文章编号:1004-0609(2010)02-0282-06
锂离子电池负极材料Li4Ti5O12的原位水解合成与表征
李运姣1,陈盼盼1,习小明2,邱文顺1
(1. 中南大学 冶金科学与工程学院,长沙 410083;2. 长沙矿冶研究院,长沙 410012)
摘 要:以TiCl4水溶液和LiOH·H2O为原料,采用原位水解与后续热处理相结合的方法制备尖晶石型锂离子电池用负极材料Li4Ti5O12。结果表明:从TiCl4水溶液原位水解合成Li4Ti5O12经历由TiCl4→TiO2→Li2TiO3→Li4Ti5O12 3个阶段的原位相转变过程;TiCl4水溶液的浓度及稳定性对合成Li4Ti5O12的结构有较大的影响;随着TiCl4浓度的增加,合成纯Li4Ti5O12所需的水解时间延长;以0.5 mol/L TiCl4水溶液水解1 h、以添加1.0 mol/L LiCl的0.5 mol/L TiCl4水溶液水解3 h、以1.0 mol/L与1.5 mol/L TiCl4水溶液水解5 h均可获得纯Li4Ti5O12;由低浓度TiCl4水溶液合成的Li4Ti5O12循环性能优良。
关键词:
中图分类号:O646;O614.1;TM912.9 文献标识码:A
Synthesis and characterization of Li4Ti5O12 anode material for
lithium ion batteries via in-situ hydrolysis
LI Yun-jiao1, CHEN Pan-pan1, XI Xiao-ming2, QIU Wen-shun1
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Changsha Research Institute of Mining and Metallurgy, Changsha 410012, China)
Abstract: Spinel Li4Ti5O12 as an anode material for lithium ion batteries was synthesized via in-situ hydrolysis followed by heat treatment using aqueous TiCl4 solution and LiOH·H2O as the raw materials. The results show that the synthesis of Li4Ti5O12 from aqueous TiCl4 solution by in-situ hydrolysis consists of three-step in-situ phase transformation, that is TiCl4→TiO2→Li2TiO3→Li4Ti5O12. The concentration and stability of aqueous TiCl4 solution have significant effects on the synthesis of Li4Ti5O12. With increasing TiCl4 concentration, the hydrolysis time of TiCl4 for the formation of Li4Ti5O12 increases. The optimal hydrolysis time for the synthesis of pure Li4Ti5O12 from TiCl4 solutions with different Ti(Ⅳ) concentrations is found to be 1 h for 0.5 mol/L TiCl4, 3 h for 0.5 mol/L TiCl4 with addition of 1.0 mol/L LiCl and 5 h for both 1.0 mol/L and 1.5 mol/L TiCl4. The products prepared from lower Ti(Ⅳ) concentration solutions show excellent cycling performance.
Key words: lithium ion batteries; anode material; spinel Li4Ti5O12; in-situ hydrolysis
尖晶石锂钛氧化物(Li4Ti5O12)是目前倍受人们青睐的最理想的锂离子电池负极材料之一[1?4]。其空间 群为Fd3m,O2?呈面心立方最紧密堆积构成FCC点 阵,占据32e位, 3/4 的Li+占据四面体空隙的8a位, 其余1/4Li+和全部Ti4+以1?5(摩尔比)的比例占据八面体空隙的16d位。在充电过程中,新嵌入的Li+占据16c位,原来位于8a位的Li+向16c位迁移,即: {(Li)8a [Li0.33Ti1.67]16dO4}→[Li1.33?0.67]16c[Ti1.67?0.33]16d O4,并在16c和16d形成空位,同时发生Ti4+向Ti3+的转变而形成相同结构的淡蓝色Li7Ti5O12[5]。由于Li4Ti5O12 与Li7Ti5O12的晶格常数相似,充放电过程结构基本不发生改变而使其具有优良的循环性能和使用寿命;其理论比容量为175 mA?h/g,实际比容量可达150~160 mA?h/g,具有良好的充放电平台,可提供稳定的工作电压(相对于Li 1.5V);且由于Li4Ti5O12不与电解液反应,与商品化的碳负极材料相比,其电化学性能和安全性能更好[6?8]。尽管Li4Ti5O12具有优良的循环性能,但用于混合电动汽车(Hybrid electric vehicle,HEV)、固态锂离子电池和锂电池等高能电池领域,高倍率充放电性能还有待改善,其合成方法有待进一步研究与开发。
目前,Li4Ti5O12的合成方法主要基于以TiO2为原料的固相反应法[9?10]和以有机化合物为原料的溶胶?凝胶?热处理法[11?13]。固相法即将纳米TiO2与LiOH·H2O或Li2CO3经充分研磨混合后在800~1 000 ℃下烧制12~20 h合成[14?15],其混合方式(如普通混合、研磨和高能球磨等)及TiO2的结构(金红石型、锐钛矿型或其混合型)与性能都直接影响到产物Li4Ti5O12的结构、形貌与性能。溶胶?凝胶?热处理法则以钛酸酊脂等钛的有机化合物与乙酸锂等锂化合物为原料,先在有机溶剂中混合制得凝胶,烘干后再进行研磨混合,然后在500~900 ℃下焙烧6~20 h制得纯Li4Ti5O12。GAO等[13]采用内凝胶与高温烧结相结合的方法,先将TiCl4水溶液与(CH2)6N4和CO(NH2)2在低温(<10 ℃)下混合后于70 ℃下加热制得球型前驱体,然后将该前驱体与Li2CO3均匀混合后在800 ℃下烧结16 h获得球型Li4Ti5O12。FATTAKHOVA等[14]采用水热法以TiO2为原料在130~200 ℃水热处理20 h合成Li4Ti5O12[14]。LI等[15]以工业TiO2粉末为原料,先在浓NaOH(10 mol/L)溶液中于120~170 ℃下处理24~72 h,借助声化学水热反应使TiO2转型成氢钛酸纳米管/线,然后将所得氢钛酸纳米管/线与0.2 mol/L LiOH溶液在120~140 ℃下水热反应24~36 h,再于300~500 ℃下热处理2~6 h获得Li4Ti5O12纳米管/线。
关于从TiCl4溶液原位水解合成Li4Ti5O12目前还未见有文献报道。本文作者研究了一种简便的湿化学合成Li4Ti5O12方法,即采用TiCl4水溶液中和水解法在水溶液中原位合成Li4Ti5O12。其实质是以LiOH为中和剂,当TiCl4发生中和水解时,利用新生成的水合氧化钛比表面积大、嵌Li+活性好等特点,在水溶液中TiO2前驱体形成时,使Li嵌入其结构中,实现原子级水平的均匀嵌Li+,并同时实现TiO2向Li4Ti5O12的原位相转化,合成锂离子电池负极材料Li4Ti5O12。在此主要报道TiCl4水解程度对产物结构与电性能的影响。
1 实验
1.1 Li4Ti5O12的制备
以TiCl4为原料,在冰水浴中配制浓度约为2 mol/L的TiCl4水溶液,置于冰箱保存备用。Li4Ti5O12的合成以TiCl4溶液和LiOH·H2O为原料,在60 ℃下,将一定浓度的LiOH·H2O溶液缓缓加入到TiCl4水解液中,使之水解原位合成Li4Ti5O12。水解反应完成后,进行真空抽滤,分离出沉淀产物,先在105 ℃下干燥。然后在800 ℃下热处理6 h,随炉冷却得到最终产物。
为了进行比较,实验过程中同时进行了TiCl4自由水解制备TiO2的研究。将上述配制的TiCl4储备液稀释成0.5和1.0 mol/L的溶液在60 ℃恒温水浴中分别进行自由水解,水解完成后经过滤和去离子水洗涤,于80 ℃下干燥2 d,得到自由水解产物。
1.2 分析与检测
采用日本理学D/max-rA X射线衍射仪分析合成样品的物相成分。采用(JEOF) JSM–56 00LV型扫描电子显微镜(日本产)表征样品的粒度及形貌。采用美国TA公司SDT Q6000型差热?热重分析仪,在室温到 1 000 ℃之间,以10 ℃/min的升温速率,在氩气保护下进行差热?热重分析(DSC-TGA)。
以制得的Li4Ti5O12样品为正极活性物质,NMP为溶剂,按正极活性物质、乙炔黑、PVDF的比例为80?12?8混合,研磨均匀后涂在铝箔上,制成正极;以金属锂片为负极,采用Celgard2400(国产)聚丙烯微孔隔膜,以1.0 mol/L LiPF6/EC(碳酸乙烯酯)+DEC(碳酸二乙酯) (体积比1?1)为电解液,在充满氩气的不锈钢手套箱中装配成CR?2025型扣式电池,室温下以恒电流/恒电压(2.5V)在LAND BTI?40程控电池测试仪上进行充放电性能测试。
2 分析与讨论
2.1 TiCl4自由水解条件下TiO2的形成
图1所示为TiCl4水溶液自由水解产物的XRD谱。由图1可知,在60 ℃下, 浓度为0.5和1.0 mol/L的TiCl4水溶液自由水解最终都生成了金红石TiO2。由于没有进行热处理,产物结晶度不高,但其XRD谱在2θ=27.480?、36.122?、41.283?、44.040?、54.377?、64.116?、68.998?和69.051?处已清晰地出现了金红石TiO2的衍射峰,说明金红石TiO2已经初步形成。在不加入中和剂的情况下,TiCl4水解生成HCl,使溶液的酸度升高(pH值都小于2)。在这种低pH值条件下,锐钛矿型TiO2不稳定,只有金红石型TiO2得以稳定存在,这与文献[16]的结果是一致的。
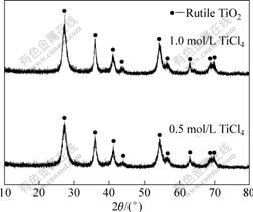
图1 TiCl4水溶液自由水解产物的XRD谱
Fig.1 XRD patterns of free hydrolysis product from aqueous TiCl4 solution
2.2 强制水解条件下Li4Ti5O12的合成
实验中以不同浓度TiCl4水溶液在不同水解时间下原位合成Li4Ti5O12。以0.5 mol/L TiCl4水溶液(以Ti-0.5表示)在不同的水解时间下合成的Li4Ti5O12的XRD谱如图2所示。从图2可以看出,水解初期(0~2/3 h)制备的Li4Ti5O12产物都不同程度地出现了Li与Ti高摩尔比的Li2TiO3相(Li与Ti的摩尔比为2)。说明水解前期得到的TiO2嵌锂活性相当好,在局部区域可
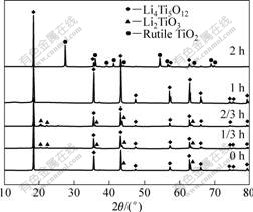
图2 Ti-0.5溶液在不同水解时间下合成的Li4Ti5O12的XRD谱
Fig.2 XRD patterns of Li4Ti5O12 obtained from aqueous Ti-0.5 solution at different hydrolysis times
以嵌入过量Li而形成Li2TiO3。水解1 h后得到了纯相的Li4Ti5O12,其XRD谱中晶体衍射峰2θ对应的d值与Li4Ti5O12标准卡片JCPDS49?0207上的d值完全吻合,且衍射峰尖锐,表明合成的粉体结晶度高。当继续延长水解时间至3 h时,所得产物中除Li4Ti5O12外,还含有一定量的金红石TiO2。表明0.5 mol/L的TiCl4水溶液水解3 h时生成的白色沉淀TiO2(或TiO2?H2O)已经具有一定的晶体结构稳定性,活性较差,这部分TiO2难以嵌锂转变成Li4Ti5O12。
图3所示为浓度为1.0 mol/L的TiCl4水溶液(以Ti-1.0表示)在不同水解时间下合成Li4Ti5O12的XRD谱。由于TiCl4水溶液的稳定性随浓度的影响较大,以不同浓度的TiCl4水溶液合成Li4Ti5O12的效果也不一样。浓度越高,溶液的稳定性越好,水解诱导期越长,水解过程进行得越缓慢。在Ti-1.0溶液刚开始水解时看不到溶液有很明显的变化,水解初期生成的沉淀也不多。与图2比较发现,随Ti浓度的提高,合成结晶度好、相纯度高的Li4Ti5O12所需水解时间延长。由图3可以看出,浓度为1.0 mol/L的TiCl4水溶液在刚发生水解(0 h)及水解3 h后所获得的样品中均出现了Li2TiO3杂相峰,在水解5 h时的合成效果较好,得到纯Li4Ti5O12,其衍射峰也较强。
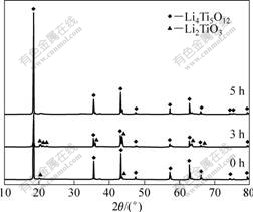
图3 Ti-1.0溶液在不同水解时间下合成的Li4Ti5O12的XRD谱
Fig.3 XRD patterns of Li4Ti5O12 obtained from Ti-1.0 aqueous solution at different hydrolysis times
当Ti的浓度达到1.5 mol/L时,同样在TiCl4水解5 h时获得纯Li4Ti5O12。为了考查溶液的稳定性对合成Li4Ti5O12的影响,对含1.0 mol/L LiCl的0.5 mol/L TiCl4溶液进行实验(以Li-1.0表示),结果发现,出现纯Li4Ti5O12的水解时间由没有添加LiCl的1 h延长至3 h。这是由于LiCl的存在在一定程度上抑制了TiCl4的水解,提高了溶液的稳定性。从不同浓度的TiCl4水溶液中和水解获得纯相Li4Ti5O12所需水解时间如表1所示,其XRD分析如图4所示。从表1和图4可以看出,溶液中Ti的浓度越高,或抑制Ti水解的添加剂(如LiCl)浓度越高,获得纯相Li4Ti5O12所需水解时间越长,但所得Li4Ti5O12的结构相同,其XRD谱基本一致。
表1 不同Ti(Ⅳ)溶液得到纯相Li4Ti5O12的水解时间
Table 1 Hydrolysis time for obtaining pure Li4Ti5O12 from different Ti(IV) solutions
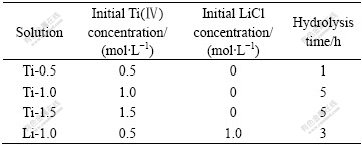
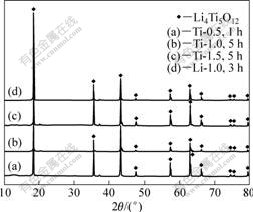
图4 以不同溶液制备的纯相Li4Ti5O12 的XRD谱
Fig.4 XRD patterns of pure Li4Ti5O12 obtained from different Ti(IV) solutions
实验研究发现,TiCl4水溶液的水解存在一个诱导期,在水解初期,由于均相成核速度缓慢,水解产生的HCl浓度较低,水解所得TiO2的结晶度低,活性较好。随着水解过程的进行,Li+嵌入TiO2(或其水合物)形成Li与Ti摩尔比较高的中间锂钛氧化物Li2TiO3。Li2TiO3在进一步的水解过程与新生成的TiO2(或其水合物)作用发生原位相转变形成Li4Ti5O12前驱体(少部分Li2TiO3在热处理过程与TiO2作用转变成Li4Ti5O12)。其过程可以用以下3个方程式来描述。
1)TiCl4水溶液水解形成活性TiO2:
TiCl4+2H2O=TiO2(or TiO2?2H2O)+4HCl (1)
2) 活性TiO2嵌锂形成中间锂钛氧化物Li2TiO3:
TiO2+2LiOH=Li2TiO3+H2O (2)
3) 中间锂钛氧化物Li2TiO3进一步与活性TiO2反应形成Li4Ti5O12前驱体:
Li2TiO3+4TiO2+2LiOH=Li4Ti5O12+H2O (3)
Li2TiO3和Li4Ti5O12都有迁移离子的能力,而且两者都具有层状结构:Li4Ti5O12为尖晶石型,Li2TiO3为岩盐型,它们的这种层状结构非常相似,只有处在8a位置的Li离子半径有所差别:Li4Ti5O12为0.76?,Li2TiO3为0.59?[17?18]。Li2TiO3(002)的晶面间距(4.80 ?)与Li4Ti5O12(111)的晶面间距(4.80 ?)非常接近[19?20]。这两种层状结构互相关联的可能性非常高。水溶液中新生成的活性TiO2通常为无定形,其结构不稳定,Ti离子很容易发生短程扩散进入Li2TiO3(002)的层间结构而转变成稳定的Li4Ti5O12(111)。
2.3 DSC-TGA分析
图5所示为TiCl4水溶液原位水解合成的Li4Ti5O12前躯体的DSC-TGA曲线。从图5可以看出,其热反应过程可以分为3个阶段:第一阶段从室温到260 ℃,这一阶段的质量损失约为28.57%,在124.3 ℃处有一个明显的吸热峰,为前躯体的脱水(包括吸附水和结晶水)过程;第二阶段从260到800 ℃,这一阶段的质量损失不明显,在602.38 ℃有一个小放热峰,为α-Li2TiO3向β-Li2TiO3转变的过程[21];此外,于607.0 ℃处还有一较强的吸热峰,这是TiO2与Li2TiO3结合形成尖晶石Li4Ti5O12过程(式(3)),反应过程由于水的生成而导致轻微的质量损失(<1%)。第三阶段在800~ 1 100 ℃之间,DSC曲线上位于947.83 ℃处有一个较大较宽的吸热峰,根据LEYKAMP等[21]的研究,此为尖晶石Li4Ti5O12向斜方锰矿型Li2Ti3O7转变的相变峰。
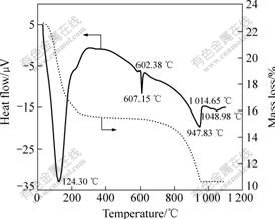
图5 Li4Ti5O12前驱体的DSC-TGA曲线
Fig.5 DSC-TGA curves of Li4Ti5O12 precursor
图6所示为合成的Li4Ti5O12前驱体在不同热处理的LiOH和LiCl量很少;550 ℃烧结6 h产物的XRD谱以Li4Ti5O12为主,但其衍射峰较宽,结晶度不太高,而且在2θ=27.5?和2θ=54.5?两处有较微弱的金红石型TiO2衍射峰,而在2θ=20.4?和2θ=37.4?处有微弱的Li2TiO3杂相峰,表明在此温度下合成产物中含有少量的金红石型TiO2和Li2TiO3杂质。前驱体在700 ℃热处理6 h后,TiO2杂相峰已经基本消失,但微弱的Li2TiO3的特征峰依然存在。随着热处理温度升高至800 ℃时,Li2TiO3与残存的TiO2及LiOH反应转变成Li4Ti5O12,Li2TiO3的特征峰消失,尖晶石Li4Ti5O12的特征峰的强度变大,衍射峰峰形变尖锐,这说明产物的结晶度变好,晶体生长逐渐完整。这与前面的DSC-TGA分析结果是一致的。
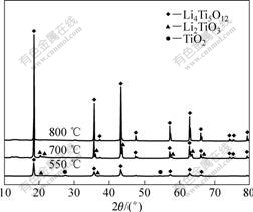
图6 不同热处理温度合成的Li4Ti5O12的XRD谱
Fig.6 XRD patterns of Li4Ti5O12 obtained at different heat treatment temperatures
2.4 电性能分析
图7所示为Ti-0.5溶液水解1 h、Ti-1.0和 Ti-1.5溶液水解5 h以及Li-1.0溶液水解3 h制备的Li4Ti5O12在0.5~2.4 V电压范围内,0.1C倍率下的首次充放电曲线。Li4Ti5O12电化学反应可以表示为
Li4Ti5O12+3Li++3e=Li7Ti5O12 E=1.56 V (4)
从图7可以看出,Ti-0.5 和Li-1.0两个试样在充放电过程中都呈现了良好的平台,Ti-0.5的充放电平台分别为1.58和1.57 V,与理论充放电平台1.56 V接近。而Li-1.0试样的充放电平台分别约为1.61和1.51 V,与1.56 V偏离稍大,这是由于电极的欧姆极化造成的。充放电曲线成L形,具有典型的两相反应特征。而Ti-1.0试样的充电平台接近1.5 V,其放电平台降至1.4 V;Ti-1.5试样的充电平台达到1.6 V,其放电平台反而靠近1.5 V。Ti-1.0和Ti-1.5两个试样的首次充放电比容量都不到100 mA?h/g。这可能与试样的粒度和形貌不均匀以及团聚现象严重有关。
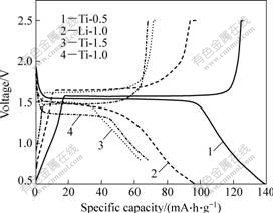
图7 以不同浓度TiCl4水溶液制备的Li4Ti5O12的首次充放电曲线
Fig.7 First charge/discharge curves of Li4Ti5O12 obtained from different TiCl4 solutions
图8所示为上述4个样品的循环比容量曲线。从图8可以看出,Ti-0.5和 Li-1.0产物的首次放电比容量分别为201.8、249.9 mA?h/g,第二次循环后,其可逆比容量分别为140.2和105.3 mA?h/g,首次比容量损失较大,这可能与生成含锂的不可逆氧化物或电解液的分解有关;经16次循环后,Ti-0.5可逆比容量仍为113.6 mA?h/g,为第二次循环容量的81.03%,经10次循环后,Li-1.0可逆比容量为90.5 mA?h/g,为第二次循环容量的85.9%。兼顾比容量和循环稳定性,以浓度为0.5 mol/L的TiCl4(Ti-0.5)水溶液水解1 h得到的尖晶石Li4Ti5O12电极材料性能最佳。
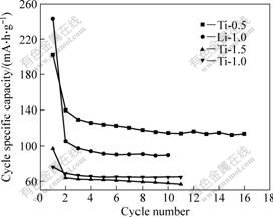
图8 以不同浓度TiCl4水溶液制备的Li4Ti5O12的循环性能
Fig.8 Cycling property of Li4Ti5O12 obtained from different TiCl4 solutions
3 结论
1) 从TiCl4水溶液原位水解合成Li4Ti5O12经历了由TiCl4→TiO2→Li2TiO3→Li4Ti5O12 3个阶段的原位相转变过程。TiCl4水溶液的稳定性对Li4Ti5O12的合成影响较大。Ti的浓度越高,溶液稳定性越好,获得纯Li4Ti5O12所需的水解时间越长。从不同浓度TiCl4水溶液合成纯Li4Ti5O12所需自由水解时间分别为:Ti-0.5水解1 h、Li-1.0水解3 h、Ti-1.0与Ti-1.5水解5 h。
2) 0.1C倍率下,低浓度TiCl4水溶液(0.5 mol/L)合成的Li4Ti5O12具有较好的循环性能,首次放电容量为201.8 mA?h/g, 循环16次后,比容量仍为113.6 mA?h/g,充放电平台与Li4Ti5O12的理论充放电平台1.56 V接近,具有Li4Ti5O12尖晶石的典型电性能特性。
REFERENCES
[1] 张汉平, 付丽君, 吴宇平, 吴浩青, 高村勉. 锂离子电池负极材料的研究进展[J]. 电池, 2005, 35(4): 571?572.
ZHANG Han-ping, FU Li-jun, WU Yu-ping, WU Hao-qing, GAO Cun-mian. Research progress in anode materials for Li-ion batteries[J]. Battery Bimonthly, 2005, 35(4): 571-?572.
[2] Jansen A N, Kahaian A J, Kepler K D, Nelson P A, Amine K, Dees D W, Vissers D R, Thackeray M M. Development of a high-power lithium-ion battery[J]. Journal of Power Sources, 1999, 81/82: 902?905.
[3] Ohzuku T, Ueda A, Yamamoto N. Zero-Strain Insertion Material of Li(Li1/3Ti5/3)O4 for rechargeable lithium cells[J]. Journal of the Electrochemical Society, 1995, 142(5): 1431?1436.
[4] Leonidov I A, Leonidova O N, Perelyaeva L A, Samigullina R F, Kovyazina S A, Patrakeev M V. Structure ionic conduction and phase transformation in lithium titanate Li4Ti5O12[J]. Physics of the Solid State, 2003, 45(11): 2183?2188.
[5] LEONIDOV I A, LEONIDOVA O N, PERELYAEVA L A, SAMIGULLINA R F, KOVYAZINA S A, and PATRAKEEV M V. Structure, ionic conduction, and phase transformations in lithium titanate Li4Ti5O12[J]. Physics of the Solid State, 2003, 45(11), 2183?2188.
[6] Kim D H, Ahn Y S, Kim J. Polyol-mediated synthesis of Li4Ti5O12 nanoparticle and its electro-chemical properties[J]. Electrochemistry Communications, 2004, 34(6): 1093?1097.
[7] Kiyoshi N, Ryosuke N, Tomoko A, Hiroshi M. Preparation of particulate Li4Ti5O12 having excellent characteristics as an electrode active material for power storage cells[J]. Journal of Power Sources, 2003, 117(12): 131?136.
[8] 徐宇虹, 巩桂英, 马 萍, 张宝宏. Li4Ti5O12的合成及其在锂离子电池中的应用[J]. 金属材料与冶金工程, 2007, 35(1): 14?18.
XU Yu-hong, GONG Gui-ying, MA Ping, ZHANG Bao- hong. Preparation of Li4Ti5O12 and application in lithium ion battery[J]. Metal Materials and Metallurgy Engineering, 2007, 35(1): 14?18.
[9] GUERFI A, CHAREST P, KINOSHITA K, PERRIER M, ZAGHIB K. Nano electronically conductive titanium-spinel as lithium-ion storage negative electrode[J]. Journal of Power Sources, 2004, 126: 163?168.
[10] HUANG Sha-hua, WEN Zhao-yin, ZHU Xiu-jian, GU Zhong-hua. Preparation and electrochemical performance of Ag doped Li4Ti5O12[J]. Electrochemistry Communications, 2004, 6: 1093?1097.
[11] DOKK K, SUGAYA J, MUNAKATA H, KANAMURA K. Preparation of micro-dot electrodes of LiCoO2 and Li4Ti5O12 for lithium micro-batteries[J]. Electrochimica Acta, 2005, 51: 966?971.
[12] VENKATESWARLU M, CHENA C H, DO J S, LIN C W, CHOU T C, HWANG B J. Electrochemical properties of nano-sized Li4Ti5O12 powders synthesized by a sol–gel process and characterized by X-ray absorption spectroscopy[J]. Journal of Power Sources, 2005, 146: 204?208.
[13] GAO J, JIANG C, YING J, WAN C. Preparation and characterization of high-density spherical Li4Ti5O12 anode material for lithium secondary batteries[J]. Journal of Power Sources, 2006, 155: 364?367.
[14] FATTAKHOVA D, KRTIL P. Electrochemical activity of hydrothermally synthesized Li-Ti-O cubic oxides toward Li insertion[J]. Journal of the Electrochemical Society, 2002, 149(9): A1224?A1229.
[15] LI J, TANG Z, ZHANG Z. Controllable formation and electrochemical properties of one-dimensional nanostructured spinel Li4Ti5O12[J]. Electrochemistry Communications, 2005, 7: 894?899.
[16] LI Y J, George P D. Precipitation of nanosized titanium dioxide from aqueous titanium(Ⅳ) chloride solutions by neutralization with MgO[J]. Hydrometallurgy, 2008, 90: 26?33.
[17] Kunimitsu K, Yasuhiko T. Crystal growth and structure refinement of monoclinic Li2TiO3[J]. Materials Research Bulletin, 2009, 44(5): 168?172.
[18] Marnix W, Ernst R H. Li-ion diffusion in the equilibrium nanomorphology of spinel Li4+xTi5O12[J]. The Journal of Physical Chemistry, 2009, 113(2): 224?230.
[19] Abe Y, Matsui E, Senna M. Preparation of phase pure and well-crystallized Li4Ti5O12 nanoparticles by precision control of starting mixture and calcining at lowest possible temperatures[J]. The Journal of Physics and Chemistry of Solids, 2007, 68(8): 681?686.
[20] Eitaro M, Yuichi A, Senna M. Solid-state synthesis of 70 nm Li4Ti5O12 particles by mechanically activating intermediates with amino acids[J]. Journal of American Ceramic Society, 2008, 91(5): 1522?1527.
[21] leykamp H. Phase equilibria in the Li-Ti-O system and physical properties of Li2TiO3[J]. Fusion Engineering and Design, 2002, 61/62: 361?366.
基金项目:国家自然科学基金资助项目(50774103)
收稿日期:2009-05-21;修订日期:2009-10-11
通信作者:李运姣,教授,博士;电话:0731-88830476;E-mail:yunjiaoli6601@hotmail.com


