
Ti3AlC2-Al2O3-TiAl3 composite fabricated by reactive melt infiltration
HE Shan-shan(何珊珊), YIN Xiao-wei(殷小玮), ZHANG Li-tong(张立同),
LI Xiang-ming(李向明), CHENG Lai-fei(成来飞)
National Key Laboratory of Thermostructure Composite Materials, Northwestern Polytechnical University,
Xi’an 710072, China
Received 15 December 2008; accepted 16 April 2009
Abstract:
Porous preforms were fabricated by cold-pressing process using powder mixture of TiC, TiO2 and dextrin. After pyrolysis and sintering, Al melt was infiltrated into the porous preforms, leading to the formation of Ti3AlC2-Al2O3-TiAl3 composite. Effects of cold-pressing pressure of preforms on microstructures and mechanical properties of the composites were studied. Synthesis mechanism and toughening mechanism of composite were also analyzed. The results shows that TiO2 is reduced into Ti2O3 by carbon, the decomposition product of dextrin, which causes the spontaneous infiltration of Al melt into TiC/Ti2O3 preform. Then, Ti3AlC2-Al2O3-TiAl3 composite is in-situ formed from the simultaneous reaction of Al melt with TiC and Ti2O3. With the increase of cold-pressing pressure from 10 MPa to 40 MPa, the pore size distribution of the preforms becomes increasingly uniform after pre-sintering, which results in the reduction of defects, and the decrease of property discrepancy of composites. Nano-laminated Ti3AlC2 grains and Al2O3 particles make the fracture toughness of TiAl3 increase remarkably by various toughening mechanisms including stress-induced microcrack, crack deflection and crack bridging.
Key words:
TiAl3; Ti3AlC2; reactive melt infiltration(RMI); toughening mechanism;
1 Introduction
The trialuminide intermetallic, TiAl3, is a potential thermo-structure composite material due to its advanced characteristics such as low density (3.3 g/cm3), high melting temperature (1 303 ℃), and good oxidation resistance. However, some weaknesses, such as low fracture toughness (2 MPa·m1/2), limit its potential applications[1-2]. Al2O3 can be used as the dispersive toughening phase of composite because of its high hardness (18 GPa) and high modulus (elastic modulus 386 GPa, shear modulus 175 GPa). Fracture toughness of Al2O3 toughened TiAl3 composite (Al2O3/TiAl3) can reach 5.0-8.6 MPa·m1/2[3-4]. Nano-laminated Ti3AlC2 is a novel polycrystalline material, which combines the best properties of both metals and ceramics[5-6]. A Ti3AlC2 unit cell is formed by two edges sharing Ti6C octahedra and close-packing Al elements arraying along carbon axis alternately. Layered Ti3AlC2 can increase fracture toughness of materials by delaminating along the weak basal plane and forming debonding, knot, bending and pullout during the fracture process. Hot-pressed sintering can be used to fabricate Ti3AlC2 with elastic modulus, compressive strength and bending strength of 289, 785 and 375 MPa, respectively[7-9]. Particularly, its fracture toughness could reach 7.2 MPa×m1/2[10], which is much higher than that of TiAl3 or Al2O3[1, 11]. Compared with TiAl3 and Al2O3, Ti3AlC2 has the most excellent thermal shock resistance[12]. Therefore, the introduction of Ti3AlC2 into Al2O3-TiAl3 may increase both toughness and thermal shock resistance of the composite.
Melt infiltration is often used to fabricate ceramic-metal and ceramic matrix composite. Al melt may be infiltrated into porous preform of TiO2, and Al2O3-TiAl3 composite may be in-situ formed from the reaction between Al and TiO2[4, 13]. Its reaction is: 13Al+3TiO2→3TiAl3+2Al2O3. Al2O3-TiAl3 composites fabricated by reactive gas-pressure infiltration and squeeze casting of Al melt into sintered porous preforms (30% TiO2 and 70% Al2O3 in volume fraction) had an interpenetrating network structure, which attained a fracture toughness of 8.6 MPa×m1/2. Reactive melt infiltration is pressureless reactive melt infiltration for short. It is a process in which the only driving force for spontaneous infiltration is the capillary force. Compared with squeeze casting and gas-pressure infiltration, reactive melt infiltration offers a possibility to produce freeform near-net shape composite at low production costs. The wettability of Al melt in the porous preform determines the extent and rate of spontaneous infiltration. When reactive melt infiltration is used to fabricate Ti3AlC2-Al2O3-TiAl3 composite, porous preform should have uniform pore size distribution and Al melt should have good wettability with ceramic phase of the preform. Powder cold-pressing is an effective process to control the pore size distribution and phase of preform, which may guarantee the spontaneous infiltration of metal melt [14].
In this work, a Ti3AlC2-Al2O3-TiAl3 composite was designed and fabricated by a combination process of powder cold-pressing and reactive melt infiltration. Effects of cold-pressing pressure of TiC/TiO2 preform on microstructure and mechanical properties of composite were studied. Synthesis mechanism of composite was studied based on the thermodynamic calculation and microstructure analysis. Toughening mechanisms of composite were investigated by analyzing its mechanical properties and crack propagation.
2 Experimental
2.1 Raw materials and preprocessing
The powder mixture was prepared by mixing TiC powder with an average particle size of 1-2 μm, nanoscale TiO2 powder with a mean crystallite size of 20 nm and dextrin powder ((C6H10O5)n?xH2O) with a mean particle size of 115 ?m. The mass ratio of TiC, TiO2, and dextrin to distilled water was 63?31?6?100. The powder mixture was wet ball-milled for 12 h and freeze-dried. Then, dry powder was ball-milled for 12 h and passed through a sieve with pore size of 200 mm. Al slices of appropriate dimensions were cut from 99.999 % Al band. The slices were ultrasonically cleaned in acetone for 20 min and dried for usage.
2.2 Composite preparation
The powder mixture was cold-pressed into three preforms, A1, B1 and C1 under a uniaxial pressure of 10, 20 and 40 MPa, respectively. Their dimensions were 70 mm×10 mm×5 mm. All of preforms were subsequently pyrolyzed in flowing Ar at 800 ℃ for 2 h, and then pre-sintered in flowing Ar at 1 400 ℃ for 0.5 h to achieve sintered preforms A2, B2 and C2. Then, Al slices were put onto sintered preforms for reactive melt infiltration. Composites were achieved by a three-step continuous annealing process in flowing Ar: firstly, the temperature was raised to 1 060 ℃ and dwelled for 20 min; secondly, raised to 1 200 ℃ and dwelled for 1 h; thirdly, raised to 1 400 ℃ and dwelled for 1.5 h. Heating rate and cooling rate were 5 ℃/min.
2.3 Thermodynamic calculation and characterization
The Gibbs free energy changes of the designed reactions at corresponding temperature were calculated, which was based on data of thermodynamic software FactSage (5.4.1, Canada) and Gibbs-Helmholtz equation [15]. For a reaction: aA+bB=cC+dD, the Gibbs free energy change of the reaction can be expressed as:
?G=c?G(C)+d?G(D)-a?G(A)-b?G(B) (1)
Microstructure of composite was examined by SEM (JEOL6700F, Japan), and elemental compositions of the fracture surface was examined by an energy dispersive X-ray spectrometer(EDS). Crystalline phase composition was analyzed by X-ray diffractometer(X’Pert Pro, Philips, Netherlands) using Cu Kα radiation at 40 kV and 35 mA. Determination of mass change was obtained by an electronic balance (DeltaRange Mettler Toledo AG204, Germany, 0.1 mg). The open porosity and the bulk density were measured by the water penetration method. Pore size distribution was measured by the automatic Hg-intrusion (Poremaster 33, Quantan, USA).
Micro-hardness was measured by analyzing the diagonals of the indentations impressed by a Vickers indenter (HBV-30A, Huayi, China) applying a load of 29.4 N for 15 s. Single edge notched bend (SENB) was used for fracture toughness evaluation following the ASTM E-399 specifications. The value of fracture toughness was calculated according to the following formula[16]:
![]() (2)
(2)
where f(c/w)=2.9(c/w)1/2-4.6(c/w)3/2+21.8(c/w)5/2-37.6×(c/w)7/2+38.7(c/w)9/2; pc is the critical load; B is the specimen width; S is the supporting span; c is the notch depth, w is the specimen height, and c/w is about 0.5. The dimensions of specimen for flexural strength and fracture toughness were 35 mm×5 mm×3 mm and 30 mm×6 mm×3 mm, respectively. The flexural strength and fracture toughness measurements were performed on a universal testing machine (SANS, CMT4304) at a cross-head speed of 0.5 mm/min and 0.05 mm/min with a loading span of 30 mm and 20 mm, respectively.
3 Results and discussion
3.1 Synthesis of Ti3AlC2- Al2O3-TiAl3 composite
Carbon produced from the decomposition of dextrin binder at 800 ℃ during pyrolysis process reduces TiO2 into Ti2O3 at 1 400 ℃ during pre-sintering in flowing argon, as shown in reaction (3)[17]:
2TiO2+C=Ti2O3+CO (3)
Composition changes of the preforms before and after sintering are shown in Fig.1. After pre-sintering, all of TiO2 is reduced into Ti2O3. During reactive melt infiltration process, Al melt is spontaneously infiltrated into the preforms containing TiC and Ti2O3 easily because the wettability of liquid Al on Ti2O3 is better than that of liquid Al on TiO2[18]. Improvement of the wettability of liquid Al on preform ensures its spontaneous infiltration. The subsequent reactions may occur:
Ti2O3+8Al=2TiAl3+Al2O3 (4)
TiAl3+2TiC=Ti3AlC2+2Al (5)
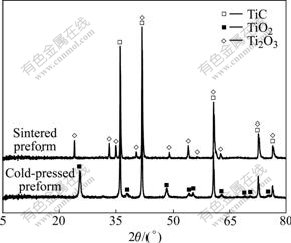
Fig.1 XRD patterns of sintered preform and cold-pressed preform
Reaction (4) and reaction (5) can be summarized as the following reaction:
2TiC+Ti2O3+6Al=Ti3AlC2+TiAl3+Al2O3 (6)
Fig.2 shows the change of Gibbs free energy(ΔG) as a function of temperature for reactions (4)-(6). Change of Gibbs free energy of Ti3AlC2 was obtained from Eq.(7) in Ref.[19] and the others were from the database of FactSage 5.4.1.
?G(MN+1AXN)=(N+l)?G(MX) (7)
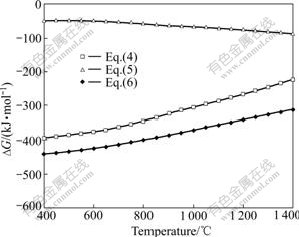
Fig.2 Change of Gibbs free energy (ΔG) as function of temperature for reactions (4)-(6)
Based on the thermodynamics analysis, reactions (4)-(6) are able to occur spontaneously in the experimental temperature range because their Gibbs free energy changes are obviously below zero. Therefore, there are two possible reaction paths in reactive melt infiltration process: 1) reaction between Al and Ti2O3 leads to the formation of TiAl3 and Al2O3, subsequently part of TiAl3 reacts with TiC to form the ternary phase Ti3AlC2; 2) Al melt is infiltrated into porous preform and reacts with TiC and Ti2O3 simultaneously, leading to the formation of Ti3AlC2-Al2O3-TiAl3 composite directly. If the former process takes place, TiAl3 may not coexist with TiC. If the latter process takes place, TiAl3 may have a chance to coexist with TiC in composite. Comparatively, the Gibbs free energy change of reaction (6) is more negative than that of reaction (4) and reaction (5). Thus, reaction (6) has priority to take place compared with reaction (4) and reaction (5), indicating that reaction path 2) is a more possible one during the fabrication procedure.
It is shown in Table 1 that densities of sintered preforms and their corresponding composites increase with increasing cold-pressing pressure while open porosities decrease. The spatial distance between particles decreases with increasing cold-pressing pressure, which results in the increase of the density of preforms. Fig.3 shows the pore size distributions of sintered preforms A2, B2 and C2. Their average pore diameters are 0.35-0.45 mm and the pore size distribution becomes narrow with the increase of cold-pressure. Sintered preform C2 has evenly distributed pores. Its compact structure is beneficial to the infiltration of Al melt. XRD result reveals that the as-received composite is mainly composed of Ti3AlC2, Al2O3, TiAl3, with residual TiC and Al (Fig.4).
Table 1 Physical properties of Ti3AlC2-Al2O3-TiAl3 composites and their sintered preforms
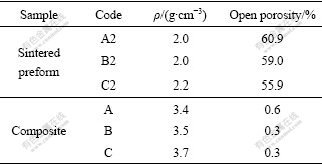
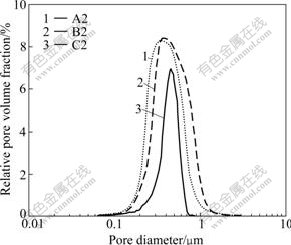
Fig.3 Pore size distributions of sintered preforms
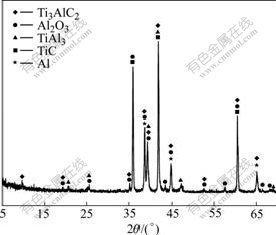
Fig.4 XRD pattern of Ti3AlC2-Al2O3-TiAl3 composite
3.2 Microstructure of Ti3AlC2- Al2O3-TiAl3 composite
Back-scattered electron(BSE) images of Ti3AlC2- Al2O3-TiAl3 composite are shown in Fig.5. With the increase of cold-pressing pressure, the microstructures of composites become increasingly compact with fewer defects. Comparatively, the composite C is free of defects such as cavity because its preform with a narrow pore size distribution is beneficial to the infiltration of Al melt. As-received composite contains two regions: M region and N region. According to XRD and EDS analysis, M region is mainly composed of Ti3AlC2, TiAl3, Al2O3 and a small amount of TiC whereas N region also contains a small amount of residual Al (Fig.6). M region can be called as TiAl3-rich region and N region can be called as TiAl3-poor region because the former has more TiAl3 than the latter. Known from Fig.6, TiAl3 can coexist with TiC in the composite, which is consistent with the result of thermodynamic calculation. This indicates that Ti3AlC2-Al2O3-TiAl3 composite is fabricated directly by Al melt reacting with TiC and Ti2O3 simultaneously. The higher cold-pressing pressure results in the formation of preform with lower porosity and smaller pore size, which is not beneficial to the flowing of TiAl3 phase (melting point of above 1 303 ℃[1]) during reactive melt infiltration up to 1 400 ℃. As a result, the composite C has the smallest amount TiAl3-rich regions, about 35%. Al2O3 particles and Ti3AlC2 grains in particulate and/or lamellar shapes are homogeneously embedded in TiAl3 matrix of both regions (Figs.6(a) and (b)). The TiAl3-poor region has more Al2O3 particles that left many pits after their pullout during fracture process (Fig.6(b)). Fig.6(c) displays the laminated structure of a Ti3AlC2 grain. This grain can absorb crack propagation energy to arrest propagating cracks and enhance the toughness of material when cracks encounter it during their propaga- tion. Known from theoretical calculations based on reaction (3) and reaction (6), as-received composite is composed (in volume fraction) of 34% Ti3AlC2, 24% Al2O3, 29% TiAl3, 8% TiC, and 5% Al.
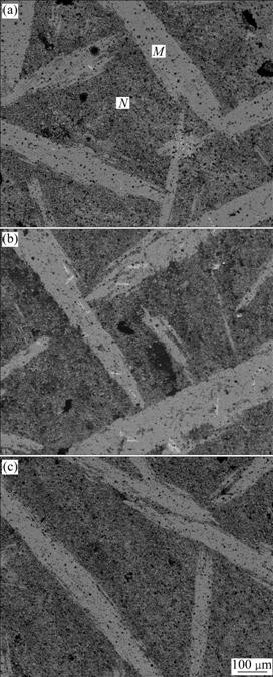
Fig.5 BSE images of polished section of composites (M denotes TiAl3-rich region, N denotes TiAl3-poor region): (a) Composite A; (b) Composite B; (c) Composite C
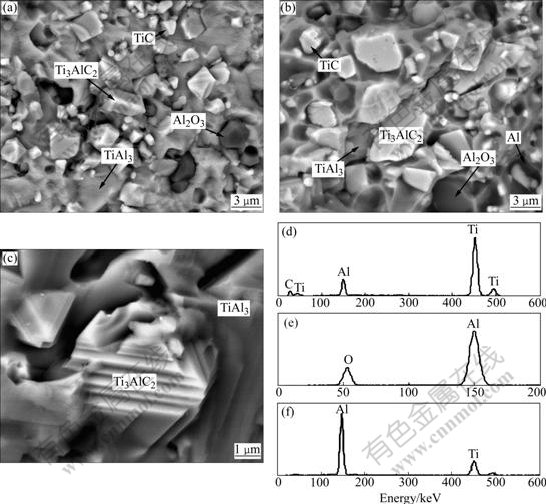
Fig.6 BSE images of fracture surface in TiAl3-rich region (a), TiAl3-poor region (b), typical morphology of Ti3AlC2 grain (c) of composite C and energy dispersive spectroscopy(EDS) microanalyses of Ti3AlC2 (d), Al2O3 (e) and TiAl3 (f)
3.3 Mechanical properties and toughening mechanisms of Ti3AlC2- Al2O3-TiAl3 composite
Table 2 lists the mechanical properties of Ti3AlC2- Al2O3-TiAl3 composites. Fracture roughnesses of composites A, B and C are close to 9 MPa·m1/2, which is much higher than that of TiAl3 or Al2O3. Bending strength of the composite can reach 288 MPa, which is close to that of Ti3AlC2. High fracture toughness of the composite indicates that Ti3AlC2 and Al2O3 had played an important role in toughening. Meanwhile, as-received composite is a soft material with a hardness of about 2 GPa, which is much lower than that of TiAl3 (6 GPa) and Al2O3 (10 GPa). Ti3AlC2 is easy to be processed because it has a special laminar structure, which is similar to the structure of graphite. Thus, the introduction of Ti3AlC2 into materials increases not only the fracture toughness but also the machinability of materials.
Table 2 Mechanical properties of Ti3AlC2-Al2O3-TiAl3 composite cold-pressed under different pressures

Mechanical property discrepancy of composite is decreased with increasing cold-pressing pressure of preform. Mechanical properties of composites A and B are similar because of their similar phase compositions. Composite C has mechanical properties with the smallest discrepancy, which is related to its narrow pore size distribution.
Toughening mechanisms of the as-received composite were revealed by analyzing their crack propagations. Microstructures of the representative crack propagation paths (Fig.7) reveal that variously complex toughening mechanisms can be found in the as-received composite including extensive crack deflection, crack bridging, crack branching, pullout and delamination of particle, which mostly results from particles toughening effect. Thermal expansion coefficient(TEC) mismatch is the root of particle toughened composite. Thermal expansion coefficient mismatch exists between the matrix TiAl3 and the secondary particles Ti3AlC2 and Al2O3. The residual stress originates upon cooling from the processing temperature because the thermal expansion coefficient mismatch can arrest crack[20]. Secondary particle will be under a residual thermal pressure(P) when it exist in a uniformly infinite matrix. Meanwhile, this pressure will produce a radial normal stress(στ) and a tangential normal stress(σt) in the matrix at the point with a distance of R from the centre of the particle. These stresses can be calculated by following equations[21-22]:
P=2?α?TEm/[(1+νm)+2β(1-2νp)] (8)
 (9)
(9)
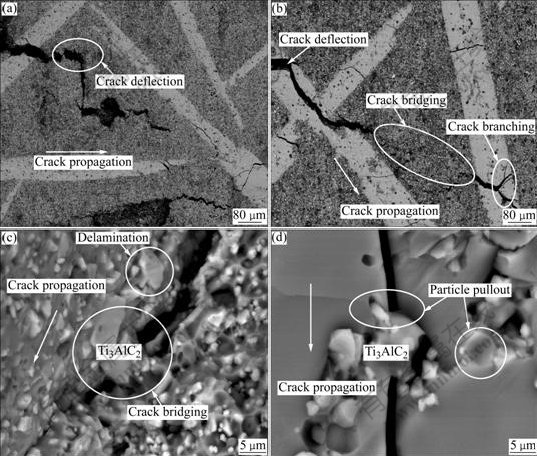
Fig.7 BSE images of crack propagations of Ti3AlC2-Al2O3-TiAl3 composites: (a) Lower magnification of TiAl3-poor region; (b) Lower magnification of TiAl3-rich region; (c) Higher magnification of TiAl3-poor region; (d) Higher magnification of TiAl3- rich region
where Δα=αp-αm, α is the thermal expansion coefficient; the subscript m and p refer to matrix and toughening particle, respectively; ΔT is the difference between the temperature TP and room temperature TR when plastic deformation can be neglected; E is the elastic modulus, β=Em/EP; ν is the Poisson’s ratio.
In Ti3AlC2-Al2O3-TiAl3 composite, the thermal expansion coefficient of TiAl3 matrix is higher than that of Ti3AlC2 grain or Al2O3 particle. Thus Δα<0, and then P<0, στ<0, σt>0, which result in a compressive stress in Ti3AlC2 and Al2O3, and a tangential tensile stress in TiAl3. In this case, spontaneously radial microcracks can be formed during cooling process if the sizes of Ti3AlC2 and Al2O3 exceed the critical size(dc), and microcracks cannot be formed if the sizes of Ti3AlC2 and Al2O3 are less than the minimum size(dmin). When the sizes of Ti3AlC2 and Al2O3 is between the minimum size(dmin) and the critical size(dc), the applied stress may induce the formation of microcracks around the main-crack tip and a corresponding associated process zone. This zone can shield the main crack with crack branching and crack deflection and enhance the resistance of crack propagation with increasing length of crack[23]. Known from SEM images (Figs.5 and 6), there are no obvious microcracks in the as-received composite before mechanical tests. However, obvious crack branching and crack deflections can be found in the composite after the bending test (Fig.7). These greatly prove the existence of stress-induced microcracks in the composite. Previous research also indicates that toughening effect could be enhanced by increasing crack deflection angle[24]. As shown in Fig.7(a), crack deflection with a big angle of 90? on the way of crack propagation constrains the crack propagation effectively. Crack deflection and crack branching are toughening processes occurring around the crack tip while crack bridging is the one occurring in a wide area after the crack tip. The mismatch of thermal expansion coefficients generates a ligament zone of residual compressive stress, resulting in interface glide, interface dissociation and secondary particles pullout to form crack bridging[25-26]. Crack propagates through the TiAl3-rich region and becomes narrow till it stops in the TiAl3-poor region, then, it continues propagating in another TiAl3-rich region in tension. The crack energy is exhausted by the alternate distribution of the two regions, and then crack propagation is stopped. Generally, Ti3AlC2-Al2O3-TiAl3 composite with high fracture toughness can be mainly ascribed to two concurrent toughening mechanisms: stress-induced microcrack, and crack deflection and bridging. Nano-laminated Ti3AlC2 grains improve the toughness of the composite by forming delamination and pullout to enrich the toughening mechanisms (Figs.7(c) and (d)).
4 Conclusions
1) Ti3AlC2-Al2O3-TiAl3 composite is in-situ fabricated by the combination process of cold-pressing and pressureless reactive melt infiltration. Thermodynamic calculation, phase analysis and microstructure analysis show that Al melt reacts with TiC and Ti2O3 simultaneously, leading to the formation of Ti3AlC2-Al2O3-TiAl3 composite directly.
2) With the increase of the cold-pressing pressure from 10 MPa to 40 MPa, the pore size distribution of the sintered preforms becomes increasingly uniform, and the capillary force for Al melt to infiltrate into preform increases, which results in the reduction of defects and the decrease of property discrepancy of composites. The composite using a cold-pressing pressure of 40 MPa attains a hardness of 2 GPa, a flexural strength of 288 MPa and a high fracture toughness of 9 MPa×m1/2.
3) Main toughening mechanisms of Ti3AlC2-Al2O3- TiAl3 composite include stress-induced microcrack, crack deflection and bridging. As a result, nano-laminated Ti3AlC2 grains and Al2O3 particles increase the fracture toughness of TiAl3 remarkably.
References
[1] MILMAN Y V, MIRACLE D B, CHUGUNOVA S I, VOSKOBOINIK I V, KORZHOVA N P, LEGK-AYA T N, PODREZOV Y N. Mechanical behaviour of Al3Ti intermetallic and Li2 phases on its basis [J]. Intermetallics, 2001, 9(9): 839-845.
[2] WANG Tao, ZHANG Jun-shan. Thermoanalytical and metallographical investigations on the synthesis of TiAl3 from elementary powders [J]. Materials Chemistry and Physics, 2006, 99(1): 20-25.
[3] WAGNER F, GARCIA D E, KPUPP A, CLAUSSEN N. Interpenetrating Al2O3-TiAl3 alloys produced by reactive infiltration [J]. J Europ Ceram Soc, 1999, 19: 2449-2453.
[4] PENG H X, WANG D Z, GENG L, YAO C K. Evaluation of the microstructure of in-situ reaction processed A13Ti-A12O3-Al composite [J]. Scripta Materialia, 1997, 37(2): 199-204.
[5] LI Shi-bo, ZHAI Hong-xiang, BEI Guo-ping, ZHOU Yang, ZHANG Zhi-li. Synthesis and microstructure of Ti3AlC2 by mechanically activated sintering of elemental powders [J]. Ceramics International, 2007, 33(2): 169-173.
[6] ZHOU Ai-guo, WANG Chang-an, HUANG Yong. A possible mechanism on synthesis of Ti3AlC2 [J]. Mater Sci Eng A, 2003, 352(1/2): 333-339.
[7] MEI Bing-chu, XU Xue-wen, ZHU Jiao-qun, LIU Jun. Study on preparation, structure and property of Ti3AlC2 fabricated by hot-pressed sintering [J]. Rare Metal Materials and Engineering, 2005, 34(5): 684-687. (in Chinese)
[8] TZENOV N V, BARSOUM M W. Synthesis and characterization of Ti3AlC2 [J]. J Am Ceram Soc, 2000, 83(4): 825-832.
[9] WANG X H, ZHOU Y C. Microstructure and properties of Ti3AlC2 prepared by the solid-liquid reaction synthesis and simultaneous in-situ hot pressing process [J]. Acta Materialia, 2002, 50(12): 3143-3151.
[10] WANG X H, ZHOU Y C. Oxidation behavior of Ti3AlC2 at 1 000- 1 400 ℃ in air [J]. Corrosion Science, 2003, 45(5): 891-907.
[11] CHEN J X, ZHOU Y C. Strengthening of Ti3AlC2 by incorporation of Al2O3 [J]. Scripta Materialia, 2004, 50(6): 897-901.
[12] BAO Y W, WANG X H, ZHANG H B, ZHOU Y C. Thermal shock behavior of Ti3AlC2 between 200 ℃ and 1 300 ℃ [J]. J Europ Ceram Soc, 2005, 25(14): 3367-3374.
[13] PAN J, LI J H, FUKUNAGA H, NING X G, YE H Q, YAO Z K, YANG D M. Microstructural study of the interface reaction between titania whiskers and aluminum [J]. Comp Sci and Tech, 1997, 57(3): 319-325.
[14] SKRINJAR O, LARSSON P L. Cold compaction of composite powders with size ratio [J]. Acta Materialia, 2004, 52(7): 1871-1884.
[15] XIAO Yan-fan, LI Wen-bin. Physicl and chemic [M]. Tianjin: Tianjin University Press, 1997: 174-175. (in Chinese)
[16] American society for testing materials (ASTM 399-74) [R]. Philadelphia, 1983: 923-936.
[17] YIN X W, TRAVITZKY N, GREIL P. Three-dimensional printing of nanolaminated Ti3AlC2 toughened TiAl3-Al2O3 composites [J]. J Amer Ceram Soc, 2007, 90(8): 2128-2134.
[18] BARIN I, KNACKE O, KUBASHEWSKI O. Thermochemical properties of inorganic substances [M]. Berlin: Springer-Verlag, 1991: 30-50.
[19] BARSOUM M W. The MN+1AXN phases: A new class of solids; thermodynamically stable nanolaminates [J]. Frog Solid State Chemistry, 2000, 28(1/4): 201-281.
[20] SAOUMA V E, CHANG S Y, SBAIZERO O. Numerical simulation of thermal residual stress in Mo- and FeAl-toughened Al2O3 [J]. Composites Part B: Engineering, 2006, 37(6): 550-555.
[21] DAVIDGE R W. Mechanical behavior of ceramics [M]. Cambridge: Cambridge University Press, 1979: 86-88.
[22] XU Zhi-lun. Elastic mechanics [M]. Beijing: Higher Education Publishing House, 1982: 279-280. (in Chinese)
[23] EVANS A G, FABER K T. The crack growth resistance of microcracking brittle materials [J]. J Amer Ceram Soc, 1984, 67(4): 255-260.
[24] FABER K T, EVANS A G. Crack deflection processes [J]. Acta Metall, 1983, 31(4): 565-566.
[25] EVANS A G. Perspective on the development of high-youghness ceramics [J]. J Amer Ceram Soc, 1990, 73(2): 187-206.
[26] BECHER P F. Microstructural design of toughened ceramics [J]. J Amer Ceram Soc, 1991, 74(2): 255-269.
Foundation item: Project(50802074) supported by the National Natural Science Foundation of China; Project(W016147) supported by the Sci-tech Innovation Foundation of Northwestern Polytechnical University, China
Corresponding author: YIN Xiao-wei; Tel: +86-29-88494947; E-mail: yinxw@nwpu.edu.cn
DOI: 10.1016/S1003-6326(08)60431-8
(Edited by YANG Hua)


