicity of magnetite-loaded polylactide, polylactide/glycolide particles and solid lipid nanoparticles [J]. International Journal of Pharmaceutics, 1996, 138(1): 85-94.
R H, MAAΒEN S, WEYHERS H, SPECHTB F, LUCKSB J S. Cytotoxicity of magnetite-loaded polylactide, polylactide/glycolide particles and solid lipid nanoparticles [J]. International Journal of Pharmaceutics, 1996, 138(1): 85-94.
[19] NEL A, XIA T, 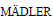 L, LI N. Toxic potential of materials at the nanolevel [J]. Science, 2006, 311(5761): 622-627.
L, LI N. Toxic potential of materials at the nanolevel [J]. Science, 2006, 311(5761): 622-627.
[20] WARHEIT D B, WEBB T R, SAYES C M, COLVIN V L, REED L K. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: Toxicity is not dependent upon particle size and surface area [J]. Toxicological Sciences, 2006, 91(1): 227-236.
[21] DENG Hong, LI Xiao-lin, PENG Qing, WANG Xun, CHEN Jin-ping, LI Ya-dong. Monodisperse magnetic single-crystal ferrite microspheres [J]. Angewandte Chemie, 2005, 117(18): 2842-2845.
[22] TING Fan, PAN Deng-ke, ZHANG Hui. Study on formation mechanism by monitoring the morphology and structure evolution of nearly monodispersed Fe3O4 submicroparticles with controlled particle sizes [J]. Industrial & Engineering Chemistry Research, 2011, 50(15): 9009-9018.
[23] SUN J, GU G, QIAN Y. Influence of different contact ways and extracting conditions on the hemolytic effect of biomaterials [J]. Journal of Biomedical Engineering, 2003, 20(1): 8-10. (in Chinese)
[24] ZHANG Wen-yun, SHEN Yi, LI Nan. Evaluation of biocompatibility of fiber-reinforced dental composites [J]. Medical Journal of Chinese People's Liberation Army, 2004, 29: 345-347. (in Chinese)
[25] PIETERS R, LOONEN A H, HUISMANS D R, BROEKEMA G J, DIRVEN M W, HEYENBROK M W, HAHLEN K, VEERMAN A J. In vitro drug sensitivity of cells from children with leukemia using the MTT assay with improved culture conditions [J]. Blood, 1990, 76(11): 2327-2336.
[26] LI Yun-tao, LIU Jing, ZHONG Yue-jiao, ZHANG Jia, WANG Zi-yu, WANG Li, AN Yan-li, LIN Mei, GAO Zhi-qiang, ZHANG Dong- heng. Biocompatibility of Fe3O4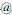 Au composite magnetic nanoparticles in vitro and in vivo [J]. International Journal of Nanomedicine, 2011, 6: 2805-2819.
Au composite magnetic nanoparticles in vitro and in vivo [J]. International Journal of Nanomedicine, 2011, 6: 2805-2819.
[27] KIM D H, LEE S H, KIM K N, SHIM I B, LEE Y K. Cytotoxicity of ferrite particles by MTT and agar diffusion methods for hyperthermic application [J]. Journal of Magnetism and Magnetic Materials, 2005, 293(1): 287-292.
[28] KARLSSON H L, GUSTAFSSON J, CRONHOLM P, 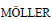 L. Size-dependent toxicity of metal oxide particles—A comparison between nano-and micrometer size [J]. Toxicology Letters, 2009, 188(2): 112-118.
L. Size-dependent toxicity of metal oxide particles—A comparison between nano-and micrometer size [J]. Toxicology Letters, 2009, 188(2): 112-118.
(Edited by FANG Jing-hua)
Foundation item: Project(2013DFA5129) supported by the International Science and Technology Cooperation Program of China
Received date: 2015-09-30; Accepted date: 2016-01-18
Corresponding author: TIAN Qing-hua, PhD, Professor; Tel: +86-731-88876795; E-mail: qinghua@csu.edu.cn
Abstract: Large scaled uniform and size-controllable magnetic submicroparticles (MSPs) were synthesized via solvothermal method with ferric chloride as iron source and sodium acetate as trapping agent. The influence of Fe3+ and NaAc contents on the size distribution of MSPs was investigated. The structural and morphological properties of the synthesized particles were studied by scanning electron microscopy (SEM), X-ray power diffraction (XRD) and vibrating sample magnetometer (VSM). The well-dispersed MSPs with size of 100-1000 nm were obtained by simply adjusting the contents of Fe3+ and NaAc. In addition, the hemolysis and cytotoxicity of Fe3O4 MSPs, and their ability to case arrest in cell life-cycles were studied. The results indicate that larger size could lead to lower hemolysis. From MTT(3-(4,5-dimethylthuazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, the interactions between MSPs and adhesive mouse fibroblast cell line(L929) were probed. Larger size of Fe3O4 MSPs demonstrates lower cell viability following an exposure to the cells.


