Trans. Nonferrous Met. Soc. China 24(2014) 2061-2066
Precipitation sequence of η phase along low-angle grain boundaries in Al-Zn-Mg-Cu alloy during artificial aging
Mao-hua LI1, Yan-qing YANG1, Zong-qiang FENG1, Bin HUANG1, Xian LUO1, Ju-hong LOU1, Ji-gang RU2
1. State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China;
2. Beijing Institute of Aeronautical Materials, Beijing 100095, China
Received 17 October 2013; accepted 30 April 2014
Abstract:
The precipitation sequence of η(MgZn2) phase along low-angle grain boundaries in Al-Zn-Mg-Cu alloy was investigated by examining samples aged at 135 °C for various times from 5 min to 6 h. High resolution transmission electron microscopy (HRTEM) observations and energy dispersive X-ray spectroscopy (EDX) analysis indicate that the precipitation sequence of η phase along low-angle grain boundaries should be supersaturated solid solution (SSS)→vacancy-rich clusters (VRC)→GP II zones→η′→η. Based on the theory of non-equilibrium grain boundary segregation (NGS) and non-equilibrium grain boundary co-segregation (NGCS), the excessive solute elements gradually segregate to the grain boundaries by the diffusion of the solute-vacancy complex during aging treatment. The grain boundary segregation plays an important role in the nucleation and growth of VRC, GP II zones, η′ phase as well as η phase.
Key words:
Al-Zn-Mg-Cu alloy; aging; low-angle grain boundaries; grain boundary segregation; precipitation sequence;
1 Introduction
Al-Zn-Mg-Cu alloys are widely used in aircraft and automotive industries due to their high strength, low density and weldability [1]. Unfortunately, Al-Zn-Mg- Cu alloys are prone to stress corrosion cracking (SCC). The major microstructure features that affect the SCC resistance are the size and dispersion of grain boundary precipitates (GBPs), the width of precipitate-free zones (PFZs) and the grain boundary segregation [2-5].
The homogeneous precipitates in Al-Zn-Mg-Cu alloys during aging treatment mainly include three phases, namely, η phase, GP zones and 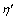 phase. The equilibrium η phase has a hexagonal lattice with a=0.521 nm and c=0.860 nm [6], which is incoherent with the Al matrix. There are 11 orientation relationships of η phase with the Al matrix termed η1-η11 [7]. Among these 11 orientaion relationships, the most frequently observed ones are η1, η2 and η4, whose specific orientation relationships with the matrix are as follows:
phase. The equilibrium η phase has a hexagonal lattice with a=0.521 nm and c=0.860 nm [6], which is incoherent with the Al matrix. There are 11 orientation relationships of η phase with the Al matrix termed η1-η11 [7]. Among these 11 orientaion relationships, the most frequently observed ones are η1, η2 and η4, whose specific orientation relationships with the matrix are as follows:


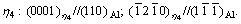
The GP zones and the metastable 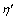 phase are the main strengthening phases in Al-Zn-Mg-Cu alloys [8]. Structure models for
phase are the main strengthening phases in Al-Zn-Mg-Cu alloys [8]. Structure models for 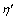 phase have been proposed from HRTEM [9] and precession electron diffraction [10]. The metastable phase
phase have been proposed from HRTEM [9] and precession electron diffraction [10]. The metastable phase 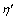 is generally recognized as a hexagonal crystal structure with lattice parameters a=0.496 nm and c=1.402 nm. The crystallographic orientation relationship of
is generally recognized as a hexagonal crystal structure with lattice parameters a=0.496 nm and c=1.402 nm. The crystallographic orientation relationship of 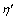 phase with the matrix is
phase with the matrix is 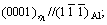

The 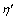 precipitates are semi-coherent with the matrix. Two types of GP zones appear during the early stage of aging, known as GP I and GP II, respectively [11]. The GP zones are completely coherent with the Al matrix. The spherical GP I zones were reported to be internally ordered with a structure similar to AuCu(I), with internal ordering of Zn and Al/Mg on the alternate {001}Al planes. The GP I zones are formed via solute-rich clusters, and in a wide temperature range from room temperature to 140-150 °C, independently of quenching temperature. The plate-like GP II zones are one or a few Zn-rich atomic layers on {111}Al planes. They are believed to be related to the VRC. The GP II zones are formed after quenching from temperatures above 450 °C and aging at temperature above 70 °C.
precipitates are semi-coherent with the matrix. Two types of GP zones appear during the early stage of aging, known as GP I and GP II, respectively [11]. The GP zones are completely coherent with the Al matrix. The spherical GP I zones were reported to be internally ordered with a structure similar to AuCu(I), with internal ordering of Zn and Al/Mg on the alternate {001}Al planes. The GP I zones are formed via solute-rich clusters, and in a wide temperature range from room temperature to 140-150 °C, independently of quenching temperature. The plate-like GP II zones are one or a few Zn-rich atomic layers on {111}Al planes. They are believed to be related to the VRC. The GP II zones are formed after quenching from temperatures above 450 °C and aging at temperature above 70 °C.
CHEN et al [2,3] investigated the effect of recrystallization and quenching rate on the copper content, the size and distribution of GBPs in Al-Zn- Mg-Cu alloys. PENG et al [4] and RANGANATHA et al [5] researched the influence of aging treatment on the size and distribution of GBPs, and the width of PFZs in Al-Zn-Mg-Cu alloys. However, few of them paid much attention to precipitation sequence along grain boundaries. Meantime, the formation process and mechanism of precipitate along grain boundaries are still unclear. In this work, therefore, the precipitation sequence of η phase along low-angle grain boundaries is investigated by HRTEM and HAADF-STEM combined with EDX. Moreover, the mechanism of grain boundary segregation and the formation mechanism of early-stage precipitation are discussed in detail.
2 Experimental
The studies were carried out on a 7050 Al alloy, whose nominal chemical composition is Al-6.29Zn- 2.22Mg-2.28Cu-0.15Zr (mass fraction, %), with a small amount of Mn, Cr, Ti, Si and Fe elements. The cast ingot of the alloy was first homogenized at 460 °C for 24 h, hot rolled to a 4 mm thin plate, then solution treated at temperature of 475 °C for 1 h, water quenched, followed by aging at 135 °C for 5 min, 20 min, 40 min, 1 h and 6 h, respectively. TEM samples were subsequently prepared by mechanical grinding and punching to 3 mm disks in diameter. The disks were finally thinned using twin jet electropolishing with an electrolyte of 30% nitric acid and 70% methanol at 15 V below -25 °C. HRTEM and HAADF-STEM observations as well as EDX analysis were carried out by Tecnai F30 G2TEM equipped with a high-angle annular dark-field detector and an X-ray energy dispersive spectrometer. 20 low- angle grain boundaries at different aging times were studied by HRTEM as well as HAADF-STEM combined with EDX.
3 Results
Figures 1(a) and (b) present HRTEM images along [011]Al direction of the samples aged at 135 °C for 5 min and 20 min, respectively, showing the morphological and crystallographic features of the GP II zones [11]. The HRTEM observations show that thin planar precipitates (marked by white arrows) are fully coherent with the Al matrix, parallel to {111}Al planes, with the size of 2-6 {111}Al atomic planes thickness and 4-7 nm width. Additionally, weak diffraction streaks are observed along {111}Al in the insets of Fig. 1, indicating the GP II zones formed. As shown in Fig. 1(b), with the increase of aging time to 20 min, the sizes of GP II zones increase to 7-12 atomic layers in thickness and 6-8 nm in width. In the present study, the GP II zones along low-angle grain boundaries consist of more atomic layers parallel to the {111}Al planes, which differ from the typical GP II zones with only 1-3 {111}Al atomic planes in thickness in the interior of grains [11].
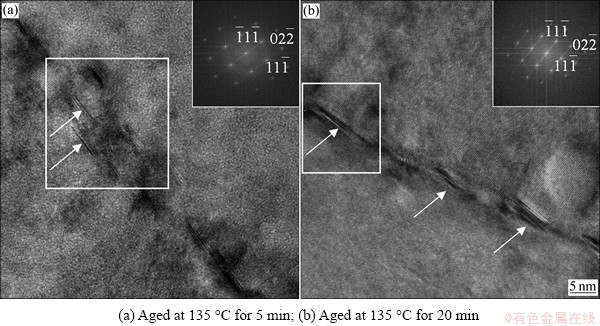
Fig. 1 HRTEM images in [110]Al projection with corresponding fast Fourier transform (FFT) spectra of white rectangular regions, showing GP II zones along low-angle grain boundaries
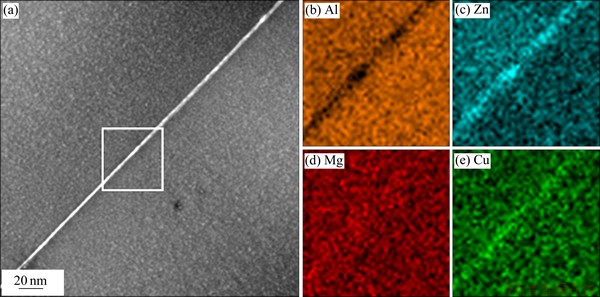
Fig. 2 HAADF-STEM image of 7050 Al alloy aged at 135 °C for 20 min (a) and corresponding EDX mapping images of white rectangular regions related to Al (b), Zn (c), Mg (d) and Cu (e)
Figure 2 shows the elemental mapping images along a low-angle grain boundary obtained by HAADF-STEM combined with EDX. In Fig. 2(a), the bright contrasts along the grain boundary are treated as precipitates. Figures 2(b)-(e) show the corresponding EDX mapping images of Al, Zn, Mg, and Cu, respectively. These mapping images clearly show that elements Zn and Cu are enriched. The element Mg is slightly enriched next to the Zn-rich region, and element Al is obviously depleted at the locations of precipitates. HRTEM image (Fig. 1(b)) and EDX analysis (Figs. 2(b)-(e)) can confirm that the precipitates along low-angle grain boundaries are GP II zones that exhibit several Zn-rich atomic layers (some Zn atoms substituted by Cu atoms) on {111}Al planes.
Figure 3 shows the  HRTEM image, which was taken from the sample aged at 135 °C for 40 min. The HRTEM observation shows that the precipitates (marked by white arrows) are semi-coherent with the Al matrix, consisting of more atomic layers (>6 layers) parallel to the {111}Al planes, and some precipitates have ring-like image contrasts. One can observe diffuse streaks and diffraction spots at the 1/3 and 2/3 {220}Al positions in the FFT spectrum (inserted in the top-right of Fig. 3), indicating that
HRTEM image, which was taken from the sample aged at 135 °C for 40 min. The HRTEM observation shows that the precipitates (marked by white arrows) are semi-coherent with the Al matrix, consisting of more atomic layers (>6 layers) parallel to the {111}Al planes, and some precipitates have ring-like image contrasts. One can observe diffuse streaks and diffraction spots at the 1/3 and 2/3 {220}Al positions in the FFT spectrum (inserted in the top-right of Fig. 3), indicating that  precipitates have been formed. Some
precipitates have been formed. Some 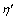 precipitates with ring-like image contrast in our observations differ from the
precipitates with ring-like image contrast in our observations differ from the 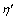 precipitates with the separate ring chains seen between two wave-like lines in Ref. [9]. HRTEM observations taken from the sample aged at 135 °C for 1 h (not shown here) indicate that the precipitates are still
precipitates with the separate ring chains seen between two wave-like lines in Ref. [9]. HRTEM observations taken from the sample aged at 135 °C for 1 h (not shown here) indicate that the precipitates are still 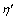 phase.
phase.
After aging at 135 °C for 6 h, equilibrium η precipitates can be observed at low-angle grain boundaries. Figure 4 shows the 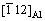 HRTEM image of the η precipitate which is incoherent with the Al matrix. It can be seen that the orientation relationship between the η precipitate and the matrix is
HRTEM image of the η precipitate which is incoherent with the Al matrix. It can be seen that the orientation relationship between the η precipitate and the matrix is 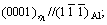
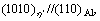 namely variant η2. Diffraction spots at the 1/3 and 2/3 {220}Al positions in the FFT spectrum (inserted in the top-left of Fig. 4) indicate that η precipitates have been formed. Moreover, the measured c parameter along the <111>Al direction is close to 0.860 nm in the enlarged portion of the white rectangular region (inserted in the bottom-right of Fig. 4), so these precipitates are identified as η phase. It is generally believed that η2 precipitates are formed by direct transformation of
namely variant η2. Diffraction spots at the 1/3 and 2/3 {220}Al positions in the FFT spectrum (inserted in the top-left of Fig. 4) indicate that η precipitates have been formed. Moreover, the measured c parameter along the <111>Al direction is close to 0.860 nm in the enlarged portion of the white rectangular region (inserted in the bottom-right of Fig. 4), so these precipitates are identified as η phase. It is generally believed that η2 precipitates are formed by direct transformation of 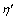 phase, since these two phases η2 and
phase, since these two phases η2 and 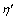 have similar orientation relationships and morphologies [7].
have similar orientation relationships and morphologies [7].

Fig. 3 HRTEM image in 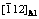 projection, showing
projection, showing 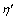 precipitates along low-angle grain boundary in sample aged at 135 °C for 40 min
precipitates along low-angle grain boundary in sample aged at 135 °C for 40 min
4 Discussion
4.1 Non-equilibrium grain boundary segregation
The diffusion of solute atoms from the vicinity of grain boundaries to grain boundaries will occur in Al-Zn-Mg-Cu alloys during aging treatment because of the occurrence of NGS. The mechanism of NGS relies on the diffusion of the vacancy-solute complex toward grain boundary. Quantification analysis of NGS has been investigated by JIANG et al [12] and XU and CHENG [13].
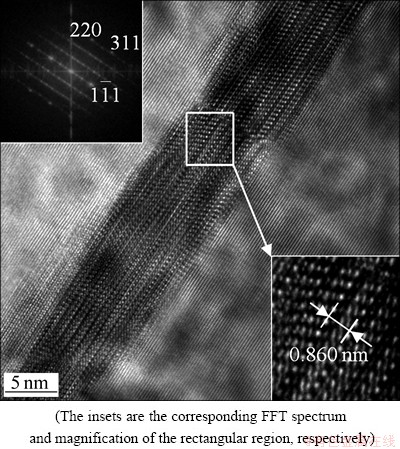
Fig. 4 HRTEM image in 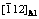 projection, showing η precipitate along low-angle grain boundary in sample aged at 135 °C for 6 h
projection, showing η precipitate along low-angle grain boundary in sample aged at 135 °C for 6 h
In Ref. [13], it was reported that the influence of the vacancy-solute binding energy, Eb, on the non- equilibrium grain boundary segregation is the most significant, and the segregation effect is large when Eb lies in the range from 0.15 to 0.6 eV.
The values of Eb can be calculated by an elasticity theory. According to the ideas of COTTRELL [14], the following formula describes approximately the binding energy Eb of a vacancy with a foreign atom:
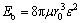
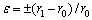 (1)
(1)
where e is the misfit of the foreign atom with the matrix lattice, m is the shear modulus of the matrix, r0 is the matrix atom radius, and r1 is the foreign atom radius. Therefore, the binding energy of a vacancy with a solute atom in 7050 Al alloy can be calculated as 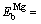 0.28 eV,
0.28 eV, 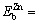 0.06 eV, and
0.06 eV, and 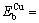 0.22 eV. The calculated results show that solutes Mg and Cu respectively have a binding energy with a vacancy in suitable range for NGS, from 0.15 to 0.6 eV.
0.22 eV. The calculated results show that solutes Mg and Cu respectively have a binding energy with a vacancy in suitable range for NGS, from 0.15 to 0.6 eV.
According to the NGS theory [13], it is postulated that in 7050 Al alloys there is an equilibrium between solute atoms (Mg and Cu), vacancy (hereafter denoted as V) and their recombined complex described as Mg-V and Cu-V, respectively.
Based on the NGS theory, in the conditions that 7050 Al alloy was first held at a solution treatment temperature 475 °C for 1 h and then rapidly water quenched, immediately aged at lower temperature 135 °C for a reasonably long time, solute atoms gradually segregate to the grain boundaries.
The vacancy equilibrium concentration in the matrix at 475 °C is obviously higher than that at 135 °C. When the samples are aged at 135 °C, vacancy gradients develop between the grain boundary and the interior of grain due to grain boundary being an ideal sink of vacancy. The complexes (Mg-V and Cu-V) are decomposed into vacancies and solute atoms (Mg and Cu) because of the decrease of the vacancy concentration near the grain boundaries. As a result, the complexes (Mg-V and Cu-V) gradients generate between the grain boundary and the interior of grain far away from the grain boundary. The complexes (Mg-V and Cu-V) diffuse from the interior of grain to the grain boundary owing to the gradients of the complexes (Mg-V and Cu-V). This diffusion causes excessive solute atoms (Mg and Cu) to segregate to the grain boundaries.
XU and CHENG [13] proposed a model for thermodynamic calculation of NGS. The maximum NGS segregation concentration, Cm(Ti +1), of a solute is
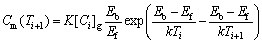 (2)
(2)
where Cm(Ti+1) is the maximum NGS segregation concentration for cooling from Ti to Ti +1; Ti is solution treatment temperature; Ti +1 is a lower temperature; [Ci]g is the equilibrium concentration of solute i within grains; Ef is the vacancy formation energy; Eb is the vacancy- solute binding energy; and k is the Boltzmann’s constant.
The interaction energy between vacancies and solute atoms Zn is rather weak (~0.06 eV), why can Zn atoms be also largely transported to grain boundaries? The reason may be that NGCS takes place, which was put forward by XU and CHENG [13]. In 7050 Al alloy, solute Mg has a binding energy (0.28 eV) with vacancy in a suitable range for NGS to occur in solvent Al, while solute Zn has a binding energy (0.06 eV) with a vacancy beyond the range for NGS to occur. Both the electronegativity difference and the atomic radii difference between Mg and Zn, between Mg and Al, and between Zn and Al are respectively 0.34 and 0.025 nm, 0.3 and 0.017 nm, 0.04 and 0.008 nm. Therefore, the attraction between Mg and Zn is stronger than that between Mg and Al, and between Zn and Al. According to the model, the non-equilibrium segregation of solute Zn occurs along with the non-equilibrium segregation of solute Mg. This implies that the diffusion of Zn atoms to grain boundaries in the 7050 Al alloy occurs via Mg-V complexes.
4.2 Vacancy-rich clusters formation
At the early stages of artificial aging, the vacancy-solute complexes (Mg-V, Mg-Zn-V and Cu-V) gradually migrate to the grain boundaries due to NGS and NGCS. In the vicinity of grain boundary, frequent collisions between these solute-vacancy complexes release vacancies which finally annihilate at the grain boundaries leaving behind mobile complexes of solute atoms which may or may not also include vacancies. As a result, vacancy-rich Zn clusters are formed, which is known to occur in most Al-Zn-Mg-Cu alloys, in which the number of Mg atoms is reduced due to the reduction in the number of vacancies [15]. In addition, Zn atoms are partially replaced by Cu atoms in VRC.
4.3 GP II zones formation
At the beginning of artificial aging (5 to 20 min at 135 °C), the GP II zones could be imaged quite readily by HRTEM in <110>Al projections which appear as thin layers on {111}Al planes (see Fig. 1). These zones are identified as Zn and Cu by EDX analysis in STEM mode, as shown in Fig. 2. Therefore, the combination of the HRTEM observations and EDX analysis can show that the GP II zones with a high content of Zn have probably started to form on {111}Al planes in the early stage of the decomposition of the supersaturated solid solution along low-angle grain boundaries.
The fine vacancy-rich Zn clusters are formed at the beginning of artificial aging, which act as GP II zones nuclei on subsequent aging. It is suggested that a possible mechanism of GP II zone growth along low-angle grain boundaries involves the diffusion of Mg-Zn-V complexes to the GP II zones boundary where the complexes dissociate, releasing Zn atoms.
The GP II zones, which are fully coherent with the matrix, therefore, have a lower interfacial energy than intermediate or equilibrium precipitate phases. As a result, the nucleation barrier for GP II zones is significantly smaller than 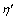 phases as well as η phases. Subsequent evolution of the microstructure involves the replacement of the GP zones with more stable phases [16].
phases as well as η phases. Subsequent evolution of the microstructure involves the replacement of the GP zones with more stable phases [16].
5 Conclusions
1) The precipitation sequence of η phase along low-angle grain boundaries in Al-Zn-Mg-Cu alloys aged at 135 °C is SSS→VRC→GP II→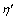 →η.
→η.
2) Quenched-in vacancies play critical roles in the processes of the grain boundary segregation. Solute atoms (Mg and Cu) gradually segregate to grain boundaries by the diffusion of the solute-vacancy complexes (Mg-V and Cu-V) in Al-Zn-Mg-Cu alloys during aging treatment. Meanwhile, solute atoms Zn segregate to grain boundaries via Mg-Zn-V complexes.
3) The grain boundary segregation of precipitate- forming elements will affect the nucleation and growth of VRC, GP II zones, 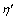 phase as well as η phase along grain boundaries in Al-Zn-Mg-Cu alloys during aging treatment.
phase as well as η phase along grain boundaries in Al-Zn-Mg-Cu alloys during aging treatment.
References
[1] STARKE E A, STALEY J T. Application of modern aluminum alloys to aircraft [J]. Progress in Aerospace Sciences, 1996, 32(2-3): 131-172.
[2] CHEN Song-yi, CHEN Kang-hua, DONG Peng-xuan, YE Sheng-ping, HUANG Lan-ping. Effect of recrystallization and heat treatment on strength and SCC of an Al-Zn-Mg-Cu alloy [J]. Journal of Alloys and Compounds, 2013, 581: 705-709.
[3] CHEN Song yi, CHEN Kang hua, PENG Guo sheng, LIANG Xin, CHEN Xue hai. Effect of quenching rate on microstructure and stress corrosion cracking of 7085 aluminum alloy [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 47-52.
[4] PENG Guo-sheng, CHEN Kang-hua, CHEN Song-yi, FANG Hua-chan. Influence of dual retrogression and re-aging temper on microstructure, strength and exfoliation corrosion behavior of Al-Zn-Mg-Cu alloy [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(4): 803-809.
[5] RANGANATHA R, ANIL KUMAR V, NANDI V S, BHAT R R, MURALIDHARA B K. Multi-stage heat treatment of aluminum alloy AA7049 [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(6): 1570-1575
[6] KOMURA Y, TOKUNAGA K. Structural studies of stacking variants in Mg-base Friauf-Laves phases [J]. Acta Crystallographica B, 1980, 36: 1548-1554.
[7] DEGISCHER H P, LACOM W, ZAHRA A, ZAHRA C Y. Decomposition processes in an Al-5%Zn-1%Mg alloy: Part II. Electronmicroscopic investigation [J]. Zeitschrift fur Metallkunde, 1980, 71: 231-238.
[8] BUHA J, LUMLEY R N, CROSKY A G. Secondary ageing in an aluminium alloy 7050 [J]. Materials Science and Engineering A, 2008, 492(1-2): 1-10.
[9] LI X Z, HANSEN V,  J, WALLENBERG L R. HREM study and structure modeling of the η' phase, the hardening precipitates in commercial Al-Zn-Mg alloys [J]. Acta Materialia, 1999, 47(9): 2651-2659.
J, WALLENBERG L R. HREM study and structure modeling of the η' phase, the hardening precipitates in commercial Al-Zn-Mg alloys [J]. Acta Materialia, 1999, 47(9): 2651-2659.
[10] KVERNELAND A, HANSEN V, THORKILDSEN G, LARSEN H B, PATTISON P, LI X Z,  J. Transformations and structures in the Al-Zn-Mg alloy system: A diffraction study using synchrotron radiation and electron precession [J]. Materials Science and Engineering A, 2011, 528(3): 880-887.
J. Transformations and structures in the Al-Zn-Mg alloy system: A diffraction study using synchrotron radiation and electron precession [J]. Materials Science and Engineering A, 2011, 528(3): 880-887.
[11] BERG L K,  J, HANSEN V, LI X Z, KNUTSON-WEDEL M, WATERLOO G, SCHRYVERS D, WALLENBERG L R. GP-zone in Al-Zn-Mg alloys and their role in artificial aging [J]. Acta Materialia, 2001, 49(17): 3443-3451.
J, HANSEN V, LI X Z, KNUTSON-WEDEL M, WATERLOO G, SCHRYVERS D, WALLENBERG L R. GP-zone in Al-Zn-Mg alloys and their role in artificial aging [J]. Acta Materialia, 2001, 49(17): 3443-3451.
[12] JIANG H, FAULKNER R G. Modelling of grain boundary segregation, precipitation and preciptate-free zones of high strength aluminium alloys: I. The models [J]. Acta Materialia, 1996, 44(5): 1857-1864.
[13] XU Ting-dong, CHENG Bu-yuan. Kinetics of non-equilibrium grain-boundary segregation [J]. Progress in Materials Science, 2004, 49(2): 109-208.
[14] COTTRELL A H. An introduction to metallurgy [M]. London: Edward Arnold, 1967: 345.
[15] LIU M, KLOBES B, MAIER K. On the age-hardening of an Al-Zn-Mg-Cu alloy: A vacancy perspective [J]. Scripta Materialia, 2011, 64(1): 21-24.
[16] RINGER S P, HONO K. Microstructural evolution and age hardening in aluminum alloys: Atom probe field-ion microscopy and transmission electron microscopy studies [J]. Materials Characterization, 2000, 44(1-2): 101-103.
Al-Zn-Mg-Cu合金在时效过程中η相沿小角晶界的析出序列
李茂华1,杨延清1,冯宗强1,黄 斌1,罗 贤1,娄菊红1,汝继刚2
1. 西北工业大学 凝固技术国家重点实验室, 西安 710072;
2. 北京航空材料研究院, 北京 100095
摘 要:采用高分辨透射电子显微镜和高角环形暗场扫描透射电子显微镜结合X射线能谱仪,研究Al-Zn-Mg-Cu合金η相沿小角晶界的析出序列。试样在135 °C分别时效5 min到6 h。结果表明,η相在小角晶界的析出序列是:SSS→VRC→GP II区→η′→η。基于非平衡晶界偏析和非平衡晶界共偏析理论,在Al-Zn-Mg-Cu合金时效过程中,通过溶质-空位对的扩散,大量的沉淀形成元素偏析到晶界。这种晶界偏析在VRC、GP II区、η′相和η相的形核和生长中起重要作用。
关键词:Al-Zn-Mg-Cu合金;时效;小角晶界;晶界偏析;析出序列
(Edited by Sai-qian YUAN)
Foundation item: Project (51071122) supported by the National Natural Science Foundation of China; Project (B08040) supported by the Program of Introducing Talents of Discipline to Universities, China (“111” Project)
Corresponding author: Mao-hua LI; Tel/Fax: + 86-29-88460499; E-mail: lmhwx@163.com
DOI: 10.1016/S1003-6326(14)63312-4
Abstract: The precipitation sequence of η(MgZn2) phase along low-angle grain boundaries in Al-Zn-Mg-Cu alloy was investigated by examining samples aged at 135 °C for various times from 5 min to 6 h. High resolution transmission electron microscopy (HRTEM) observations and energy dispersive X-ray spectroscopy (EDX) analysis indicate that the precipitation sequence of η phase along low-angle grain boundaries should be supersaturated solid solution (SSS)→vacancy-rich clusters (VRC)→GP II zones→η′→η. Based on the theory of non-equilibrium grain boundary segregation (NGS) and non-equilibrium grain boundary co-segregation (NGCS), the excessive solute elements gradually segregate to the grain boundaries by the diffusion of the solute-vacancy complex during aging treatment. The grain boundary segregation plays an important role in the nucleation and growth of VRC, GP II zones, η′ phase as well as η phase.


