文章编号:1004-0609(2012)08-2283-06
液氮淬火法制备高性能锂离子电池正极材料LiFePO4/C
张 明1, 2,尹周澜1,郭学益3,张 宝3
(1. 中南大学 化学化工学院,长沙 410083;
2. 肇庆学院 化学化工学院,肇庆 526061;
3. 中南大学 冶金科学与工程学院,长沙 410083)
摘 要:
以FePO4、Li2CO3和葡萄糖为原料,用液氮急速淬火法制备单一橄榄石结构的锂离子电池正极材料LiFePO4/C。结果表明:淬火使得LiFePO4晶格中产生Li空位,有利于提高其电子导电性。淬火样品的一次颗粒细小(100~500 nm),无明显团聚,并形成多孔结构;该样品在1C、2C和4C倍率下的首次放电比容量分别为151.4、138.0和116.7 mA·h/g,循环100次后的容量保持率高达99.3%、98.6%和94.5%。
关键词:
中图分类号:TM912.9 文献标志码:A
Synthesis of high-performance LiFePO4/C by liquid nitrogen quenching method
ZHANG Ming1, 2, YIN Zhou-lan1, GUO Xue-yi3, ZHANG Bao3
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. College of Chemistry and Chemical Engineering, Zhaoqing University, Zhaoqing 526061, China;
3. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: A single olivine-type LiFePO4 was synthesized by liquid nitrogen rapid quenching method, using FePO4, Li2CO3 and glucose as raw materials. XRD refinement results indicate that rapid quenching leads to the formation of a number of Li vacancies in LiFePO4 crystals. These defects are beneficial to improving the electronic conductivity of LiFePO4. The quenched sample shows the primary particles size of 100-500 nm. The particles exhibit little agglomeration, and form a porous structure. The sample exhibits an initial discharge capacity of 151.4, 138.0 and 116.7 mA·h/g at 1C, 2C and 4C rate, and shows the capacity retention of 99.3%, 98.6% and 94.5% after 100 cycles, respectively.
Key words: lithium-ion batteries; cathode materials; lithium iron phosphate; liquid nitrogen; quenching
橄榄石结构的LiFePO4因具有理论比容量高(170 mA·h/g)、循环性能和安全性能好、原料来源广等优点,成为新一代锂离子电池正极材料的有力竞争 者[1-3]。然而,极低的电子导电率(10-9~10-10 S/cm)和锂离子扩散速率(1.8×10-14 cm2/s)[4-5]阻碍了其大规模应用,因此,如何提高LiFePO4的电子电导率和锂离子扩散速率成为研究热点。目前,提高LiFePO4电导率的方法主要如下:高价金属阳离子掺杂[6-9]、制备LiFePO4/金属粉末[10-11]或LiFePO4/C[12-14]复合材料,而提高锂离子扩散速率则主要通过优化合成工 艺,制备纳米级粉末[15-17]以及复合快离子导体[18-19]等来解决。
在此,本文作者提出一种可以同时提高LiFePO4电子电导率和锂离子扩散速率的新方法。即通过改变煅烧后物料的冷却速度,再用液氮急速淬火以获得高性能LiFePO4。一方面,急速淬火使得LiFePO4晶格中产生缺陷,如空穴等,可大大提高其电导率;另一方面,急速淬火使得LiFePO4颗粒变得更加细小,有利于锂离子的扩散。最近,ZHENG等[20-21]用液氮淬火法制备了高性能Li3V2(PO4)3/C正极材料,研究表明液氮淬火后Li3V2(PO4)3/C的倍率性能和循环性能得到了极大改善。然而,迄今为止,尚未见液氮淬火法在其他正极材料方面的应用报道,在此,本文作者将其用于LiFePO4的合成改性,重点研究液氮淬火对LiFePO4的晶体结构、形貌及电化学性能的影响。
1 实验
按Li、Fe、C摩尔比为1:1:0.1称取FePO4(自制)、Li2CO3(AR)和葡萄糖(AR),以乙醇(AR)为介质,在常温下球磨2 h得前驱体混合物,将混合物于80 ℃烘干后置入程序控温管式炉,在高纯氩气(99.999%,质量分数)的保护下于650 ℃煅烧10 h,然后迅速将炽热的粉末倒入液氮中,液氮迅速气化,收集粉末即为LiFePO4(记为LFP-NQ)。另外,按上述相同流程获得前驱体混合物,于氩气中650 ℃下煅烧10 h后随炉冷却得到的LiFePO4作为对比样品(记为LFP)。
采用日本Rigaku D/max2550VB+18kW转靶X射线衍射仪进行物相分析, 分析条件如下:Cu Kα辐射,40 kV,300 mA,步宽0.02°;用Rietveld方法对XRD进行精修,计算晶格常数;采用JEOL公司生产的JSM-5600LV扫描电镜在20 kV下观察材料的表面形貌;用 Tecnai G12型透射电镜(TEM)表征材料的微观形貌;用德国CS800碳硫检测仪分析样品的碳含量;用四探针法测定样品的电导率。
将LiFePO4、导电炭黑和黏结剂(PvDF)按质量比9:0.5:0.5混合,以铝箔为基体制备成d 14 mm的正极片,将正极片与负极片(Li,d 14 mm)、电解液(1 mol/L的LiPF6/EC+EMC+DMC (体积比1:1:1) )和隔膜(Celgard 2300 PP/PE/PP)在充满氩气的手套箱中组装成CR2025型扣式电池,电池静置12 h后进行电化学测试。用兰电测试系统对电池的首次充放电性能和循环性能进行测试,测试条件如下:室温,2.5~4.1 V,以0.1C、1C、2C和4C恒流充放电。循环伏安和交流阻抗测试在华晨CHI660D电化学工作站上完成,循环伏安测试条件如下:电压扫描范围2.5~4.5 V,扫描速率0.1 mV/s;交流阻抗测试条件如下:频率范围0.01 Hz~100 kHz,正弦波振幅5 mV。
2 结果与讨论
2.1 X射线衍射分析
图1所示为样品LFP和LFP-NQ的XRD谱。与LiFePO4的标准图谱(PDF#40-1499)相比,两样品均为有序的橄榄石结构,且均未发现杂相的存在,说明液氮淬火没有改变LiFePO4的晶体结构。然而,LFP-NQ的衍射峰强度明显低于LFP的,说明淬火使得LiFePO4的结晶度降低。另外,碳-硫分析表明:样品LFP和LFP-NQ的碳含量分别为3.51%和3.62%(质量分数),然而XRD中均未出现碳的衍射峰,说明其为无定形结构。
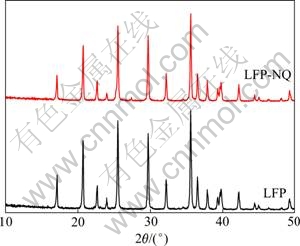
图1 样品LFP和LFP-NQ的XRD谱
Fig. 1 XRD patterns of samples LFP and LFP-NQ
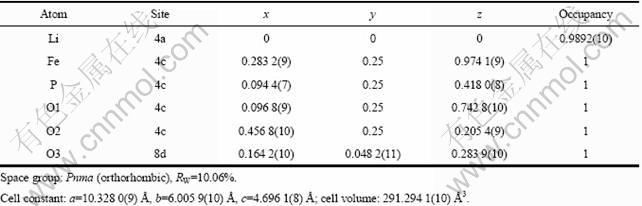
为了进一步研究液氮淬火对LiFePO4结构的影响,用Rietveld方法对晶格常数、原子位置、半峰宽、占位率和各向同性温度因子等参数进行了精修,精修结果如图2和表1和2所示。由图2可知,精修曲线和测试曲线基本吻合,说明精修结果可靠。由表1和2可知,淬火使得LiFePO4的晶格常数减小,晶胞体积收缩。另外,样品LFP的Li位占位率为1,而LFP- NQ的Li位占位率为0.989 2,说明LFP-NQ中存在Li空位。这是由于液氮的急速淬火使得样品的结晶突然停止,从而导致缺陷的产生。四探针法测得样品LFP和LFP-NQ的电导率分别为2.6×10-4和9.8×10-3 S/cm,这说明Li空位的产生可以大大提高LiFePO4的电导率[4, 9, 20]。
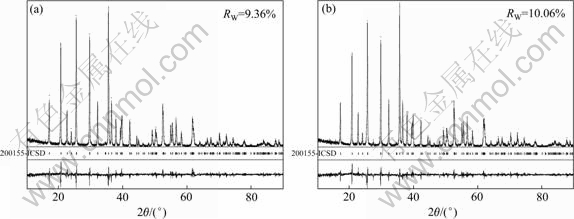
图2 样品LFP和LFP-NQXRD谱的Rietveld精修曲线
Fig. 2 Rietveld-refined XRD patterns of samples LFP (a) and LFP-NQ (b)
表1 LFP的XRD-Rietveld精修结果
Table 1 Rietveld refinement results of LFP
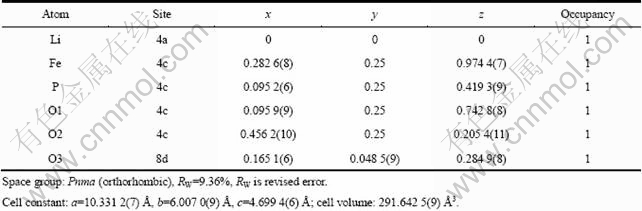
表2 LFP-NQ的XRD-Rietveld精修结果
Table 2 Rietveld refinement results of LFP-NQ
2.2 样品的扫描电镜分析
图3所示为样品LFP和LFP-NQ的SEM像。由图3可知,随炉冷却的样品(LFP)一次颗粒粒径为0.2~ 1.5 μm,且颗粒的团聚严重。而淬火冷却样品(LFP-NQ)一次颗粒粒径为100~500 nm,颗粒无明显团聚,并组成多孔的结构,这种结构使得电解液与LiFePO4接触更加充分,有利于充放电过程中锂离子的传递。这表明淬火能极大地改变LiFePO4的形貌,使得材料的一次颗粒来不及长大,并能有效防止其团聚。
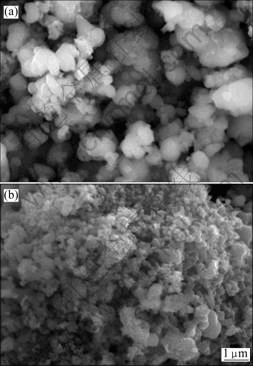
图3 样品LFP和LFP-NQ的SEM像
Fig. 3 SEM images of samples LFP (a) and LFP-NQ (b)
2.3 样品的透射电镜分析
图4所示为样品LFP和LFP-NQ的TEM像。LFP-NQ的颗粒粒径要明显小于LFP的,这与SEM的观测结果一致。二者的颗粒周围均有絮状的碳网相连,这有利于提高材料的电子导电性。
2.4 样品的电化学性能
图5所示为LFP和LFP-NQ在不同倍率下的首次充放电曲线。由图5可知,二者在0.1C倍率下的首次放电比容量相当,分别为163.5和163.4 mA·h/g。但是,在高倍率下LFP-NQ的放电比容量和循环性能均明显优于LFP的,如LFP-NQ在1C、2C和4C倍率下的首次放电比容量分别为151.4、138.0和116.7 mA·h/g,比LFP的首次放电比容量(分别为142.8、123.5 和97.0 mA·h/g)分别提高了6.0%、11.7% 和20.3%。
图6所示为LFP和LFP-NQ在不同倍率下的循环性能曲线。液氮淬火后的样品在高倍率下的循环性能明显优于随炉冷却的样品。LFP在1C、2C和4C倍率下循环100次后的放电比容量分别为133.3、112.2和75.4 mA·h/g,容量保持率分别为93.3%、90.9%和77.7%;而LFP-NQ在1C、2C和4C倍率下循环100次后的放电比容量分别为150.4、136.0和110.3 mA·h/g,容量保持率高达99.3%、98.6%和94.5%。根据前面的分析,液氮淬火对LiFePO4电化学性能显著改善的原因如下:1) 淬火使得晶体中产生缺陷(如Li空位),有助于改善LiFePO4的导电性;2) 淬火使得LiFePO4的一次颗粒减小,防止其团聚,有利于充放电过程中锂离子的传递。
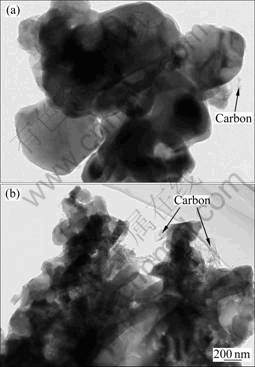
图4 样品LFP和LFP-NQ的TEM像
Fig. 4 TEM images of samples LFP (a) and LFP-NQ (b)
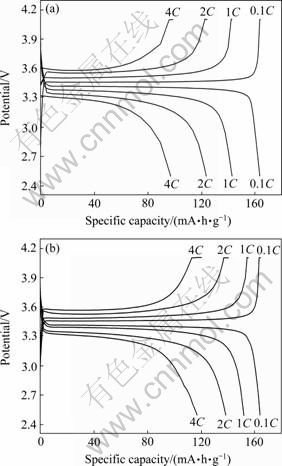
图5 样品LFP和LFP-NQ在不同倍率下的首次充放电曲线
Fig. 5 Initial charge and discharge curves of LFP(a) and LFP-NQ(b) at various rates
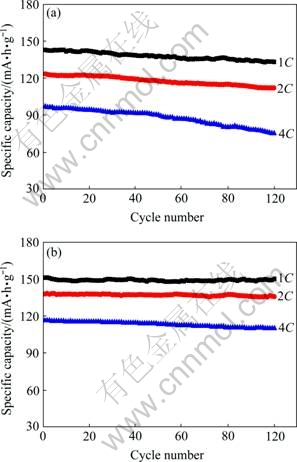
图6 样品LFP和LFP-NQ在不同倍率下的循环性能曲线
Fig. 6 Cycle performance profiles of LFP(a) and LFP-NQ(b) at various rates
2.5 样品的循环伏安分析
图7所示为LFP和LFP-NQ电极的循环伏安曲线。由图7可知,各样品均有且仅有一对氧化还原峰,氧化峰在3.6 V附近,还原峰在3.4 V附近。但是,LFP- NQ电极氧化还原峰之间的电势差明显小于LFP电极的电势差,说明LFP-NQ电极的极化更小,反应可逆性更好,电极反应更易进行。
2.6 样品的交流阻抗分析
图8所示为LFP和LFP-NQ电极的交流阻抗谱。图8中两条谱线均由一个半圆和一条直线组成,高频区的半圆对应于电解质/电极材料界面的电荷转移阻抗(Zct),低频区的直线代表Li+在电极材料中扩散所引起的Warburg阻抗(ZW)[22]。由图8可知,LFP-NQ电极的Zct(112 Ω)低于LFP电极的(261 Ω),电荷转移阻抗的减小有利于克服充放电过程中的动力学限制,从而有助于材料比容量的提高和循环性能的改善。
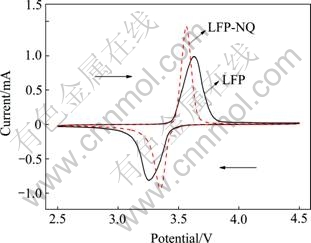
图7 样品LFP和LFP-NQ的循环伏安曲线
Fig. 7 Cyclic voltammetry profiles of samples LFP and LFP-NQ (Sweep rate: 0.1 mV/s, sweep potential: 2.5-4.5 V)
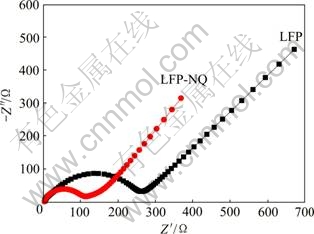
图8 样品LFP和LFP-NQ的交流阻抗谱
Fig. 8 EIS curves of samples LFP and LFP-NQ (Discharge state frequency range: 0.01 Hz-100 kHz; amplitude: 5 mV)
3 结论
1) 用液氮淬火法制备了锂离子电池正极材料LiFePO4/C,淬火并未改变LiFePO4的晶体结构,但使得晶体产生缺陷(如Li空位),提高了其电子导电性。
2) 淬火使得LiFePO4的形貌发生较大变化,使得其一次颗粒细小,并且可有效防止其团聚。液氮淬火制备的LiFePO4具有优异的倍率性能和循环性能。
REFERENCES
[1] PADHI A K, NANJUNDASWAMY K S, GOODENOUGH G B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries[J]. J Electrochem Soc, 1997, 144(4): 1188-1194.
[2] PROSINI P P, CAREWSKA M, SCACCIA S, WISNIEWSKI P, PASQUALI M. Long-term cyclability of nano-structured LiFePO4[J]. Electrochim Acta, 2003, 48(28): 4205-4211.
[3] CABALLERO A, CRUZ-YUSTA M, MORALES J, SANTOS-PENA J, RODRIGUEZ-CASTELLON R. A new and fast synthesis of nanosized LiFePO4 electrode materials[J]. Eur J Inorg Chem, 2006, 2006(9): 1758-1764.
[4] CHUNG S Y, BLOKING J T, CHIANG Y M. Electronically conductive phospho-olivines as lithium storage electrodes[J]. Nat Mater, 2002, 1(2): 123-128.
[5] PROSINI P P, LISI M, ZANE D, PASQUALI M. Determination of the chemical diffusion coefficient of lithium in LiFePO4[J]. Solid State Ionics, 2002, 148: 45-51.
[6] WANG D, LI H, SHI S, HUANG X, CHEN L. Improving the rate performance of LiFePO4 by Fe-site doping[J]. Electrochim Acta, 2005, 50(14): 2955-2958.
[7] SHI S, LIU L, OUYANG C, WANG D S, WANG Z, CHEN L, HUANG X. Enhancement of electronic conductivity of LiFePO4 by Cr doping and its identification by first-principles calculations[J]. Phys Rev B, 2003, 68(19): 195108.
[8] 伍 凌, 王志兴, 李新海, 李灵均, 郑俊超, 郭华军, 刘久清. 前驱体掺杂-常温球磨还原制备Ti4+掺杂LiFePO4[J]. 中南大学学报: 自然科学版, 2009, 40(2): 288-293.
WU Ling, WANG Zhi-xing, LI Xin-hai, LI Ling-jun, ZHENG Jun-chao, GUO Hua-jun, LIU Jiu-qing. Preparation of Ti4+-doped LiFePO4 by precursor-doping and room temperature reduction via ball-milling[J]. Journal of Central South University: Science and Technology, 2009, 40(2): 288-293.
[9] MEETHONG N, KAO Y H, SPEAKMAN S A, CHIANG Y M. Aliovalent substitutions in olivine lithium iron phosphate and impact on structure and properties[J]. Adv Funct Mater, 2009, 19: 1060-1070.
[10] MI C H, CAO Y X, ZHANG X G, ZHAO X B, LI H L. Synthesis and characterization of LiFePO4/(Ag+C) composite cathodes with nano-carbon webs[J]. Powder Technol, 2008, 181(3): 301-306.
[11] CROCE F, EPIFANIO A D, HASSOUN J, DEPTULA A, OLCZAC T, SCROSATI B. A novel concept for the synthesis of an improved LiFePO4 lithium battery cathode[J]. Electrochem Solid-State Lett, 2002, 5(3): A47-A50.
[12] CHEN Z, DAHN J R. Reducing carbon in LiFePO4/C composite electrodes to maximize specific energy, volumetric energy, and tap density[J]. J Electrochem Soc, 2002, 149(9): A1184-A1189.
[13] LIU H P, WANG Z X, LI X H, GUO H J, PENG W J, ZHANG Y H, HU Q Y. Synthesis and electrochemical properties of olivine LiFePO4 prepared by a carbothermal reduction method[J]. J Power Sources, 2008, 184: 469-472.
[14] 张 宝, 李新海, 朱炳权, 王志兴, 郭华军. 低温合成LiFePO4/C正极材料及其电化学性能[J]. 中南大学学报: 自然科学版, 2006, 37(3): 505-508.
ZHANG Bao, LI Xin-hai, ZHU Bing-quan, WANG Zhi-xing, GUO Hua-jun. Low temperature synthesis and electrochemical properties of LiFePO4/C cathode[J]. J Cent South Univ: Science and Technology, 2006, 37(3): 505-508.
[15] YU F, ZHANG J, YANG Y, SONG G. Preparation and characterization of mesoporous LiFePO4/C microsphere by spray drying assisted template method[J]. J Power Sources, 2009, 189: 794-797.
[16] 郑俊超, 李新海, 王志兴, 郭华军, 王丹琴. 制备过程pH值对FePO4·xH2O及LiFePO4性能的影响[J]. 中国有色金属学报, 2008, 18(5): 867-872.
ZHENG Jun-chao, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun, WANG Dan-qin. Effect of pH value on performance of FePO4·xH2O and LiFePO4 in synthesis process[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(5): 867-872.
[17] KIM D H, KIM J. Synthesis of LiFePO4 nanoparticles in polyol medium and their electrochemical properties[J]. Electrochem Solid-State Lett, 2006, 9(9): A439-A442.
[18] ZHENG J C, LI X H, WANG Z X, LI J H, LI L J, WU L, GUO H J. Characteristics of xLiFePO4·yLi3V2(PO4)3 electrodes for lithium batteries[J]. Ionics, 2009, 15: 753-759.
[19] WANG L, LI Z, XU H, ZHANG K. Studies of Li3V2(PO4)3 additives for the LiFePO4-based Li ion batteries[J]. J Phys Chem C, 2008, 112: 308-312.
[20] ZHENG J C, LI X H, WANG Z X, LI L J, LI J H, WU L, GUO H J. Li3V2(PO4)3 composite material with porous structure and nano-carbon webs synthesized through liquid nitrogen quenching[J]. Chem Lett, 2009, 38(8): 818-819.
[21] ZHANG B, ZHENG J C. Synthesis of Li3V2(PO4)3/C with high tap-density and high-rate performance by spray drying and liquid nitrogen quenching method[J]. Electrochim Acta, 2012, 67: 55-61.
[22] CHANG Y C, SOHN H J. Electrochemical impedance analysis for lithium ion intercalation into graphitized carbons[J]. J Electrochem Soc, 2000, 147(1): 50-58.
(编辑 龙怀中)
基金项目:中南大学博士后基金资助项目
收稿日期:2011-03-15;修订日期:2011-11-14
通信作者:张 明,博士;电话:13760027473;E-mail: zhangming_zq@163.com
摘 要:以FePO4、Li2CO3和葡萄糖为原料,用液氮急速淬火法制备单一橄榄石结构的锂离子电池正极材料LiFePO4/C。结果表明:淬火使得LiFePO4晶格中产生Li空位,有利于提高其电子导电性。淬火样品的一次颗粒细小(100~500 nm),无明显团聚,并形成多孔结构;该样品在1C、2C和4C倍率下的首次放电比容量分别为151.4、138.0和116.7 mA·h/g,循环100次后的容量保持率高达99.3%、98.6%和94.5%。


